Abstract
The Ebola outbreak that has devastated parts of west Africa represents an unprecedented challenge for research and ethics. Estimates from the past three decades emphasise that the present effort to contain the epidemic in the three most affected countries (Guinea, Liberia, and Sierra Leone) has been insufficient, with more than 24 900 cases and about 10 300 deaths, as of March 25, 2015. Faced with such an exceptional event and the urgent response it demands, the use of randomised controlled trials (RCT) for Ebola-related research might be both unethical and infeasible and that potential interventions should be assessed in non-randomised studies on the basis of compassionate use. However, non-randomised studies might not yield valid conclusions, leading to large residual uncertainty about how to interpret the results, and can also waste scarce intervention-related resources, making them profoundly unethical. Scientifically sound and rigorous study designs, such as adaptive RCTs, could provide the best way to reduce the time needed to develop new interventions and to obtain valid results on their efficacy and safety while preserving the application of ethical precepts. We present an overview of clinical studies registered at present at the four main international trial registries and provide a simulation on how adaptive RCTs can behave in this context, when mortality varies simultaneously in either the control or the experimental group.
Introduction
The crushing Ebola virus disease outbreak that has devastated parts of west Africa is the largest recorded in history and represents an unprecedented challenge for health policy, research, and ethics.1
Although Ebola has affected people of all ages and both sexes, many of the people affected by the epidemic are young adults (aged 15–44 years)2 who represent the social and economic backbone of already fragile local communities. The best available figures to estimate the size of the outbreak are chilling and underline a strong geographical inequity that shows the uneven capability of different countries to afford interventions, to contain transmission, and to care for infected people.2, 3 In the three most affected countries (Guinea, Liberia, and Sierra Leone), the effort to contain human-to-human transmission has been grossly insufficient. As of March 25, 2014, WHO4 reported 24 907 Ebola virus disease cases and 10 326 deaths (41% mortality): 3429 cases from Guinea (2263 deaths), 9602 cases from Liberia (4301 deaths), and 11 841 cases from Sierra Leone (3747 deaths). Other cases have been reported from Mali (eight cases, six deaths), Nigeria (20 cases, eight deaths), Senegal (one case), Spain (one case), UK (one case), and USA (four cases, one death).4
Faced with such an exceptional event, WHO declared that it “is ethical to offer unproven interventions with as yet unknown efficacy and adverse effects, as potential treatment or prevention”.5 Although this is a reasonable statement under the circumstances, some experts argued that well designed randomised controlled trials (RCTs) are both unethical and infeasible in the present circumstances and that researchers should first try to ascertain which intervention is efficacious by doing observational or non-comparative studies.6 We argue that this represents an inferior strategy and that instead, RCTs should be used from the early stages of human experimentation of candidate Ebola interventions.7, 8
What has been done until now
The most sensible, and ethically acceptable, strategy for planning interventions during the largest Ebola virus disease outbreak ever recorded should have been to favour clinical studies located in the most affected areas to assess whether new therapeutic options could help those who are in the greatest need—ie, patients with acute Ebola virus disease—although little was done in this vein.
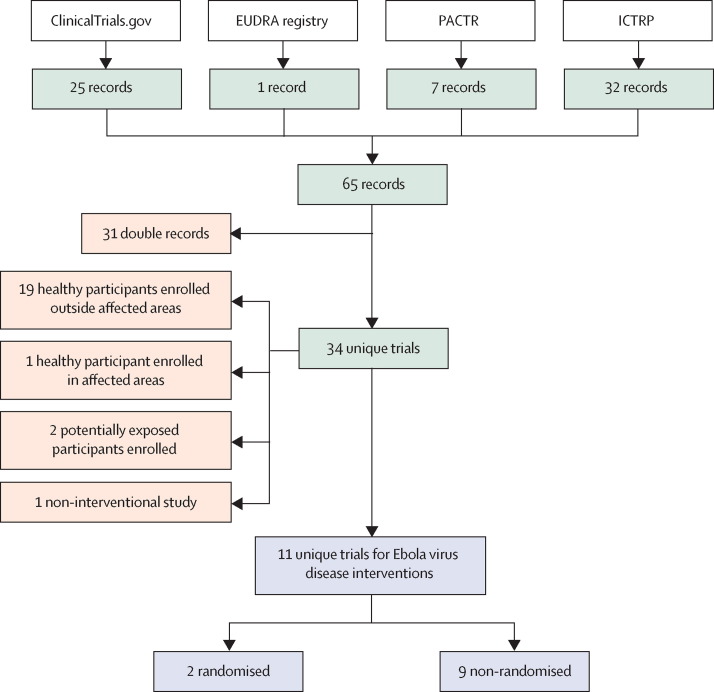
Of the 34 clinical studies submitted to the four main international registries (figure 1 ): 20 are enrolling healthy participants (only one is located in the main outbreak area), one is an observational study, two are studies launched to assess isolation measures in contacts, and only 11 (a third) are studies aimed to assess the efficacy of new interventions for patients with acute Ebola virus disease.
