Abstract
Purpose
To investigate the imaging features of emerging COVID-19 pneumonia on chest ultrasound (US), radiographs (CXR) and computed tomography (CT) examinations performed at admission and to provide a comprehensive radiological literature review on ongoing radiological data from recent publications.
Materials and methods
In this retrospective single-center study, we enrolled consecutive patients from February 15, 2020, to March 15, 2020, with laboratory-confirmed SARS-CoV-2 hospitalized in Valduce Hospital (Como, Italy). Multi-modality imaging findings were evaluated and compared. Literature research was conducted through a methodical search on Pubmed and Embase databases.
Results
Fifty-eight patients (36 men, 22 women; age range, 18–98 years) were included in the study. Among these, chest US, CXR, and CT were performed respectively in twenty-two, thirty-two and forty-two patients. Lung US findings were consistent with diffuse B lines (100%) and subpleural consolidations (27.3%). CXR showed prevalent manifestations of consolidations (46.9%) and hazy increased opacities (37.5%). Typical CT features included bilateral and multilobar ground-glass opacities (GGO) with (59.5%) and without (35.7%) consolidations having a predominantly peripheral distribution (64.3%). Other imaging features included crazy paving pattern (57.1%), fibrous stripes (50%), subpleural lines (35.7%), architectural distortion (28.6%), air bronchogram sign (26.2%), vascular thickening (23.8%) and nodules (2.4%). Also, enlarged lymph nodes (14.3 %) and pleural effusion (7.1%) were observed. The literature review identified twenty-six original studies supporting our imaging chest findings.
Conclusion
The spectrum of chest imaging manifestations of COVID-19 pneumonia upon admission includes B-lines and consolidations on US, consolidations and hazy increased opacities on CXR, and multifocal GGO with consolidations on CT.
Keywords: coronavirus disease, COVID-19, Computed Tomography (CT), pneumonia, SARS-CoV-2, ultrasound (US), radiographic chest examination (CXR)
1. Introduction
Coronavirus disease-19 (COVID-19) is the first pandemic infectious disease caused by a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Its genome arises from the same group of RNA-viruses that caused severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [1]. Infection can result in a range of clinical outcomes, from asymptomatic to severe life-threating course. Dominant clinical presentation is characterized by high temperature and cough [2].
At present, the diagnostic strategy is based on the combination of a history of exposure, clinical characteristics and RT-PCR (real-time polymerase chain reaction) assay from specimens obtained by oropharyngeal or nasopharyngeal swab, bronchoalveolar lavage, or tracheal aspirate, followed by imaging tests including ultrasound (US), Chest X-Ray (CXR) and Computed Tomography (CT) [3].
In this study we aimed to systematically assess the chest imaging manifestations of COVID-19 pneumonia with the description and comparison of CXR, US and CT findings detected in 58 patients admitted in our hospital. Furthermore, we provided a comprehensive insight into the literature to compare our results to the recent evidence regarding the radiological key features of this novel respiratory infectious disease.
2. Material and methods
2.1. Patients
This retrospective study was approved by our local institutional review board.
From February 15, 2020, to March 15, 2020, a total of fifty-eight patients (36 men, 22 women; age range, 18 to 98 years; mean age 66.3 years ± 16.6 standard deviation) with confirmed COVID-19 by RT-PCR assay and hospitalized in Valduce Hospital (Como, Italy), were included. All patients underwent a chest radiological examination upon admission to our emergency department including US, and/or CXR, and/or CT. Clinical data, symptoms and laboratory results were also observed.
2.2. Image acquisition
Bedside US was performed in the emergency room using linear or convex probes. For each exam, B-mode evaluation was performed and serial images and video clips were saved.
Digital CXR was acquired in the routine posteroanterior projection at full inspiration or in the anteroposterior projection in the case of supine patients.
CT examinations were performed using a multi-detector CT scanner with 64 channels. The detailed parameters for CT acquisition were as follows: tube voltage, 120 kVp; tube current, standard (reference mAs, 60–120); slice thickness, 1.0 mm; reconstruction interval, 1.0 mm. All CT images were acquired at full inspiration with the patient in the supine position and without contrast medium.
2.3. Image analysis
All US, CXR and CT images were stored and reviewed by two thoracic radiologists in consensus (C.B. and A.M. with 12 and 32 years of experience in thoracic imaging).
US images were examined for the assessment of the following signs: thickened pleural line, A-lines, B-lines, consolidation and pleural effusion.
Chest CXR and CT manifestations were classified according to the Fleischner Society Glossary [4]. CXR images were evaluated for the presence and distribution of the following abnormalities: hazy increased lung opacity, consolidation, and pleural effusion. CT images were evaluated on a PACS workstation using a width of 1500 Hounsfield units (HU) and a level of -600 HU for the pulmonary window and, a width 350 HU and a level of 50 HU for the mediastinal window. The following CT characteristics were evaluated: distribution (peripheral, central, or both), involved lung lobes (right upper, middle, lower lobes and left upper, lower lobes), number of lobes involved (one, two, three, four or five), appearance (ground-glass opacity, consolidation, or ground-glass opacity with consolidation), specific signs (“crazy-paving” pattern, air bronchogram sign, architectural distortion, fibrous stripes, subpleural lines, vascular thickening, and nodules), and extrapulmonary manifestations (mediastinal enlarged lymph nodes and pleural effusion).
2.4. Literature search and data extraction
We aimed to provide an updated and exhaustive literature review of imaging manifestations of COVID-19 pneumonia including CXR, ultrasound and CT examinations. We performed a literature search using the terms “coronavirus”, “SARS-CoV-2” and “COVID-19” on Pubmed and Embase, to identify fitting original research studies published up to 16th March 2020. We included studies with more than 10 patients with COVID-19 infection and we excluded studies on pediatric populations.
Consistent data were extracted from articles using a standardized form. This included clinical data (i.e. number of patients, age, sex), CXR findings (i.e. consolidation, pleural effusion), chest CT data (time between onset and the first scan, consolidation, lesion predominant distribution, ground-glass opacity, crazy paving, interstitial thickening or reticulation, air bronchograms, reversed halo sign, vascular enlargement, vacuolar sign, fibrotic streaks or linear opacities, spider web sign, lymphadenopathy, pleural effusion, pleural retraction sign and/or pleural thickening).
2.5. Statistical Analysis
Continuous variables were presented as mean ± standard deviation (SD) values. Categorical variables were reported as whole numbers and percentages in brackets.
3. Results
3.1. Patient population and radiological examinations
All the fifty-eight patients presented clinical symptoms at admission, with fever in fifty-eight patients (100%), cough in thirty-three patients (57%), dyspnea in twenty-four patients (41%) and fatigue in eighteen patients (31%). Laboratory results showed a low lymphocyte count in forty-eight patients (83%) and the elevated C-reactive protein in fifty-six patients (96.5%).
Available radiological examinations for each patient were retrieved as follows: CXR alone from eleven patients, CT alone from fifteen patients, whereas a combination of CXR and CT were performed in ten patients, US and CXR in five patients, US and CT in eleven patients and US, CXR and CT in six patients.
3.2. US findings
Lung US was performed in a total of twenty-two patients. In all cases (100%), US showed various B lines patterns (focal, multifocal, and confluent) due to interlobular septa thickening or hazy opacities that did not obscure the underlying bronchial structures or pulmonary, with the bilateral distribution. Subpleural consolidation was observed in six patients (27.3%) and a thickened pleural line in three patients (13.6%). In only one case (4.5%), US examination showed a mixed pattern with A- and B-lines. Among these patients, CXR was performed in eleven patients and chest CT examination in seventeen patients; CXR showed bilateral consolidations or hazy increased opacities (Fig. 1) while CT demonstrated bilateral ground-glass opacities (GGO) with consolidations (Fig. 2, Fig. 3). Detailed results about US findings are reported in Table 1.
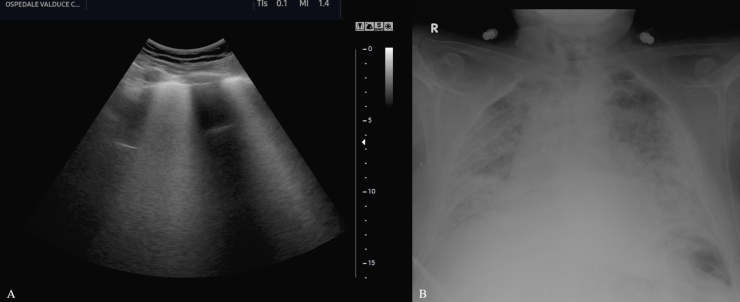
Fig. 1.
Ultrasound evaluation of lung parenchyma using the convex transducer in a patient with Coronavirus disease-19 (A) shows the simultaneous co-existence of multiple lung B-lines and A-lines. Chest x-ray (B) shows bilateral lung opacities which appear more evident in the peripheral areas.
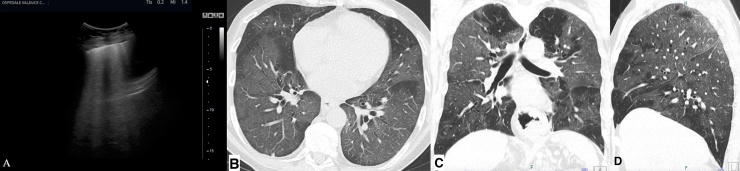
Fig. 2.
Ultrasound lung evaluation using a convex and linear transducer (A) of a patient with Coronavirus disease-19 shows compact B-line without horizontal reverberation (white lung sign), with tiny areas of subpleural consolidation. Axial CT evaluation of the same region shows bilateral and diffuse ground-glass opacities (B). Coronal and sagittal chest CT images (C and D) also demonstrate the diffuse extension of these findings.
Fig. 3.
Ultrasound thoracic examination using a linear transducer of a Patient with Coronavirus disease-19 (A) shows the irregular appearance of the pleural line with multifocal B-line. Axial CT image of the same region shows patchy bilateral ground-glass opacities and consolidations (B) with air bronchogram (arrow in B).
Table 1.
US findings and results.
| US findings (No. of Patients: 22) | ||
|---|---|---|
| Appearance of the lesions | No. | % |
| Thickened pleural line | 3 | 13.6 |
| A-lines | 1 | 4.5 |
| B-lines in various patterns | 22 | 100 |
| Consolidation | 6 | 27.3 |
| Pleural Effusion | 1 | 4.5 |
3.3. CXR manifestations
A total of thirty-two patients had CXR. Of these, five patients exhibited normal CXR despite chest CT scan acquired on the same day demonstrated bilateral ground-glass opacities (GGO). In the remaining twenty-seven cases, we observed consolidation in fifteen patients (46.9%) and hazy increased opacity in twelve patients (37.5%); no pleural effusion was identified. The distribution of these findings was bilateral in twenty-five patients (78.1%) and unilateral in two patients (6.2%). Besides, CXR showed a lower zone involvement in fifteen patients (52%), a similar lower and upper zone involvement in eleven patients (34.4%) and an upper involvement in one patient (3.1%). Moreover, eleven patients of this cohort performed CT along CXR on the same day and showed correlated findings such as bilateral GGO with consolidations (Fig. 4) and a predominant lower zone localization. The CXR findings and distribution results are reported in Table 2.
Fig. 4.
18-year-old female with a fever and cough for 7 days non-responsive to antibiotic therapy, without other known comorbidities neither relatives with similar symptoms. Chest x-ray shows bilateral opacities mainly peripheral and more evident in the left lung. Axial chest CT shows peripheral ground-glass opacities in the right upper lobe and consolidation in the left upper lobe (B). These findings are more evident in the lower lobes (C), especially in the left lower lobe, which appears almost completely consolidated. Air bronchogram sign is also evident (arrows in C). Diagnosis of Coronavirus disease-19 with the atypical presentation was confirmed with an oropharyngeal swab.
Table 2.
Chest X-Ray (CXR) findings and results.
| CXR findings (No. of Patients: 32) | ||
|---|---|---|
| Distribution of the lesions | No. | % |
| Bilateral | 25 | 78.1 |
| Unilateral | 2 | 6.2 |
| Neither bilateral or unilaterala | 5 | 15.6 |
| Lobes | ||
| Upper | 1 | 3.1 |
| Lower | 15 | 46.9 |
| Similar Upper or Lower | 11 | 34.4 |
| Neither upper or lowera | 5 | 15.6 |
| Appearance of lesions | ||
| Hazy increased opacity | 12 | 37.5 |
| Consolidation | 15 | 46.9 |
| Pleural Effusion | 0 | 0 |
| No abnormalities | 5 | 15.6 |
CXR showed no abnormalities.
3.4. CT features
Chest CT examinations were obtained in a total of forty-two patients. Among these cases, two chest CT showed no abnormalities and no previous US or CXR were available for comparison. The most common chest CT features of the remaining cases were characterized by GGO in 40 patients (95.2%), of whom 25 (25.9%) presented concomitant consolidations (Fig. 5); in all cases lesions were bilateral, showing lung involvement, of two or more lobes (right lower lobe, 92.9% and left upper lobe, 92.9%) with a predominantly peripheral distribution in twenty-seven patients (64.3%) whilst a diffuse distribution was depicted in twelve patients (28.6%). Additional lung CT manifestations were observed such as “crazy paving” (Fig. 6) pattern in twenty-four patients (57.1%), fibrous stripes (Fig. 7) in twenty-one patients (50%), subpleural lines (Fig. 7) in fifteen patients (35.7%), architectural distortion (Fig. 8) in twelve patients (28.6%), air bronchogram sign (Fig. 5) in eleven patients (26.2%), perilesional vascular thickening (Fig. 9) in ten patients (23.8%) and scattered nodules in one case (2.4%). Among extrapulmonary abnormalities, enlarged mediastinal lymph nodes were encountered in six cases (14.3 %) and pleural effusion in three cases (7.1%). The distribution and appearance of CT imaging findings are shown in Table 3.
Fig. 5.
A 67-year-old male with a history of fever for 12 days. An oropharyngeal swab performed a couple of days after the onset of symptoms was negative for Coronavirus disease-19. He was hospitalized for the persistence of symptoms and the onset of dyspnea. Axial chest CT images show patchy bilateral ground-glass opacities in the upper lobes mainly peripheral (A and B) and more consolidated areas along with ground glass opacities in the lower lobes (C). A new oropharyngeal swab confirmed Coronavirus disease-19. A follow-up CT performed 8 days after his hospitalization shows consolidation of the involved lung parenchyma. Air bronchogram sign is also evident (arrow in E).
Fig. 6.
61-year-old female with a history of fever, cough and mild pharyngodynia for 2 days. Axial chest CT images show ground-glass opacities (arrows, A and B). Coronal CT image clearly shows the thickness of interlobular and intralobular septa, “crazy paving” (arrow, C). Coronavirus disease-19 was confirmed on the oropharyngeal swab.
Fig. 7.
Axial CT image shows fibrous stripes in the right lower lobe (arrow, A). Axial CT scan shows subpleural lines in bilateral lower lobes (arrow, B).
Fig. 8.
Coronal and Axial Minimum-intensity-projection CT images show bronchus architectural distortion (arrows, A and B).
Fig. 9.
Axial chest CT images of four different patients with confirmed coronavirus disease-19 show ground-glass opacities with perilesional vascular thickening (arrows in A-D).
Table 3.
Computed Tomography (CT) Chest Findings and results.
| CT findings (No. of Patients: 42) | ||
|---|---|---|
| Distribution of the lesions | No. | % |
| Peripheral | 27 | 64.3 |
| Central | 1 | 2.4 |
| Peripheral and Central | 12 | 28.6 |
| Neither peripheral or centrala | 2 | 4.8 |
| Lobes | ||
| Right upper lobe | 39 | 92.9 |
| Right middle lobe | 35 | 83.3 |
| Right lower lobe | 39 | 92.9 |
| Left upper lobe | 39 | 92.9 |
| Left lower lobe | 39 | 90.5 |
| No. of lobes | ||
| 1 | 0 | 0 |
| 2 | 1 | 2.4 |
| 3 | 2 | 4.8 |
| 4 | 3 | 7.1 |
| 5 | 34 | 85.7 |
| Appearance of the lesions | ||
| Ground-glass opacity | 15 | 35.7 |
| Consolidation | 0 | 0 |
| Ground-glass opacity with consolidation | 25 | 59.5 |
| No abnormalitiesa | 2 | 4.8 |
| Specific signs | ||
| “Crazy-paving” pattern | 24 | 57.1 |
| Air bronchogram sign | 11 | 26.2 |
| Architectural distortion | 12 | 28.6 |
| Fibrous stripes | 21 | 50 |
| Subpleural lines | 15 | 35.7 |
| Vascular thickening | 10 | 23.8 |
| Nodules | 1 | 2.4 |
| Extrapulmonary manifestations | ||
| Mediastinal enlarged lymph node Pleural effusion |
6 3 |
14.3 7.1 |
CT scans showed no lesions.
3.5. Literature review synthesis
We identified 26 articles [2,[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]] with more than 10 patients reporting the CXR or CT findings of SARS-CoV-2 infected patients. The search encompassed a total of 3886 patients, with a mean age of 49.39 years old, male: 2059 and female: 1827. All revised articles reported chest CT abnormalities and only two studies [16,27] included CXR findings; besides, no original studies regarding lung US were found, with exception of two letters to the editor [30,31].
CXR manifestation: Chen et al. [16] reported bilateral pneumonia as the most common finding on chest radiograph. In NG Ming-Yen et al. [27] study only five patients underwent CXR and of these, three had parenchymal consolidations, two showed no alterations, none had pleural effusion. The CXR findings of the included studies are summarized in Table 4.
Table 4.
Literature review of chest radiological examination (CXR) findings of COVID-19 pneumonia.
Chest CT abnormalities: data regarding the time between symptom onset and 1 st CT were not available for all included studied, therefore an estimated time range was calculated as from 1 to 14 days. The most common described chest CT findings associated with COVID-19 pneumonia were: isolated GGO, followed by consolidations and GGO in combination with consolidative opacities; the predominant distribution was bilateral, peripheral, subpleural and of the lower lobes. Among all the analyzed CT abnormalities, it was interesting to note that the presence of the air bronchogram was identified in few studies, resulting in percentages up to 80% with an interval between 8% to 80%. Heterogeneous results were found in works describing the crazy-paving pattern, which showed very large values ranging from 5% to 75% and interstitial thickening or reticulation pattern with ranging from 17.5% to 81.8%. A further result showing diversified values was pleural involvement with values ranging from 8.4% to 56.5%. Of note, the reversed halo sign was described in only three studies: Bernheim et al. found it in 2% [10], Yan Li et al. in 3.9% [12], Harrison X. Bai in 5% [20]. According to the revised studies, lymphadenopathy and pleural effusion were rarely shown. Detailed CT characteristics of the included studies are reported in Table 5.
Table 5.
Literature review of CT imaging manifestations of COVID-19 pneumonia.
| Patient Characteristics |
CT findings |
|||||||
|---|---|---|---|---|---|---|---|---|
| First author, Publication data [Reference No.] | No. of Patients | Age (mean) | Sex | Time Between Onset & 1 st | GGO | Consolidation | Distribution and/or Location | Other findings |
| Huang, 24 January 2020 [5] | 41 | 49 | F: 11 M: 30 | 8 | Typically present | Typically present | Bilateral: 40 (98%) | |
| Chen, 29 January 2020 [16] | 99 | 55.5 | F: 32 M: 67 | N.R. | 14 (14%) | 99 (100%) | Bilateral 74 (75%) Unilateral 25 (25%) | |
| M. