Abstract
The novel severe acute respiratory syndrome (SARS) coronavirus caused severe disease and heavy economic losses before apparently coming under complete control. Our understanding of the forces driving seasonal disappearance and recurrence of infectious diseases remains fragmentary, thus limiting any predictions about whether, or when, SARS will recur. It is true that most established respiratory pathogens of human beings recur in wintertime, but a new appreciation for the high burden of disease in tropical areas reinforces questions about explanations resting solely on cold air or low humidity. Seasonal variation in host physiology may also contribute. Newly emergent zoonotic diseases such as ebola or pandemic strains of influenza have recurred in unpredictable patterns. Most established coronaviruses exhibit winter seasonality, with a unique ability to establish persistent infections in a minority of infected animals. Because SARS coronavirus RNA can be detected in the stool of some individuals for at least 9 weeks, recurrence of SARS from persistently shedding human or animal reservoirs is biologically plausible.
“Whoever wishes to investigate medicine properly should proceed thus: in the first place to consider the seasons of the year…” Hippocrates (circa 400BC)
1 year after the devastating severe acute respiratory syndrome (SARS) outbreak of 2003, reports of four confirmed SARS cases in China's Guangdong province prompted widespread speculation that the disease was making a seasonal resurgence. There was no epidemic spread, however, and the source or sources of the 2004 viruses have not been definitively established. In one case, the virus was identified as genetically related to animal isolates.1 In the other cases, it is unclear whether the viruses were descendants of the 2003 human isolates or different lineages newly emergent from an animal reservoir.
The episode serves to illustrate one persisting mystery surrounding the emergence and sudden disappearance of the first major emerging infectious disease of the 21st century. It also highlights how little is understood about the underlying causes of annual variations in infectious diseases. Nearly every important respiratory pathogen of human beings exhibits distinct seasonal variations, yet after hundreds of years of observing and documenting this phenomenon modern science has only superficial observations and largely untested theories about the underlying causes. Is it the cold? Dry air? Crowding together of people indoors in winter? Where do pathogens such as influenza and respiratory syncytial virus (RSV) go in the summertime? Do they migrate across the equator and return the following winter, or do they remain present at low levels in human or animal populations until environmental or host conditions are suitable for re-emergence? With the spread of the SARS coronavirus halted, at least temporarily, and the devastating impact of the spring 2003 emergence still fresh in the minds of public-health authorities, an understanding of respiratory virus seasonality and an ability to predict how the novel coronavirus may act assumes greater importance.
Most established respiratory pathogens are seasonal
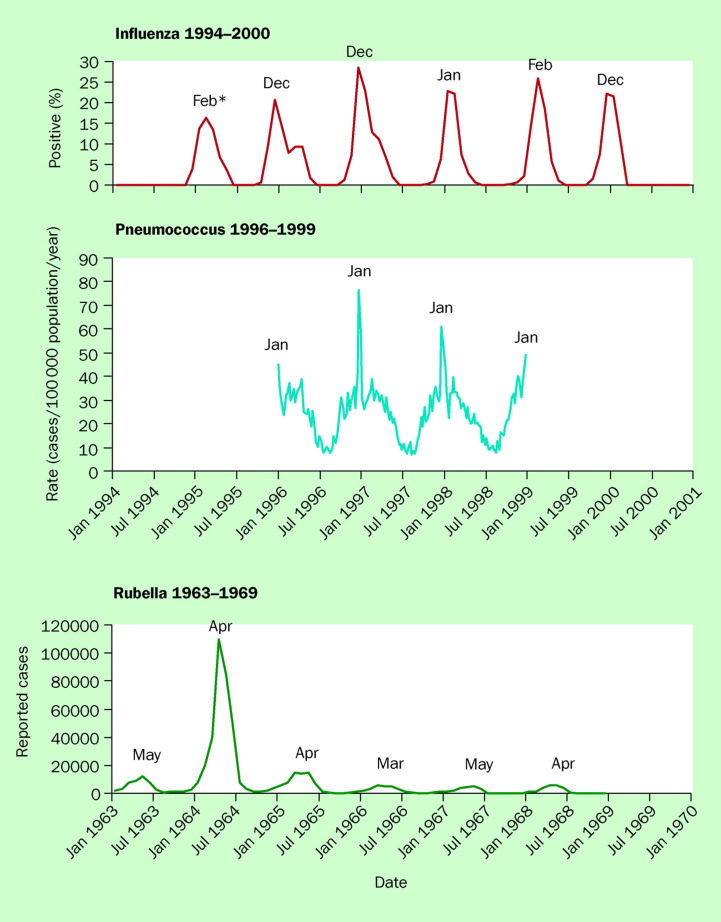
Most human respiratory pathogens exhibit an annual increase in incidence each winter, although there are variations in the timing of onset and magnitude of the increases (figure 1 ). This is true for bacterial pathogens such as the pneumococcus as well as for viruses transmitted by respiratory routes, such as rubella and influenza.
Figure 1.
Seasonal patterns of three established human respiratory pathogens: percentage of specimens testing positive for influenza viruses among specimens tested by WHO and US National Respiratory and Enteric Virus Surveillance System collaborating laboratories, month of peak incidence; weekly rates of invasive pneumococcal disease in the USA, January 1996–December 1998; and annual cycle of rubella activity, 1963–1969. Adapted from 2, 3, 4.
Influenza incidence peaks between November and April in northern temperate latitudes, with the seasonal increase coming between May and September in the southern hemisphere. Nearer the equator, it is commonly reported that influenza can occur year-round, but regular annual variation has nonetheless been documented in tropical areas such as Hong Kong, and even Singapore at just 3 degrees north latitude.5, 6, 7 In recent years it has also been recognised that small influenza outbreaks can occur in temperate latitudes during the summertime, although the effect of such outbreaks has been limited and generalised spread has not resulted.8, 9, 10, 11 The explanation for the lack of spread in the summer remains elusive.
Experiments in the 1940s and 1950s are widely cited as indicating that influenza virus is more stable in cool, dry air.12, 13 Later experiments indicated that this explanation might be insufficient, because when temperature and humidity were kept constant in a controlled laboratory setting, mice were still substantially more susceptible to influenza infection in winter than in summer.14 Many features of influenza seasonality are not satisfactorily addressed by explanations that rest on cold temperature or low humidity. For example, recent comprehensive influenza studies from Hong Kong have documented a burden of disease that is as high or higher than in the USA, despite the fact that Hong Kong is nearly always more warm and more humid.7 In addition, marked annual variations in incidence persist, even in areas where many spend summers in air-conditioned spaces, and similar strains of virus appear almost simultaneously across vast stretches of oceans in areas of similar latitude around the globe.15, 16 This latter feature has been particularly difficult to explain, leading some authorities to throw up their hands, and at least one investigator to propose the trans-Pacific migration of aerosolised virus on high-altitude trade winds.15, 17, 18
RSV activity also peaks in the late autumn, winter, or early spring in temperate latitudes, with the period of maximum activity often distinct from that of influenza.19 A recent comprehensive review of global RSV seasonal distributions identified consistent annual variation in incidence in tropical and temperate latitudes alike, with onset of activity occurring earlier in areas nearer to the equator, and moving from south to north during October through to April in North America, and north to south during July through to September in South America.20 The spread of the epidemic does not seem to be associated with the spread of the virus, since outbreaks of genetically similar strains appear simultaneously at similar latitudes across different continents. Recent findings of patients with chronic airways disease who were PCR-positive in all seasons have raised the novel possibility that RSV establishes a state of low-level presence in the population, emerging in epidemic form when conditions are right.21 Such a mechanism for seasonal recurrence is not widely accepted for human RSV disease, but there is some evidence from the annual re-emergence of bovine RSV in closed cattle herds that chronic low-level infections might be responsible for the persistence of the viruses in these herds through the off-season.22, 23 Conditions promoting the annual re-emergence of RSV might include climatic conditions, behaviour changes such as crowding, a susceptible birth cohort, or a seasonal physiological increase in host susceptibility.20, 21
A recent hypothesis proposes that the seasonal variation in many infectious diseases may result from variation in the susceptibility of the animal or human host.24 Under laboratory conditions, mice exhibit clear circadian rhythms in susceptibility to invasive pneumococcal disease (higher in the early morning hours than any other time of day).25 This biological variation in susceptibility seems also to be true for circumannual rhythms, such as the increased susceptibility to influenza in winter despite controlled temperature and humidity.14 The underlying physiological changes governing the seasonal variation in susceptibility have become an intensive area for research among experimental biologists,26, 27 but have received less attention from medical researchers. It remains to be seen whether SARS will prompt further investigations by highlighting the importance of an improved understanding of this area of medical research.
Newly emergent pathogens are unpredictable
The SARS coronavirus may have emerged from animal reservoirs to affect human beings as incidental hosts, and may therefore exhibit patterns of recurrence that are difficult to predict. In a similar example, the ebolavirus emerged from a presumed but still unidentified animal reservoir to cause large outbreaks in Zaire and Sudan in 1976 and then largely disappeared until its recurrence 18 years later (figure 2 ).30 The irregular pattern of ebola recurrence since that time indicates that if SARS behaves like a newly emergent zoonotic pathogen it may be difficult to predict when, or if, it will re-emerge.