Figure 1.
Flowchart for selection of clinical trials enrolling patients with acute Ebola virus disease to assess efficacy or safety, or both, of new interventions
Records or trial to be selected (green boxes); excluded records or trials (orange boxes); analysed trials (blue boxes). EUDRA=European Union Drug Regulatory Authorities. PACTR=Pan African Clinical Trial Registry. ICTRP=International Clinical Trials Registry Platform (WHO).
The analysis of these 11 studies is even more dissatisfying. Only two are RCTs and only one is located in the outbreak area but is not yet recruiting. The other nine studies are aimed at assessing efficacy against non-randomised controls—three of these studies are enrolling participants at present. Table 1 outlines the study design and enrolment status of each study, as of March 1, 2015, and table 2 reports present knowledge about the potential safety and efficacy of the experimental interventions under investigation.
Table 1.
Description of registered clinical trials that enrol participants with acute Ebola virus disease to assess efficacy or safety, or both, of new therapies by registration number
| Ebola virus disease diagnosis | Location | Recruitment status | Sponsor | Main outcome | Size (design)* | Intervention model | Patent | First received | Anticipated completion date | |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomised | ||||||||||
| NCT02307591; PACTR201501001014425 | Laboratory confirmed | Sierra Leone | Not recruiting | Emergency Onlus | Safety, efficacy | Up to 132 in two groups (parallel assignment)† | Amiodarone + sSC vs sSC alone | Expired | Nov 21, 2014 | August, 2015 |
| NCT02363322 | Laboratory confirmed | USA | On invitation | NIAID, USA | Safety, efficacy | Up to 1000 in two groups (parallel assignment)† | ZMapp + sSC vs sSC alone | Mapp Bio | Feb 13, 2015 | December, 2016 |
| Non-randomised | ||||||||||
| PACTR201411000939962 | Laboratory confirmed | Liberia | Withdrawn | University of Oxford | Safety, efficacy | 140 (single arm) | 140 brincidofovir vs HCC | Chimerix‡ | Nov 14, 2014 | June, 2015 |
| NCT02342171 | Laboratory confirmed | Guinea | Not recruiting | ITM, Belgium | Safety, efficacy | Up to 400 in two groups (convenient allocation)§ | ECP + sSC vs sSC in HCC | NA | Jan 12, 2015 | October, 2015 |
| ChiCTR-OON-14005558 | Clinical | Sierra Leone | Recruiting | China Army | Efficacy | Up to 60 in two groups (convenient allocation)¶ | QBD + XBJ + ST vs western drugs‖ | NA | Nov 29, 2014 | .. |
| NCT02333578 | Laboratory confirmed | Liberia | Recruiting | Clinical RM | Safety, efficacy | 70 (single arm) | ECP vs HCC | NA | Jan 5, 2015 | June, 2015 |
| NCT02329054 | Laboratory confirmed | Guinea | Recruiting | INSERM, France | Safety, efficacy | 225 (single arm)†** | Favipriravir + sSC vs HCC | Toyama Chemical | Dec 16, 2014 | June, 2015 |
| NCT02295501 | Laboratory confirmed | USA | Recruiting | Cerus Corporation | Safety, efficacy | 12 (single arm) | INTERCEPT†† ECP | Cerus Corporation | Nov 4, 2015 | January, 2016 |
| NCT02271347; EUDRA-2014–004450–33 | Laboratory confirmed | Europe, North America | Withdrawn | Chimerix UK Limited | Safety, efficacy | 50 (single arm) | Brincidofovir | Chimerix‡ | Oct 7, 2014 | .. |
| PACTR201501000997429 | Laboratory confirmed | Sierra Leone | Not recruiting | University of Oxford | Safety, efficacy | 100 (single arm) | TKM-Ebola | Tekmira | Jan 16, 2015 | June, 2015 |
| JPRN-UMIN000016101 | Laboratory confirmed | Japan | Not recruiting | NCGHM, Japan | Safety, efficacy | 5 (single arm) | Favipriravir | Toyama Chemical | Jan 2, 2015 | .. |
sSC=standardised supportive care (ie, in comparative studies when standardised control treatment is reported). HCC=historical or concurrent controls (non-random). ECP=Ebola convalescent plasma. NA=not applicable. QBD=Qingwenbaidu decoction (herbal product). XBJ=Xuebijing injection (herbal product). ST=symptomatic therapy.
No study uses allocation concealment (masking).
Adaptive design (ie, any deign that uses interim analyses to modify study design).
On Feb 1, 2015, Chimerix said it would stop participation in clinical studies because of a substantial decrease in the number of new cases of Ebola virus disease.
No patient will be refused ECP; control will be patients with Ebola virus disease recruited during the period before ECP becomes available or for whom no compatible convalescent plasma is available.
Allocation on voluntary base.
If western drug (ie, not traditional Chinese medicine) and ST are unspecified the study is reported as observational.
Final analysis will be done according to three different groups: (A1) adults with time between first symptoms and first dose of favipiravir (<72 h); (A2) adults with time between first symptoms and first dose of favipiravir ≤72 h; and (C) all children.
INTERCEPT is a US Food and Drug Administration approved system for ex-vivo preparation of plasma to reduce the risk of transfusion-transmitted infection during treatment of patients needing therapeutic plasma transfusion.
Table 2.
Interventions for which a clinical trial has been proposed during the present outbreak
|
Present knowledge |
Potential issues for large-scale use in Africa | |||
|---|---|---|---|---|
| Mechanism of action | Safety | Efficacy | ||
| Amiodarone | Inhibition of viral entry | Widespread human use for more than 30 years; toxic effects are mainly reported for long-lasting use; potential acute toxic effects in case of low potassium concentrations in blood | In-vitro data show significant suppression of viral replication and infectivity at the same plasma concentration reached for clinical management of arrhythmia;9 unpublished data on case-by-case use has not provided clear evidence for or against efficacy so far | No available in-vivo evidence for efficacy; however, the drug is easy to administer (available in both intravenous and oral routes, and is thermostable); the drug is low cost and already available for large-scale use |
| ZMapp | Neutralising antibody | Data on human beings are very restricted | 100% efficacy on NHP;10 case-by-case experiences on human beings are very restricted but promising11 | Difficult to administer, potentially very expensive, and no guarantee exists that production can be scaled for wide use |
| Brincidofovir | Unclear | Tested in a clinical trial for DNA viruses; generally better tolerated than the already approved cidofovir12 | Unpublished data from Viral Special Pathogens Branch (USA) revealed that in-vitro activity of brincidofovir against the Ebola virus is similar to that reported against other viral diseases; no animal data;13 was used on two occasions in human beings with Ebola virus disease (one died and one survived) | The manufacturer has recently decided to stop experimentation in human beings |
| ECP | Neutralising antibody | Mainly transfusion related | Whole blood and ECP have been already used as empirical treatments with promising results in a small group of cases of Ebola virus disease12, 14, 15 | WHO has already developed a guidance for use of ECP;15 potential limitations are related to availability and risk for transmission of infections other than Ebola virus disease |
| QBD + XBJ | Immunomodulators16, 17 | Not assessed according to stringent regulatory authority requirements; however, human use is presumed to be widespread in China; both drugs are sold online | No available data on patients with Ebola virus disease | .. |
| Favipiravir | Inhibitor of viral RNA-dependent RNA polymerase | Well tolerated in patients without Ebola virus disease; evidence from large clinical trials; the drug is approved for human use in Japan at present; preliminary data exist on patients with Ebola virus disease18 | Evidence from studies in vitro and in small animals for activity against Zaire ebolavirus;19 preliminary data on human use (case-by-case use and early analysis of trials) has not provided evidence on efficacy so far | Favipiravir is conveniently formulated in oral thermostable tablets, but cost might be high as it is a patented drug; Toyama Chemical announced in October, 2014, that it had 20 000 courses of treatment in stock |
| TKM-Ebola | Cleaves Ebola RNA inside the cell | Increased cytokines in safety studies on human beings;12 FDA suspended phase 1 in July, 2014; in August, 2014, the FDA changed the status to partial hold, allowing the drug to be used under expanded access in people infected with Ebola virus but with the phase 1 trial still suspended (NCT02041715) | Up to 100% efficacy in NHP12 | Potentially very expensive and no guarantee exists that production for wide use can be scaled |
NHP=non-human primates. ECP=Ebola convalescent plasma. QBD=Qingwenbaidu decoction (herbal product). XBJ=Xuebijing injection (herbal product). FDA=US Food and Drug Administration.
14 months after the start of the outbreak—which has caused more than 10 300 deaths as of March 25, 2015,4—the best evidence base for Ebola virus disease treatment is a handful of anecdotal experiences in high-resource settings,11, 13, 20, 21 which are hardly reproducible in Africa. The unrealistic notion that three uncontrolled studies (one of which is testing herbal remedies against supportive therapy) could succeed in showing an intervention to have substantial effectiveness against one of the deadliest human infections shows the exceptional scarcity of trial investments made so far in the face of the an outbreak that is still not under control. Even if somehow the present epidemic is eventually contained (something that is far from certain), the world will still be totally unprepared for the next epidemic that could strike again at any time in an equally explosive manner.
