Abstract
Clin Microbiol Infect 2012; 18: 67–73
Abstract
Solid organ transplant recipients (SOTR) are at risk of serious influenza‐related complications. The impact of respiratory co‐infection in SOTR with 2009 pandemic influenza A(H1N1) is unknown. A multicentre prospective study of consecutive cases of pandemic influenza A(H1N1) in SOTR was carried out to assess the clinical characteristics and outcome and the risk factors for co‐infection. Overall, 51 patients were included. Median time from transplant was 3.7 years, 5.9% of the cases occurred perioperatively and 7.8% were hospital‐acquired. Pneumonia was diagnosed in 15 (29.4%) patients. Ten cases were severe (19.6%): 13.7% were admitted to intensive care units, 5.9% suffered septic shock, 5.9% developed acute graft rejection and 7.8% died. Co‐infection was detected in 15 patients (29.4%): eight viral, six bacterial and one fungal. Viral co‐infection did not affect the outcome. Patients with non‐viral co‐infection had a worse outcome: longer hospital stay (26.2 ± 20.7 vs. 5.5 ± 10.2) and higher rate of severe diseases (85.7% vs. 2.3%) and mortality (42.8% vs. 2.3%). Independent risk factors for non‐viral co‐infection were: diabetes mellitus and septic shock. Other factors associated with severe influenza were: delayed antiviral therapy, diabetes mellitus, time since transplantation <90 days and pneumonia. In conclusion, pandemic influenza A can cause significant direct and indirect effects in SOTR, especially in the early post‐transplant period, and should be treated early. Clinicians should be aware of the possibility of non‐viral co‐infection, mainly in diabetic patients and severe cases. An effort should be made to prevent influenza with immunization of the patient and the environment.
Keywords: Co‐infection, outcome, pandemic influenza A(H1N1), solid organ transplant
Introduction
In 2009, a new pandemic influenza A(H1N1) virus was detected [1, 2] and propagated throughout the world [3]. Although severe pandemic influenza A(H1N1) has been detected in younger patients compared with seasonal influenza, 20% of cases occurred in immunosuppressed patients [4, 5]. In this setting, solid organ transplant recipients (SOTR) were included among the risk groups to receive the pandemic vaccine [6, 7], given the related direct and indirect morbidity of seasonal influenza in this population [8].
Recently, a retrospective study has described the demographic and clinical characteristics and prognostic factors of intensive care unit (ICU) admission and death in 237 adults and children with influenza A and organ transplantation [9]. Other authors, in small series, have observed a longer duration of symptoms, higher frequency of pneumonia and higher mortality rate than in the general population [10, 11].
Influenza viral infection is known to predispose to subsequent lung bacterial superinfection [12]. The initial descriptions of severe cases of 2009 pandemic influenza showed evidences of primary viral pneumonia with poor relevance of bacterial co‐infection [13, 14, 15]. However, in later studies using PCR and immunochemistry methods, bacterial co‐infection was found in 28–55% of fatal cases [16, 17]. Some of the previous studies in SOTR [9, 11] describe the viral or bacterial co‐infections, but their impact on the prognosis and clinical care of the influenza was not analyzed.
The aim of this study was to prospectively assess the clinical characteristics and outcome of pandemic influenza A(H1N1) infection in SOTR, and the risk factors for co‐infection by other pathogens.
Methods
Setting, patients and study design
This prospective observational study was conducted at nine teaching hospitals belonging to the Spanish Network for Research in Infectious Diseases (REIPI). All SOTR older than 16 years, diagnosed with influenza A(H1N1), and hospitalized at the participating centres from June 2009 to January 2010, were included. A confirmed case was defined in the presence of influenza‐like illness with laboratory‐confirmed 2009 pandemic influenza A(H1N1) virus infection. The study was approved by the coordinator local ethics committee and informed consents were obtained from all subjects.
Clinical assessment and follow‐up
In cases of respiratory symptoms, SOTR were advised to seek care at the hospital. Thereafter, an investigator evaluated and followed them until cure or death in the case of influenza diagnosis, and clinical data were recorded in a standardized protocol. Data were collected on demographic characteristics, co‐morbidities, body mass index (BMI), type of transplant and immunosuppressive therapy, previous vaccination, clinical signs and symptoms, biochemical analysis, chest X‐ray findings, antiviral and antibacterial therapy, concomitant and/or secondary infection, and outcomes, including mortality.