Figure 2.
Seasonal patterns of two presumed zoonotic pathogens: recognised outbreaks of ebola, 1976–2003; and recognised outbreak of the SARS coronavirus. Compiled from 28, 29.
Influenza behaves both as an established human respiratory pathogen, with predictable seasonal recurrences,31, 32 and periodically as a newly emergent zoonotic disease, when shifted strains emerge from reassortment of avian or other animal influenza viruses to cause pandemics.31 Under the latter circumstance, seasonality is not predictable. The 1918 pandemic swept around the globe without regard to season, and planning for the 1957 pandemic in the USA was severely disrupted when the virus spread months earlier than predicted.15
In a recent example of newly emergent pathogens violating seasonal predictions, the first southern hemisphere occurrence of echovirus type 33, typically a summertime enteroviral infection, occurred in New Zealand during the winter of 2000.33 Illness was unusually severe, the novel seasonal pattern was puzzling, and it remains difficult to predict if or when cases will recur.
Animal coronaviruses are often seasonal and can establish persistence
A great deal is known about a range of animal coronaviruses, and this understanding may provide some insights into the possible future behaviour of the newest coronavirus. Many of these coronaviruses do exhibit a distinct seasonal pattern of incidence in their natural hosts. This is true for the bovine coronavirus associated with winter dysentery of calves,34, 35 as well as for the feline infectious peritonitis virus and others.36 The two previously described human coronaviruses (229E and OC43) both show distinct winter seasonality.37
The forces underlying these seasonal patterns are not well understood, but a common feature of most coronaviruses is their ability to establish long-term persistence in a subset of infected animals. For example, most cats recover from their feline influenza virus (FEIV) infection and stop shedding virus, but a minority are persistently infected, shedding virus in their stools for up to 7 years or more, and it is these animals that are the continuing source for FEIV outbreaks in catteries.36, 38 Symptomless and intermittent shedding of porcine coronavirus was documented on 24 occasions over a period of 18 months in one sow, and virulence of the shed virus was documented by the ability to infect 3-day-old piglets.39 Human coronaviruses may also establish persistent infections in vitro.40 Persistence of these RNA viruses involves changes in both virus and host cell, but evidence points to features of the host immune response to the initial infection that determines whether the animal will become a persistent shedder or will eradicate the infection.38, 40
It is plausible that the infection of animal SARS coronavirus in its natural host, be it palm civets or other animals yet to be identified, also assumes seasonality in such a way that it might increase the likelihood of animal-to-human transmission in the winter season. In January 2004, after the first two cases of SARS were reported in Guangdong, the Chinese government banned trading and farming of all civets in Guangdong province as soon as it was noted that civets shed high titres (109) of virus. Decision making for such a policy clearly included considerations for the impending winter high season of SARS virus activity.
Will SARS recur?
The observation that some patients shed detectable SARS viral RNA in their stools for at least 9 weeks after recovery is entirely consistent with what would be predicted based on the behaviour of other animal coronaviruses.41, 42 There is ample evidence that immune suppression may induce the persistence or recurrence of herpes viruses and other viral infections, and the high dosages of corticosteroids given to some of the SARS patients in the above studies may have artificially prolonged the shedding of viral RNA.
Nevertheless, if SARS has established persistent infections in a minority of those exposed during the 2003 outbreak, it may be that SARS will follow the model of other human respiratory pathogens and animal coronaviruses and re-emerge at a later time. Critical questions about what would prompt the re-emergence (weather, host susceptibility, host behaviour, virus factors) remain unanswered.
With a single year of experience with SARS and great uncertainties about its origins and previous seasonal patterns, it is very difficult to predict whether it will recur, and preparations for its recurrence are hampered. Will it emerge from an animal reservoir, will it break out from a research laboratory, or will it spread silently from persistently infected people and resume its global spread? Or will it eventually behave like many other seasonal respiratory viruses and emerge simultaneously, inexplicably, in bands of latitude around the globe at essentially the same time and in essentially the same format as it took when it went quietly into hibernation several months earlier?
Uncertainties about the seasonal recurrence of SARS are more than academic questions about the mechanisms underlying basic patterns of epidemiology. With the estimated economic impact of the first few months of SARS ranging from US$10 billion to 100 billion, and the accumulation of more than 8000 human cases and more than 700 deaths, the consequences of our ignorance regarding the seasonality of infectious diseases are glaringly apparent.43, 44 Major vaccine manufacturers lack persuasive information on predicted incidence to guide the enormous investments that would be required to rapidly develop a vaccine and make it available, and pharmaceutical companies, uncertain if a market for a new product will exist may delay developing antiviral drugs effective against coronaviruses.