Ethical considerations on RCTs
RCTs are widely deemed to be the most important vehicle for generating evidence about the efficacy and safety of novel interventions. The ethical basis of RCTs relies on the principle of clinical equipoise (ie, no genuine evidence exists that an experimental treatment is better than the standard of care) and individual uncertainty (ie, clinical investigators and enrolled patients are substantially uncertain about the merits of the experimental treatment). By providing a virtually unbiased comparator, RCTs guarantee, at best, robustness of results about both the efficacy and safety of investigational drugs. Thus, since the inception of RCTs, researchers have acknowledged that until an intervention has been proven beneficial, randomisation is the most ethical approach and provides the best answer soonest.7, 22, 23, 24, 25, 26 The idea that RCTs are ethically unjustified in the present Ebola outbreak might be based on several widespread misconceptions.
The first is a somewhat fatalistic assumption that case fatality rates always exceed 70% because no standard of care exists that can substantially affect the clinical outcomes of patients with Ebola virus disease.6 This assumption is incorrect because enough evidence exists from previous27 and present Ebola outbreaks2 that standard supportive therapy can significantly reduce mortality.28, 29 Remarkably, reported case fatality rates range widely between less than 50% to more than 70% according to the different countries where patients are treated.2, 7 This variability is probably because of the application of supportive therapy and other intangible differences across studies and settings.
The second is the overoptimistic assumption that drug efficacy in preclinical studies unequivocally translates into significant benefits towards the clinical outcomes of patients.30
The third is that in phase 1 and 2 research, non-randomised designs are preferable, merely because they are widely used8 or easily accepted by local communities.
We believe that when ethical aspects of non-randomised studies are considered in the midst of the most terrifying Ebola virus outbreak ever recorded several topical answers arise.31 First, how will non-randomised studies affect global capability to manage present and future Ebola outbreaks? Second, will non-randomised studies guarantee a reliable assessment of safety of new treatments? Third, will non-randomised studies have an immediate effect of ameliorating health-care standards in the location where the study is set, when general improvements in patient care might be as important for reducing mortality as any experimental intervention? Fourth, are non-randomised trials ethical for testing treatments that still do not have a line of mass-scale production and thus can only be used for a few, selected patients? Finally, which kinds of so-called alternative study designs should be approved, which kinds should not be acceptable, and, most importantly, what will the selection criteria be for distinguishing between these choices?
Previous experiences with the severe acute respiratory syndrome outbreak and H1N1 pandemic influenza32 suggest that these issues can be reasonably met by well designed RCTs, whereas no guarantee is provided by the possibly disorganised implementation of non-randomised studies based on subjective perceptions rather than scientifically sound and rigorous methods.
Randomised designs are better and safer than non-randomised studies
In the present situation of perceived impotence and absence of reliable estimates about the real efficacy of present medical interventions, no guarantee exists that the efficacy and safety of the new therapies can be assessed without a comparable control group—only randomisation can provide this guarantee.7, 33 Non-randomised studies will inevitably produce contrasting results with the risk of fostering uncertainty among experts while ultimately jeopardising the effort to produce clear and feasible clinical guidance.
Similar to other investigators,34 we think that well designed RCTs with adaptive study design35 should be endorsed in this crisis and preferred to non-randomised designs for several reasons.
First, no unequivocal data about the case fatality rates of Ebola virus disease exist. The most reliable estimates from WHO range between 46% in Nigeria (ie, the most affluent setting) to 72% in Liberia (ie, the least affluent setting).2 In such circumstances, non-randomised studies will provide clinicians with no reliable means to assess promptly the safety and efficacy of new treatments, thus making room for larger ethical dilemmas than those arising from random allocation of interventions.
Unless a new treatment has great effectiveness (eg, by decreasing mortality to close to 0% in series of hundreds of patients), we might continue to have substantial uncertainty about whether a treatment works at all for many years after the trial finishes. If a treatment does confer such great effectiveness, an RCT should be able to detect and document the effectiveness very quickly. However, if effectiveness is only slight, external comparators (both concurrent or historical) in observational series or non-comparative studies could be severely biased because of high and undocumented patient heterogeneity and will probably differ systematically between patients who received treatment and patients who did not. By contrast, interim analyses in RCTs can, at best, inform clinical investigators on whether to proceed with the same randomised allocation, either shifting all patients to an intervention group with an effective treatment or stopping the treatment with a potentially unsafe drug.7 The focus of an interim analysis is to respond rapidly to high quality data emerging from an RCT, rather than in an RCT without an interim analysis wherein researchers blindly treat and hope for a positive outcome.
Second, non-randomised or compassionate use of various interventions might happen anyhow. The challenge is not to encourage additional compassionate use and so-called hints and guesses, but to put together a robust RCT agenda. In fact, for proposed interventions that do not have an established line of large-scale production, and whose availability is very restricted (eg, ZMapp), use in non-randomised studies, instead of RCTs, is not straightforward. Such use wastes the already small sample size of potential RCTs that could have been done with the restricted available stock and thus negates the chances of being able to understand whether these treatments are effective or are not.33
Third, as recommended by WHO,5, 34 well designed RCTs will ensure that all patients receive at least the best feasible care, which at present is standard supportive care,28, 29 and that “investigational therapeutic or prophylactic options should not divert attention or resources from the public health measures that remain the main priority in outbreak control”.5 In this view, RCTs should be designed to have a control group with standard supportive care (as deemed feasible in the sites where the trials are done) and an experimental group, consisting of one or more new drugs in addition to the standard care. Moreover clinical centres should be able to implement infection control measures in advance to prevent health-care associated transmission of infection. The sponsors of such studies should provide the clinical facilities and adequate resources to ensure that the standard supportive care meets minimum requirements of good clinical practice. The rigorous implementation and monitoring of interventions that are agreed by consensus as being practicable in the context of local care, would help to set minimum ethically acceptable practices for treatment and infection control. By contrast, the deregulated scenario, where any new drugs with unproven efficacy can be used, provides no such guarantee. Of course, we do not advocate that resources for RCTs should be drawn from the restricted health-care resources of Ebola-stricken sites. RCTs should bring along additional resources to this acute crisis.
Fourth, as also acknowledged by others,36 some non-randomised clinical study designs could even undermine present thinking. Particularly, when scarcely available treatment is given to consecutive series of patients without them being randomly assigned to a treatment group; the patients selected might be systematically different from their historical or contemporary control groups and the selection rules might choose sicker patients than the population average who might have the worst probability of responding, thus potentially condemning to failure even treatments that could have been effective in earlier stages of the disease than were treated.
Finally, in the present context of emergency, much of the information about the outcome of patients who receive the so-called new treatment might not be systematically collected and analysed in the absence of a clear study framework.
Testing the feasibility of adaptive RCTs with a standard simulation approach
The major challenge in the present Ebola crisis is not whether RCTs should be used or not, but how their efficiency can be increased, producing the desirable answers faster.
In view of the idea that a scientifically sound and rigorous method does not compromise ethics but in fact is the prerequisite to implement ethical precepts by production of valid and reproducible results,31 we propose that adequate and well controlled studies can be safely done in the present Ebola outbreak and argue that the adaptive RCT approach is better than approaches proposed at present (table 1). Adaptive RCTs, particularly, can reasonably overcome the main objections raised against randomisation in emergencies and related to the ethical issue that a substantial number of patients would not receive a treatment with potentially (extraordinary) effectiveness. As acknowledged by the European Medicines Agency, “adaptive design would be best utilised as a tool for planning clinical trials in areas where it is necessary to cope with difficult experimental situations”.37
To lend support to our idea we tested the performance of an ideal two-arm adaptive RCT aimed at assessing the efficacy of the addition of a specific investigational drug to the present standard of care (ie, supportive therapy) by comparison with the standard of care alone. The RCT design and simulation has been done according to requisites for “generally well understood adaptive designs with valid approaches to implementation”.38
The proposed RCT will have a maximum sample size of 210 participants and two interim analyses, allowing early cessation for efficacy (group sequential design) and toxicity stopping rules (decided by an independent data monitoring committee). The simulation was done to show how the RCT will behave in response to the simultaneous variation of the reported mortality in either treatment or control group. The full description of the RCT is reported in the panel and the results of the simulations are reported in figure 2 .
Panel. Randomised controlled trial description.
A priori assumption
Scenario 1: π1=50% and π2=30%
Scenario 2: π1=60% and π2=40%
Scenario 3: π1=70% and π2=50%
Efficacy (as difference): π2 – π1=–20%
Efficacy (as ratio): odds ratio between 0·43 and 0·44—ie,
Minimum and maximum sample size
70–210 participants
Intervention and drugs
Control group: standard supportive care
Experimental group: standardised supportive care + experimental drug
Study design
Intervention model: parallel assignment
Allocation ratio: 1:1
Masking: open
Primary purpose: treatment
Early stopping for efficacy: sequential group design
Stopping for toxicity: establishment and operation of clinical trial data monitoring committee
Adaptive framework
Null hypothesis: π2 – π1=0
Conditional power: 80%
Test: two-sided χ2 with continuity correction
Number of interim analyses: two (ie, three stage design)
Sample size per stage: 35 per group fixed (ie, no sample size recalculation)
Information rate per stage: 33·3% (uniform)
α spending model: O'Brien and Fleming design
α spending function: 0·0005 (stage 1); 0·0143 (stage 2); 0·0500 (stage 3)
Potential inflation in comparison with standard design: 1·7% (to accept H0 with 80% power)
Follow-up (maximum)
14 days after enrolment
Approximate study duration
2–6 months; depending on:
-
•
Study location
-
•
Phase of outbreak and effective reproduction number
-
•
True mortality in overall population (figure 2)
-
•
True efficacy of the experimental drug (figure 2)
Simulator specification
Software: ADDPLAN TM 6.1.1 ADDPLAN (approved by FDA, EMA, and PMDA)
π1=morality in control group. π2=mortality in experimental group. FDA=US Food and Drug Administration. EMA=European Medicines Agency. PMDA=Pharmaceuticals and Medical Devices Agency, Japan.
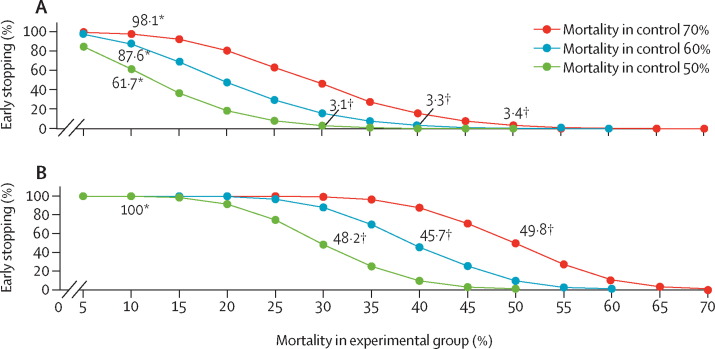
Figure 2.
Trial simulation to estimate probability of early stopping
(A) Early stopping estimate at the first interim analysis (overall sample size=70). (B) Early stopping estimate at the second interim analysis (overall sample size=140). *Punctual estimate for early stopping if reported mortality in control group is equal to 10% (ie, extraordinary unexpected efficacy). †Punctual estimate for early stopping if reported mortality is equal to the a priori assumptions.
Consistent with our hypothesis, an extraordinary unexpected efficacy of the experimental treatment will result in a very early RCT cessation. For example, if mortality in the experimental group is only 10% the probability of stopping the RCT at the first interim analysis will be 98·1%, 87·6%, and 61·7% for mortality in the control equal to 70%, 60%, and 50%, respectively (see figure 2A). With such an extraordinary efficacy no RCT will go beyond the second interim analysis (see figure 2B). Moreover, if a priori hypotheses are confirmed the chance that a significant effect will be reported at the second interim analysis is still about 50% (see figure 2B). As the likelihood of the study cessation is positively associated with the mortality in the control group, very few patients (in absolute terms) who did not receive such an extraordinary intervention will actually die. In view of the availability of a reliable comparator, interim analyses in RCTs are much stronger than analyses in non-comparative studies to inform investigators about any unexpected toxic effects of the investigational drug.
A singular confirmation of our argument is provided by the preliminary results (interim analysis) of the JIKI trial (NCT02329054; table 1) presented at the last Conference on Retroviruses and Opportunistic Infections, held in Seattle in February, 2015.18 JIKI is a non-comparative, proof-of-concept trial aimed to enrol 225 participants to assess the benefit of high-dose favipiravir in reducing mortality and decreasing Ebola viral load in patients with acute Ebola virus disease. At present JIKI is the most ambitious trial in progress and since the start of enrolment, on Dec 17, 2014, the investigators have enrolled 80 participants after just 36 days. The main results presented were that mortality was positively associated with Ebola viral load; that favipiravir was generally well tolerated; and that a non-significant trend for reduced mortality was reported in those with the lowest Ebola viral load who received the drug by comparison with historical controls, whereas an opposite trend was reported in those with the highest Ebola viral load who received the drug.
In view of the absence of an extraordinary efficacy or a reliable comparator, the analysis provided hardly any conclusive evidence. An association between mortality and Ebola viral load and the good tolerability of favipiravir are all expected findings, whereas researchers remain uninformed about the efficacy of favipiravir. These results leave ethical uncertainty for the researchers about whether to continue the study as it is, change the enrolment according to baseline viral load, or to interrupt the study and divert resources for tests of new experimental drugs. Should the JIKI trial have been designed within an adaptive framework, the maximum sample size would have been reduced (210 vs 225) and, having gone through an adaptation iteration, the researchers would have been able to discuss truly comparative evidence for this kind of treatment by the time 80 patients had been assessed.
In our opinion this trial exemplifies the idea that when considerations related to the urgency of actions are preferred over scientific hypotheses the efforts to obtain new evidence could be jeopardised without the realisation of any ethical advantage.
Potential limitations of the adaptive design
Adaptive study designs remain attractive because of their flexibility, which could provide several practical and ethical advantages compared with standard RCT design. The primary goal of adaptive trials is to minimise the harm to study participants by exposing fewer participants to the burden and risks of research, and to benefit more participants with the favourable treatment, by reducing time to obtain conclusive evidence compared with conventional RCTs. Nevertheless this flexibility comes at a cost. Several issues could hinder the implementation of adaptive RCTs and reduce the internal and external validity of these studies.39
First, adaptive trial designs are more complex to implement and analyse than standard RCTs.34, 37, 38 Second, adaptive study designs seem most suitable for situations where endpoints can be quickly and reliably assessed. This restricted suitability implies that adaptive RCTs might be unable to assess long-term outcomes. Third, results of interim analyses might influence decisions of the data and safety monitoring boards, researchers, and study participants. Finally, adaptive designs usually include multiple interim analyses, which often leads to an inflated type-I error; as such the adaptive framework should be kept as simple as possible.35 However, a prudent adaptive design including a small number of clinical centres, a restricted number of interim analyses, and a well understood and validated adaptive framework could easily address all these issues.37, 38
Conclusions
For life-threatening diseases under the conditions of suboptimum standards of care40 (such as with the present Ebola outbreak) RCTs that take a very long time to do would not be straightforward or would be unacceptable.33, 39 However, dependence on non-randomised studies to assess the efficacy and harms of interventions might be even worse than RCTs. New RCT designs, such as adaptive designs, can provide the best solution for researchers to obtain robust evidence on the merits of candidate interventions.
Acknowledgments
Acknowledgments
We have received grants from the Italian Ministry of Health (ricerca corrente and ricerca finalizzata).
Contributors
All authors contributed equally to the Personal View.
Declaration of interests
SK is part of a consortium (VEBCON) funded by the WHO/Wellcome Trust to do phase 1 studies on a VSV-ZEBOV vaccine. EG reports personal fees from Janssen-Cilag, Abbot Diagnostics, Gilead Sciences, ViiV Healthcare, and BMS Europe, grants from Gilead Sciences, and non-financial support from BMS Europe, outside the submitted work. All other authors declare no competing interests.
References
- 1.Hooper C, Krishna S. This must be the year we beat Ebola in West Africa. https://theconversation.com/this-must-be-the-year-we-beat-ebola-in-west-africa-35832 (accessed March 31, 2015).
- 2.WHO Ebola Response Team Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouqui P, Ippolito G. Ebola and travel—management of imported cases. Travel Med Infect Dis. 2014;12:561–562. doi: 10.1016/j.tmaid.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 4.WHO Ebola Situation Report. 25 March 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-25-march-2015 (accessed March 30, 2015).
- 5.WHO . Ethical considerations for use of unregistered interventions for Ebola viral disease: report of an advisory panel to WHO. World Health Organization Press; Geneva: Aug 11, 2014. http://www.who.int/csr/resources/publications/ebola/ethical-considerations/en/ (accessed Dec 1, 2014). [Google Scholar]
- 6.Adebamowo C, Bah-Sow O, Binka F. Randomised controlled trials for Ebola: practical and ethical issues. Lancet. 2014;384:1423–1424. doi: 10.1016/S0140-6736(14)61734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox E, Borio L, Temple R. Evaluating Ebola therapies—the case for RCTs. N Engl J Med. 2014;371:2350–2351. doi: 10.1056/NEJMp1414145. [DOI] [PubMed] [Google Scholar]
- 8.Djulbegovic B, Hozo I, Ioannidis JP. Improving the drug development process: more not less randomized trials. JAMA. 2014;311:355–356. doi: 10.1001/jama.2013.283742. [DOI] [PubMed] [Google Scholar]
- 9.Gehring G, Rohrmann K, Atenchong N. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother. 2014;69:2123–2131. doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu X, Wong G, Audet J. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyon GM, Mehta AK, Varkey JB, the Emory Serious Communicable Diseases Unit Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371:2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 12.Bishop BM. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother. 2015;49:196–206. doi: 10.1177/1060028014561227. [DOI] [PubMed] [Google Scholar]
- 13.Kreuels B, Wichmann D, Emmerich P. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2014;371:2394–2401. doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 14.Mupapa K, Massamba M, Kibadi K, the International Scientific and Technical Committee Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis. 1999;179(suppl 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 15.WHO Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease: empirical treatment during outbreaks. 2014. http://www.who.int/csr/resources/publications/ebola/convalescent-treatment/en/ (accessed March 31, 2015).
- 16.Yu ZM, Liu ZH, Chen J, Zeng Q. Anti-inflammatory effect of Qingwen Baidu Decoction in sepsis rats. Chin J Integr Med. 2014;20:934–943. doi: 10.1007/s11655-014-1863-x. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Liu J, Guo X. Xuebijing injection reduces organ injuries and improves survival by attenuating inflammatory responses and endothelial injury in heatstroke mice. BMC Complement Altern Med. 2015;15:4. doi: 10.1186/s12906-015-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sissoko D, Anglaret X, Malvy D, et al. Favipiravir in patients with Ebola virus disease: early results of the JIKI trial in Guinea. Conference on Retroviruses and Opportunistic Infections: Seattle, WA. February 23–26, 2015. 103-ALB.