Microbiological studies
Pandemic influenza A(H1N1) was confirmed with RT‐PCR (Inf A/H1N1 Detection, Roche, Basel, Switzerland), in naspopharyngeal smears or aspirates.
Blood cultures, standard studies for bacterial and fungi of respiratory samples (sputum, bronchial aspirates, bronchoalveolar lavage, protected specimen brush and transbronchial biopsy), and urinary antigen tests for Streptococcus pneumoniae and Legionella pneumophila serogroup 1 were performed. Sputum samples were processed if they contained >25 polymorphonuclears and <10 epithelial cells per high‐power field.
Viral co‐infection was investigated in nasopharyngeal samples using a PCR multiplex RV 12 ACE detection seeplex kit (Seegene Inc., Seul, Corea) including metapneumovirus, adenovirus, coronavirus 229E/NL63 and OC43/HKU1, parainfluenza virus 1/2/3, rhinovirus A/B, respiratory syncytial viruses (RSV) A/B, and influenza virus A/B.
Definitions
Obesity was defined as a BMI ≥30. Hospital‐acquired influenza was considered when symptoms began more than 7 days after admission. Pneumonia was defined by the presence of clinical symptoms (fever, dyspnoea, cough and/or expectoration) and a new pulmonary infiltrate in the chest X‐ray for which other non‐infectious causes were excluded. Severity of pneumonia was assessed by the PSI and CURB‐65 scores [18, 19]. Non‐viral co‐infection was considered if bacteria or fungi were isolated from blood or respiratory samples, or with positive urinary antigen detection tests of S. pneumoniae or L. pneumophila. Severe infections were defined as cases that were admitted to ICU, developed graft rejection or died.
Statistical analysis
A descriptive analysis was performed. Continuous variables are expressed as median and range. All proportions were calculated as percentages of the patients with available data. The chi‐square or Fisher’s exact tests were used for categorical variables and the Student‐t, Mann–Whitney and Wilcoxon tests for continuous variables, when appropriate. The multivariate logistic regression analysis of factors potentially associated with non‐viral co‐infection included significant variables in the univariate analyses and clinically relevant variables. Statistical significance was established at p <0.05 (two‐tailed). The SPSS software (version 15.0; SPSS Inc., Chicago, IL, USA) was used.
Results
Fifty‐one SOTR, with a median age of 48 years (16–73) and including 28 (54.9%) men were diagnosed with influenza A (H1N1). The types of transplant were 24 kidney, 11 liver, eight lung, five heart, two combined pancreas‐kidney and one combined liver‐kidney. Most patients received triple therapy with tacrolimus, mycophenolate mofetil and steroids. One patient had an allograft rejection treated with a corticosteroid bolus in the month prior to influenza. Influenza occurred at 3.7 years after transplantation (1 day to 21.5 years) and in 40 cases (78.4%) occurred beyond the first year (Fig. 1). In three patients (5.9%), symptoms started 1–5 days after the transplant, without identification of the source of influenza infection; all had pneumonia, one died and another lost the graft because of acute rejection. Four cases (7.8%) were hospital acquired.
Figure 1.
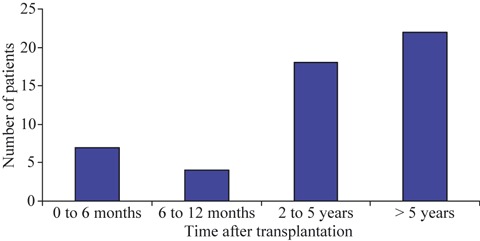
Pandemic influenza A(H1N1) virus infection in SOTR. Time after transplantation.
Co‐morbidities, vaccination history and previous use of antibiotics are detailed in Table 1. Only one patient had received pandemic influenza A(H1N1) vaccine, having normal gammaglobulin levels and no prior rejection episodes.
Table 1.