It seems likely that SARS has not disappeared from the face of the earth forever, and biologically plausible that it will emerge again, perhaps when the changing seasons induce the physiological changes in its natural or incidental host that are necessary for epidemic spread to occur. Meanwhile, it is time for a critical examination of the state of knowledge on seasonality of infectious diseases and to give this ancient epidemiological phenomenon the modern attention it deserves.
Conflicts of interest
The authors were funded by their respective institutions and have no relevant conflicts of interest to declare.
References
- 1.Chinese SARS Molecular Epidemiology Consortium Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous Update: Influenza activity–United States, 1999–2000 season. MMWR Morb Mortal Wkly Rep. 2000;49:173–177. [PubMed] [Google Scholar]
- 3.Dowell SF, Whitney CG, Wright C, Rose CE, Jr, Schuchat A. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9:573–579. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witte J, Karchmer A, Case M. Epidemiology of rubella. Am J Dis Child. 1969;118:107–111. doi: 10.1001/archpedi.1969.02100040109018. [DOI] [PubMed] [Google Scholar]
- 5.Doraisingham S, Goh KT, Ling AE, Yu M. Influenza surveillance in Singapore: 1972–86. Bull World Health Organ. 1988;66:57–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Chew FT, Doraisingham S, Ling AE, Kumarasinghe G, Lee BW. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiol Infect. 1998;121:121–128. doi: 10.1017/s0950268898008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu SS, Lau YL, Chan KH, Wong WH, Peiris JS. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 8.Uyeki TM, Zane SB, Bodnar UR. Large summertime influenza A outbreak among tourists in Alaska and the Yukon Territory. Clin Infect Dis. 2003;36:1095–1102. doi: 10.1086/374053. [DOI] [PubMed] [Google Scholar]
- 9.Kohn M, Farley T, Sundin D, Tapia R, McFarland L, Arden N. Three summertime outbreaks of influenza type A. J Infect Dis. 1995;172:246–249. doi: 10.1093/infdis/172.1.246. [DOI] [PubMed] [Google Scholar]
- 10.Dowell SF. Low attack rate of summertime influenza: could it be the host? Clin Infect Dis. 2001;33:1951–1952. doi: 10.1086/324091. [DOI] [PubMed] [Google Scholar]
- 11.Laurel V, De Witt C, Geddie Y. An outbreak of influenza A caused by imported virus in the United States, July 1999. Clin Infect Dis. 2001;32:1639–1642. doi: 10.1086/320513. [DOI] [PubMed] [Google Scholar]
- 12.Hemmes J, Winkler K, Kool S. Virus survival as a seasonal factor in influenza and poliomyelitis. Nature. 1960;188:430–431. doi: 10.1038/188430a0. [DOI] [PubMed] [Google Scholar]
- 13.Loosli C, Lemon H, Robertson O, Appel E. Experimental airborne influenza infection. I. Influence of humidity on survival of virus in air. Proc Soc Exp Biol. 1943;53:205–206. [Google Scholar]
- 14.Schulman J, Kilbourne E. Experimental transmission of influenza virus infection in mice. II. Some factors affecting the incidence of transmitted infections. J Exp Med. 1963;118:267–275. doi: 10.1084/jem.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langmuir A, Schoenbaum S. The epidemiology of influenza. Hosp Pract. 1976;11:49–56. doi: 10.1080/21548331.1976.11707011. [DOI] [PubMed] [Google Scholar]
- 16.Thacker SB. The persistence of influenza A in human populations. Epidemiol Rev. 1986;8:129–142. doi: 10.1093/oxfordjournals.epirev.a036291. [DOI] [PubMed] [Google Scholar]
- 17.Hammond G, Raddatz R, Gelskey D. Impact of atmospheric dispersion and transport of viral aerosols on the epidemiology of influenza. Rev Infect Dis. 1989;11:494–497. doi: 10.1093/clinids/11.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hope-Simpson R, Golubev D. A new concept of the epidemic process of influenza A virus. Epidemiol Infect. 1987;99:5–54. doi: 10.1017/s0950268800066851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson WW, Shay DK, Weintraub E. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 20.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22(suppl):S21–S32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 21.Seemungal T, Harper-Owen R, Bhowmik A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 22.Van der Poel WH, Kramps JA, Middel WG, Van Oirschot JT, Brand A. Dynamics of bovine respiratory syncytial virus infections: a longitudinal epidemiological study in dairy herds. Arch Virol. 1993;133:309–321. doi: 10.1007/BF01313771. [DOI] [PubMed] [Google Scholar]
- 23.Larsen LE, Tjornehoj K, Viuff B. Extensive sequence divergence among bovine respiratory syncytial viruses isolated during recurrent outbreaks in closed herds. J Clin Microbiol. 2000;38:4222–4227. doi: 10.1128/jcm.38.11.4222-4227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7:369–374. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feigin RD, San Joaquin VH, Haymond MW, Wyatt RG. Daily periodicity of susceptibility of mice to pneumococcal infection. Nature. 1969;224:379–380. doi: 10.1038/224379a0. [DOI] [PubMed] [Google Scholar]
- 26.Nelson R, Drazen D. Melatonin mediates seasonal adjustments in immune function. Reprod Nutr Dev. 1999;39:383–398. doi: 10.1051/rnd:19990310. [DOI] [PubMed] [Google Scholar]
- 27.Demas G, Nelson R. Exogenous melatonin enhances cell-mediated, but not humoral, immune function in adult male deer mice (Peromyscus maniculatus. J Biol Rhythms. 1998;13:245–252. doi: 10.1177/074873098129000084. [DOI] [PubMed] [Google Scholar]
- 28.Arthur RR. Ebola in Africa–discoveries in the past decade. Euro Surveill. 2002;7:33–36. doi: 10.2807/esm.07.03.00342-en. [DOI] [PubMed] [Google Scholar]
- 29.WHO . Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) World Health Organization; Geneva: 2003. [Google Scholar]
- 30.Khan AS, Tshioko FK, Heymann DL. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(suppl 1):S76–S86. doi: 10.1086/514306. [DOI] [PubMed] [Google Scholar]
- 31.Cox N, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 32.Anonymous Update: influenza activity–United States and worldwide, 1995–96 season, and composition of the 1996–97 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1996;45:326–329. [PubMed] [Google Scholar]
- 33.Huang QS, Carr JM, Nix WA. An echovirus type 33 winter outbreak in New Zealand. Clin Infect Dis. 2003;37:650–657. doi: 10.1086/376915. [DOI] [PubMed] [Google Scholar]
- 34.Traven M, Naslund K, Linde N. Experimental reproduction of winter dysentery in lactating cows using BCV–comparison with BCV infection in milk-fed calves. Vet Microbiol. 2001;81:127–151. doi: 10.1016/S0378-1135(01)00337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho KO, Halbur PG, Bruna JD. Detection and isolation of coronavirus from feces of three herds of feedlot cattle during outbreaks of winter dysentery-like disease. J Am Vet Med Assoc. 2000;217:1191–1194. doi: 10.2460/javma.2000.217.1191. [DOI] [PubMed] [Google Scholar]
- 36.Foley JE, Poland A, Carlson J, Pedersen NC. Risk factors for feline infectious peritonitis among cats in multiple-cat environments with endemic feline enteric coronavirus. J Am Vet Med Assoc. 1997;210:1313–1318. [PubMed] [Google Scholar]
- 37.Hendley JO, Fishburne HB, Gwaltney JM., Jr Coronavirus infections in working adults. Eight-year study with 229 E and OC 43. Am Rev Respir Dis. 1972;105:805–811. doi: 10.1164/arrd.1972.105.5.805. [DOI] [PubMed] [Google Scholar]
- 38.Addie DD, Schaap IA, Nicolson L, Jarrett O. Persistence and transmission of natural type I feline coronavirus infection. J Gen Virol. 2003;84:2735–2744. doi: 10.1099/vir.0.19129-0. [DOI] [PubMed] [Google Scholar]
- 39.Woods RD, Wesley RD. Transmissible gastroenteritis coronavirus carrier sow. Adv Exp Med Biol. 1998;440:641–647. doi: 10.1007/978-1-4615-5331-1_83. [DOI] [PubMed] [Google Scholar]
- 40.Arbour N, Cote G, Lachance C, Tardieu M, Cashman NR, Talbot PJ. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J Virol. 1999;73:3338–3350. doi: 10.1128/jvi.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren Y, Ding HG, Wu QF. Detection of SARS-CoV RNA in stool samples of SARS patients by nest RT-PCR and its clinical value. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:368–371. [PubMed] [Google Scholar]
- 42.Leung WK, To KF, Chan PK. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan E. SARS: economic impacts and implications. Asian Development Bank; Manila: 2003. pp. 1–10. [Google Scholar]
- 44.Pearson H, Clarke T, Abbott A, Knight J, Cyranoski D. SARS: what have we learned? Nature. 2003;424:121–126. doi: 10.1038/424121a. [DOI] [PMC free article] [PubMed] [Google Scholar]