- 19.Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Wolf T, Kann G, Becker S. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet. 2014 doi: 10.1016/S0140-6736(14)62384-9. published online Dec 18. [DOI] [PubMed] [Google Scholar]
- 21.Parra JM, Salmerón OJ, Velasco M. The first case of Ebola virus disease acquired outside Africa. N Engl J Med. 2014;371:2439–2440. doi: 10.1056/NEJMc1412662. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LW, Chalmers TC. Ethics in cooperative clinical trials. Ann N Y Acad Sci. 1970;169:487–495. doi: 10.1111/j.1749-6632.1970.tb54759.x. [DOI] [PubMed] [Google Scholar]
- 23.Byar DP, Simon RM, Friedewald WT. Randomized clinical trials. Perspectives on some recent ideas. N Engl J Med. 1976;295:74–80. doi: 10.1056/NEJM197607082950204. [DOI] [PubMed] [Google Scholar]
- 24.Spodick DH. The randomized controlled clinical trial. Scientific and ethical bases. Am J Med. 1982;73:420–425. doi: 10.1016/0002-9343(82)90746-x. [DOI] [PubMed] [Google Scholar]
- 25.Royall RM, Bartlett RH, Cornell RG. Ethics and statistics in randomized clinical trials. Stat Sci. 1991;6:52–88. doi: 10.1214/ss/1177011934. [DOI] [PubMed] [Google Scholar]
- 26.Colli A, Pagliaro L, Duca P. The ethical problem of randomization. Intern Emerg Med. 2014;9:799–804. doi: 10.1007/s11739-014-1118-z. [DOI] [PubMed] [Google Scholar]
- 27.Borchert M, Mutyaba I, Van Kerkhove MD. Ebola haemorrhagic fever outbreak in Masindi District, Uganda: outbreak description and lessons learned. BMC Infect Dis. 2011;11:357. doi: 10.1186/1471-2334-11-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Ebola virus disease. September 2014. http://www.who.int/mediacentre/factsheets/fs103/en (accessed Dec 1, 2014).
- 29.Lamontagne F, Clément C, Fletcher T, Jacob ST, Fischer WA, 2nd, Fowler RA. Doing today's work superbly well—treating Ebola with current tools. N Engl J Med. 2014;371:1565–1566. doi: 10.1056/NEJMp1411310. [DOI] [PubMed] [Google Scholar]
- 30.Meslin EM, Blasimme A, Cambon-Thomsen A. Mapping the translational science policy ‘valley of death’. Clin Transl Med. 2013;2:14. doi: 10.1186/2001-1326-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283:2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 32.Yong E. Trials at the ready: preparing for the next pandemic. BMJ. 2012;344:e2982. doi: 10.1136/bmj.e2982. [DOI] [PubMed] [Google Scholar]
- 33.Shaw D. Randomisation is essential in Ebola drug trials. Lancet. 2014;384:1667. doi: 10.1016/S0140-6736(14)61735-9. [DOI] [PubMed] [Google Scholar]
- 34.WHO . Ethical issues related to study design for trials on therapeutics for Ebola Virus Disease. World Health Organization Press; Geneva: Oct 21, 2014. http://www.who.int/csr/resources/publications/ebola/ethical-evd-therapeutics/en/ (accessed March 31, 2015). [Google Scholar]
- 35.Chow SC, Chang M. Adaptive design methods in clinical trials— a review. Orphanet J Rare Dis. 2008;3:11. doi: 10.1186/1750-1172-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joffe S. Evaluating novel therapies during the Ebola epidemic. JAMA. 2014;312:1299–1300. doi: 10.1001/jama.2014.12867. [DOI] [PubMed] [Google Scholar]
- 37.European Medicines Agency Committee for Medicinal Products for Human Use Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design. Oct 18, 2007. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003616.pdf (accessed March 31, 2015).
- 38.US Food and Drug Administration Federal Drug Administration Guidance for Industry Adaptive Design Clinical Trials for Drugs and Biologics Center for Drug Evaluation and Research Rockville. 2010. http://www.fda.gov/downloads/Drugs/Guidances/ucm201790.pdf (accessed March 31, 2015).
- 39.van der Graaf R, Roes KC, van Delden JJ. Adaptive trials in clinical research: scientific and ethical issues to consider. JAMA. 2012;307:2379–2380. doi: 10.1001/jama.2012.6380. [DOI] [PubMed] [Google Scholar]
- 40.Ippolito G, Feldmann H, Lanini S. Viral hemorrhagic fevers: advancing the level of treatment. BMC Med. 2012;10:31. doi: 10.1186/1741-7015-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]