Demographics and underlying medical conditions
| Variable | Value |
|---|---|
| Male sex, No. (%) | 28 (54.9%) |
| Age in years, median (range) | 48 (16–73) |
| Contact with children <3 years, No. (%) | 11 (21.5%) |
| Tobacco smoker, No. (%) | 5 (9.8%) |
| Type of transplant | |
| Kidney transplant, No. (%) | 24 (47.1%) |
| Liver transplant, No. (%) | 11 (21.6%) |
| Lung transplant, No. (%) | 8 (15.7%) |
| Heart transplant, No. (%) | 5 (9.8%) |
| Combined pancreas‐kidney transplant, No. (%) | 2 (3.9%) |
| Combined liver‐kidney transplant, No. (%) | 1 (1.9%) |
| Immunosuppression | |
| Ciclosporin, No. (%) | 13 (25.4%) |
| Tacrolimus, No. (%) | 31 (60.7%) |
| Mycophenolate mofetil, No. (%) | 38 (74.5%) |
| m‐TOR inhibitors, No. (%) | 8 (15.7%) |
| Azathioprine, No. (%) | 1 (1.9%) |
| Corticosteroids >20 days, No. (%) | 30 (58.8%) |
| Triple immunosuppressive therapy, No. (%) | 28 (54.9%) |
| Double immunosuppressive therapy, No. (%) | 21 (41.1%) |
| Induction therapy in the previous 6 months, No. (%) | 3 (5.9%) |
| Allograft rejection in the previous month, No. (%) | 1 (2%) |
| Co‐morbidities | 44 (86.3%) |
| Chronic pulmonary disease, No. (%) | 8 (15.7%) |
| Diabetes mellitus, No. (%) | 12 (23.5%) |
| Chronic heart disease, No. (%) | 14 (27.5%) |
| Cerebrovascular disease, No. (%) | 1 (1.9%) |
| Chronic renal failure, No. (%) | 21 (41.5%) |
| Chronic liver disease, No. (%) | 8 (15.7%) |
| HIV infection, No. (%) | 1 (1.9%) |
| Connective tissue disease, No. (%) | 3 (5.9%) |
| Obesity, No. (%) | 2 (3.9%) |
| Previous vaccines | |
| Seasonal influenza vaccine 2008/2009, No. (%) | 26 (50.9%) |
| Seasonal influenza vaccine 2009/2010, No. (%) | 24 (47.1%) |
| Pandemic influenza A(H1N1) vaccine, No. (%) | 1 (1.9%) |
| Pneumococcal vaccine, No. (%) | 19 (37.2%) |
| Other treatments | |
| Previous antibiotics, No. (%) | 5 (9.8%) |
| Previous statins, No. (%) | 17 (33.3%) |
| Previous angiotensin converter inhibitors, No. (%) | 14 (27.4%) |
Clinical manifestations
The most common symptoms were fever (94.1%) and cough (80.4%). The median delay between the onset of symptoms and diagnosis was 2 days (1–15). Pneumonia occurred in 15 patients (29.4%). Severity of pneumonia was assessed at admission with a median PSI of 72 (20–147) and median CURB‐65 score of 1 (0–4). Three and two patients had septic shock (5.9%) and cardiac failure (3.9%), respectively. The main clinical manifestations and radiological and analytical findings are detailed in Table 2.
Table 2.
Clinical, laboratory and radiological findings in solid organ transplant recipients at pandemic influenza A (H1N1) virus infection diagnosis
| Variables | Values |
|---|---|
| Clinical manifestations | |
| Duration of symptoms, median (range) | 2 days (1–15 days) |
| Fever, No. (%) | 48 (94.1%) |
| Temperature, median (range) | 38°C (35.5–39.8°C) |
| Chills, No. (%) | 18 (35.3%) |
| Cough, No. (%) | 41 (80.4%) |
| Expectoration, No. (%) | 19 (37.2%) |
| Purulent, No. (%) | 16 (31.4%) |
| Mucous, No. (%) | 3 (5.9%) |
| Nasal congestion, No. (%) | 11 (21.6%) |
| Sore throat, No. (%) | 6 (11.8%) |
| Dyspnoea, No. (%) | 15 (29.4%) |
| Arthralgias, No. (%) | 32 (62.7%) |
| Headache, No. (%) | 16 (31.4%) |
| Altered mental status, No. (%) | 2 (3.9%) |
| Diarrhoea, No. (%) | 5 (9.8%) |
| Vomiting, No. (%) | 9 (17.6%) |
| Septic shock, No. (%) | 3 (5.9%) |
| Radiological findings | |
| Pneumonia, No./Total No. (%) | 15 (29.4%) |
| Alveolar infiltrates, No./Total No. (%) | 11 (73.3%) |
| Bilateral infiltrates, No./Total No. (%) | 11 (73.3%) |
| Analytical findings | |
| Partial oxygen saturation <90%, No. (%) | 4 (7.8%) |
| Leukocyte count, per mm3, median (range) | 6400 (1800–21450) |
| <4000/mm3, No. (%) | 10 (19.7%) |
| >12000/mm3, No. (%) | 9 (17.6%) |
| Neutrophil count, per mm3, median (range) | 4108 (66–13100) |
| <1000/mm3, No. (%) | 6 (11.7%) |
| Lymphocyte count, per mm3, median (range) | 750 (7–9180) |
| <1500/mm3 | 36 (70.5%) |
| Hematocrit, %, median (range) | 37.4 (24.4–53) |
| <30%, No. (%) | 9 (17.6%) |
| Platelet count, per mm3, median (range) | 169000 (230–526000) |
| <100 000/mm3, No. (%) | 7 (13.7%) |
| AST >40 U/L, No. (%) | 10 (19.6%) |
| ALT >40 U/L, No. (%) | 5 (9.8%) |
| Creatine kinase >100 U/L, No. (%) | 3 (5.9%) |
| Sodium, mEq/L, median (range) | 138 (124–146) |
| Potassium, mEq/L, median (range) | 4.3 (2.5–7.1) |
| Creatinine, mg/dL, median (range) | 1.5 (0.6–5.9) |
| Lactate dehydrogenase, U/L, median (range) | 236 (175–496) |
| Glucose, mg/dL, median (range) | 107 (69–285) |
| C‐reactive protein >20 mg/L, No. (%) | 11 (21.5%) |
At the diagnosis, the only laboratory findings that differed from previous basal values were the lymphocyte and platelet counts (1504 vs. 750/μL (p <0.001) and 193 800 vs. 169 000/μL (p 0.004), respectively).
Treatment
All patients received oseltamivir a median of 2 days after the onset of symptoms (0–16 days), for a median of 5 days (3–24 days), at a dosage of 75 mg twice daily in all but four patients, who received 150 mg twice daily. None of the patients discontinued treatment due to adverse effects. Antibiotics were used empirically in 33 patients (64.7%), the most common being levofloxacin (16 cases, 31.2%) followed by amoxicillin‐clavulanate (8 cases, 15.6%). They were administered at a median of 5 h after admission (0–96 h) for a median of 7 days (1–16 days). Decisions regarding adjustment of immunosuppressive therapy were taken by the clinicians in charge. In eight severe influenza cases (16.7%), corticosteroids were added.
Co‐infection
Viral co‐infection was evaluated in 32 patients (62.7%). In eight patients other respiratory viruses were detected: parainfluenza 2 in two cases, RSV B in two cases, and parainfluenza 1, parainfluenza 3, rhinovirus, adenovirus and RSV A in one case each.
Non‐viral co‐infection was detected in seven patients (13.7%): Staphylococcus aureus in two cases (sputum and blood/bronchoalveolar lavage), S. aureus (sputum) and Enterococcus faecium (blood) in one case, S. pneumoniae in two cases (blood and urinary antigen detection), Streptococcus viridans in one case (pleural fluid), and Aspergillus fumigatus (bronchoalveolar lavage) in one case. Factors associated with the presence of non‐viral co‐infection were: diabetes mellitus (57.1% vs. 18.1%, RR 4.2, 95% CI 1.1–16.2, p 0.04), duration of symptoms at influenza diagnosis (3 days vs. 2 days, p 0.03), pneumonia (100% vs. 18.2%, RR undefined, p <0.001) and septic shock (28.5% vs. 2.3%, RR 6.4, 95% CI 2.0–20.2, p 0.04). No other predictors were observed in terms of underlying diseases, immunosuppressive therapy, types of organ, time since transplantation and clinical findings. In the multivariate analysis, variables independently related to non‐viral co‐infection were diabetes mellitus (OR 16.6, 95% CI 1.4–197.2, p 0.026) and septic shock (OR 67.2, 95% CI 1.8–2454.8, p 0.022).
Clinical outcomes
The median duration of fever and cough were 2 days (0–10) and 3 days (0–18), respectively. The median length of hospital stay was 4 days (2–71). In 10 patients influenza infection was severe (19.6%) (Table 3). Seven patients required admission to ICU (13.7%) for a median of 7 days.
Table 3.
Clinical outcomes in solid organ transplant recipients with pandemic influenza A (H1N1) virus infection
| Variable | Value |
|---|---|
| Duration of fever, days, median (range) | 2 (0–10) |
| Duration of cough, days, median (range) | 3 (0–18) |
| Length of hospital stay, days, median (range) | 4 (2–71) |
| Severe influenza, No. (%) | 10 (19.6%) |
| Intensive care unit admission, No. (%) | 7 (13.7%) |
| Length of ICU stay, days, median (range) | 7 (1–33) |
| APACHE II score, median (range) | 12 (8–24) |
| Acute respiratory failure, No. (%) | 5 (9.8%) |
| Invasive mechanical ventilation, No. (%) | 2 (3.9%) |
| Allograft rejection, No. (%) | 3 (5.9%) |
| Graft loss, No. (%) | 1 (1.9%) |
| Mortality, No. (%) | 4 (7.8%) |
Four patients died (7.8%). Two of them died 4 and 7 days after influenza diagnosis, with septic shock and S. aureus bacteraemic pneumonia and S. viridans empyema, respectively. A kidney recipient patient died from pulmonary aspergillosis 31 days after influenza. The fourth patient, a kidney recipient, had pneumococcal bacteraemic pneumonia 8 days after influenza diagnosis and Escherichia coli pneumonia 40 days after influenza.
Three patients had allograft rejection in the month following the influenza diagnosis: a pancreas recipient and a kidney recipient transplanted 4 and 2 months ago, respectively, who responded to a corticosteroid bolus, and a 7‐day liver recipient who lost the graft. Immunosuppressants were not reduced in any of these patients after influenza diagnosis.
Factors related to severe influenza are detailed in Table 4. No other predictors were observed in terms of underlying diseases, previous rejection, immunosuppressive therapy (type of calcineurin inhibitor, steroids and induction therapy), types of organ, and clinical manifestations.
Table 4.
Factors associated with severe pandemic influenza A(H1N1) virus infection in SOTR
| Variable | Severe | Non‐severe | RR CI 95% | p |
|---|---|---|---|---|
| Diabetes mellitus, No. (%) | 6 (60%) | 6 (14.6%) | 4.9 (1.6–14.4) | 0.007 |
| Time since transplant <90 days, No. (%) | 3 (30%) | 1 (2.4%) | 5.0 (2.1–12.2) | 0.02 |
| Septic shock, No. (%) | 3 (30%) | 0 (0%) | 6.8 (3.4–13.6) | 0.005 |
| Pneumonia, No. (%) | 9 (90%) | 6 (14.6%) | 21.6 (2.9–155.8) | <0.001 |
| Non‐viral co‐infection, No. (%) | 6 (60%) | 1 (2.4%) | 9.4 (3.5–25.1) | <0.001 |
| Time from onset of symptoms to antiviral therapy, days, median (range) | 1 (0–13) | 3.5 (1–16) | 0.033 | |
| Heart rate, bpm, median (range) | 97 (70–135) | 83.5 (54–130) | 0.02 | |
| Haematocrit, %, median (range) | 29.8 (25–46) | 38.8 (24.4–53) | 0.01 | |
| Serum creatinine, mg/dL, median (range) | 1.84 (1.1–5.9) | 1.31 (0.6–5.3) | 0.04 |
Bpm: beats per minute.
The chi‐square or Fisher’s exact tests were used for categorical variables and the t‐test, Mann–Whitney test and Wilcoxon test for continuous variables, when appropriate. Statistical significance was established at α = 0.05. All reported p values are two‐tailed.
Patients with non‐viral co‐infection had a longer hospital stay (26.2 ± 20.7 vs. 5.5 ± 10.2, p 0.001), and higher rate of severe disease (85.7% vs. 2.3%, RR 9.4, 95% CI 3.5–25.2, p <0.001) and mortality (42.8% vs. 2.3%, RR 18.1, 95% CI 2.3–156.7, p 0.006). The presence of viral co‐infection had no influence on the outcomes.
Discussion
The present study shows that one‐third of SOTR with pandemic influenza had pneumonia, and that mortality is higher than in the general population [20]. Influenza infection is more severe in patients with recent transplants and in those with delayed onset of antiviral therapy, among other factors. Besides this, influenza may be associated with acute allograft rejection. Co‐infections were common in SOTR, and non‐viral co‐infections were associated with a worse outcome. Diabetes mellitus and septic shock were associated with non‐viral co‐infections.
The emergence of the pandemic influenza A virus raised important questions regarding its impact on the transplant population, with an expected high associated morbidity and mortality, as confirmed by this and other studies [9, 10, 11].
Seasonal influenza can occur at any time after the transplant. Vilchez et al. [8] observed a median time since transplantation of 34.5–38.6 months, with the earliest cases occurring 1.5–2 months after the transplant, in 30 SOTR with seasonal influenza. In the case of influenza A(H1N1), our data and other studies show that it may occur at any time after the transplant, with a median time of 27–44 months [9, 10, 11]. Moreover, the present prospective study shows that some cases occurred in the first week after transplant, and it was associated with a poor prognosis; thus, two of three patients lost the graft and died, respectively. Some studies suggested that seasonal influenza severity and mortality increase among transplant recipients in the early post‐transplant period [21]. Other studies on pandemic influenza in SORT have not reported data regarding this aspect [9, 10, 11]. In the present study, it was observed that time since transplantation shorter than 3 months was associated with severity.
Hospital‐acquired seasonal influenza in SOTR has been documented [22]. Within the recent pandemic, hospital‐acquired cases of influenza were reported in immunocompromised patients [23], and isolated cases were described in SOTR [24, 25]. In the same way, we found that 8% of the pandemic influenza cases were hospital‐acquired. Hospital‐acquired influenza infection in SOTR, even in the early postoperative period, and the adverse outcomes in cases occurring within the first 3 months after the transplant, prompt some considerations. First, early influenza diagnosis and treatment should be considered in the presence of influenza‐like symptoms, especially in cases of pneumonia, in pandemic or seasonal influenza periods, even in the immediate post‐transplant period. Secondly, these patients should be considered a high‐risk population, and therefore measures to avoid in‐hospital transmission should be rigorous, with implementation of infection control measures, such as isolation of infected patients, and avoidance of contact with symptomatic caregivers. In this sense, it is necessary to increase the rate of vaccination among SOTR and close contacts to confer indirect protection for those who are non‐responders to vaccine.
In the present study, we found that symptoms and signs were similar to those described in the general population [26]. Moreover, SOTR with pandemic influenza showed frequent haematological and biochemical abnormalities. Some of them, such as renal failure, mild hepatolysis and anaemia, were present before influenza infection and might possibly be associated with the transplant and concomitant immunosuppressive drugs. However, others, such as lymphopenia and thrombocytopenia, significantly worsen after influenza.
Transplant recipients have more seasonal influenza‐related complications than the general population, including primary viral and secondary bacterial pneumonias [8, 27]. The proportion of pneumonia in our cohort was in the reported range (22–49%) in SOTR [9, 10, 11]. With respect to the severity of pneumonia at admission, three of the four cases who died had a PSI risk class of IV or V; accordingly, the CURB‐65 score of these patients was 2–4. With the limitations of the reduced number of patients, these data suggest the usefulness of these severity scores for pneumonia in pandemic influenza.
In the general population, delayed hospital admission and antiviral therapy have been associated with an unfavourable outcome [28, 29, 30]. In the present study, time to antiviral therapy was also associated with severe influenza, being the only modifiable prognostic factor. These data agree with those from a retrospective study showing that delayed antiviral therapy is associated with intensive care unit admission and mortality [9]. It must be noted that most of the patients included received antiviral therapy within 48 h after the onset of symptoms, many of them before virological confirmation, which might have contributed to an improvement in the outcome.
Bacterial pneumonia is a known complication of influenza. In the present study, patients with non‐viral co‐infection, in contrast to those with viral co‐infection, experienced a worse outcome despite early antibacterial therapy. Risk factors for non‐viral co‐infection in patients with pandemic influenza A have not been previously described. In this study, two associated factors at influenza diagnosis have been identified: diabetes mellitus and septic shock. On the other hand, non‐viral co‐infections were not diagnosed in mild cases without pneumonia. These findings may suggest that non‐severe cases could be managed without antibacterial therapy, reducing the number of patients exposed to antibiotics, in our case two‐thirds of the patients.
Our data are subject to several limitations. First, as in other studies carried out in the general population, the data are from hospitalized patients with pandemic influenza A. However, they offer information regarding this illness in transplant patients with severe disease needing hospital admission. Second, as susceptibility to oseltamivir was not performed, we could not conclude whether fatal cases were related to oseltamivir resistance. Third, the possibility of graft rejection was only histologically evaluated if it was clinically evident, which could lead to misdiagnoses of mild allograft rejections.
In summary, our findings indicate that pandemic influenza A can not only cause significant direct morbidity and mortality in SOTR, especially in the early post‐transplant period, but also indirect effects with possibly devastating consequences for graft function. Clinicians should be aware of the possibility of non‐viral co‐infection in these patients, mainly in diabetic patients and severe cases. An effort should be made to increase vaccination among SOTR and caregivers.
Transparency Declaration
This work was supported by the ‘Programa de Investigación sobre gripe A/H1N1, Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación’ (Grant GR09/0041) and by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III – co‐financed by the European Development Regional Fund ‘A way to achieve Europe’ ERDF, Spanish Network for Research in Infectious Diseases (REIPI RD06/0008). The funding sources had no role in the study design, data collection, data analysis or interpretation, or writing of the manuscript. No commercial relationship or potential conflict of interest of any nature is related to the present manuscript.
Acknowledgement
The results of the study have been presented at the 50th ICAAC, September 2010, Boston.
Appendix: Members of the Novel Influenza A(H1N1) Study Group
Teresa Allende Aydillo, Ana Pérez‐Ordoñez, Laura Merino, Miguel Angel Gentil, Ernesto Lage, Carmen Bernal‐Bellido: Infectious Diseases Unit, Hospital Universitario Virgen del Rocío, Instituto de Biomedicina Sevilla (IBiS).
María Angeles Marcos, Inmaculada Hoyo, Carlos Cervera: Infectious Diseases Unit, Hospital Clinic.
Evelyn Cabral, Joan Gavaldá, Albert Pahissa: Infectious Diseases Unit, Hospital Vall d′Hebron.
Julio Goikoetxea: Infectious Diseases Unit, Hospital Universitario de Cruces.
Julián Torre‐Cisneros, Rosario Lara: Infectious Diseases Unit, Hospital Universitario Reina Sofía‐IMIBIC, Universidad de Córdoba.
Jesús Fortún, Mario Rodríguez, Cristina Galeano: Infectious Diseases Unit, Hospital Ramón y Cajal.
José Daniel García‐Palomo, María Victoria San Juan, María Arnaíz: Infectious Diseases Unit, Hospital Universitario Marqués de Valdecilla.
Jordi Carratalá: Infectious Diseases Unit, Hospital Universitario de Bellvitge‐IDIBELL.
Patricia Muñoz, Emilio Bouza: Infectious Diseases Unit, Hospital General Universitario Gregorio Marañón.
References
- 1. Outbreak of swine‐origin influenza A (H1N1) virus infection – Mexico, March–April 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 467–470. [PubMed] [Google Scholar]
- 2. Garten RJ, Davis CT, Russell CA et al. Antigenic and genetic characteristics of swine‐origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Update: novel influenza A (H1N1) virus infections – worldwide, May 6, 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 453–458. [PubMed] [Google Scholar]
- 4. Dawood FS, Jain S, Finelli L et al. Emergence of a novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360: 2605–2615. [DOI] [PubMed] [Google Scholar]
- 5. Thompson WW, Shay DK, Weintraub E et al. Influenza‐associated hospitalizations in the United States. JAMA 2004; 292: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 6. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58: 1–8. [PubMed] [Google Scholar]
- 7. Kumar D, Morris MI, Kotton CN et al. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant 2010; 10: 18–25. [DOI] [PubMed] [Google Scholar]
- 8. Vilchez RA, McCurry K, Dauber J et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2: 287–291. [DOI] [PubMed] [Google Scholar]
- 9. Kumar D, Michaels MG, Morris MI et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid‐organ transplants: a multicentre cohort study. Lancet Infect Dis 2010; 10: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Low CY, Kee T, Chan KP et al. Pandemic (H1N1) 2009 Infection in Adult Solid Organ Transplant Recipients in Singapore. Transplantation 2010; 90: 1016–1021. [DOI] [PubMed] [Google Scholar]
- 11. Smud A, Nagel CB, Madsen E et al. Pandemic influenza A/H1N1 virus infection in solid organ transplant recipients: a multicenter study. Transplantation 2010; 90: 1458–1462. [DOI] [PubMed] [Google Scholar]
- 12. Small CL, Shaler CR, McCormick S et al. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol 2010; 184: 2048–2056. [DOI] [PubMed] [Google Scholar]
- 13. Hospitalized patients with novel influenza A (H1N1) virus infection – California, April–May, 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 536–541. [PubMed] [Google Scholar]
- 14. Intensive‐care patients with severe novel influenza A (H1N1) virus infection – Michigan, June 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 749–752. [PubMed] [Google Scholar]
- 15. Jain S, Kamimoto L, Bramley AM et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April‐June 2009. N Engl J Med 2009; 361: 1935–1944. [DOI] [PubMed] [Google Scholar]
- 16. Gill JR, Sheng ZM, Ely SF et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med 2010; 134: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) – United States, May‐August 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 1071–1074. [PubMed] [Google Scholar]
- 18. Fine MJ, Auble TE, Yealy DM et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med 1997; 336: 243–250. [DOI] [PubMed] [Google Scholar]
- 19. Lim WS, van der Eerden MM, Laing R et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viasus D, Pano‐Pardo JR, Pachon J et al. Factors associated with severe disease in hospitalized adults with pandemic (H1N1) 2009 in Spain. Clin Microbiol Infect 2010. Sep 3. doi: 10.1111/j.1469‐0691.2010.03362.x [e publ. ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Ljungman P, Andersson J, Aschan J et al. Influenza A in immunocompromised patients. Clin Infect Dis 1993; 17: 244–247. [DOI] [PubMed] [Google Scholar]
- 22. Malavaud S, Malavaud B, Sandres K et al. Nosocomial outbreak of influenza virus A (H3N2) infection in a solid organ transplant department. Transplantation 2001; 72: 535–537. [DOI] [PubMed] [Google Scholar]
- 23. Cunha BA, Thekkel V, Krilov L. Nosocomial swine influenza (H1N1) pneumonia: lessons learned from an illustrative case. J Hosp Infect 2010; 74: 278–281. [DOI] [PubMed] [Google Scholar]
- 24. Seville MT, Blair JE, Vikram HR, Kusne S. 2009 H1N1 Influenza in Hospitalized Transplant Recipients. Transplantation 2010; 90: 571–574. [DOI] [PubMed] [Google Scholar]
- 25. Zapata R, Uribe M, Martinez W, Andrade A, Leal JL, Gomez F. Severe novel H1N1 influenza A infection in the immediate postoperative period of a liver transplant patient. Liver Transpl 2010; 16: 447–452. [DOI] [PubMed] [Google Scholar]
- 26. Perez‐Padilla R, de lR‐Z, Ponce deLS et al. Pneumonia and respiratory failure from swine‐origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361: 680–689. [DOI] [PubMed] [Google Scholar]
- 27. Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest 2001; 119: 1277–1280. [DOI] [PubMed] [Google Scholar]
- 28. Patients hospitalized with 2009 pandemic influenza A (H1N1) – New York City, May 2009. MMWR Morb Mortal Wkly Rep 2010; 58: 1436–1440. [PubMed] [Google Scholar]
- 29. Campbell A, Rodin R, Kropp R et al. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ 2010; 182: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hackett S, Hill L, Patel J et al. Clinical characteristics of paediatric H1N1 admissions in Birmingham, UK. Lancet 2009; 374: 605. [DOI] [PubMed] [Google Scholar]


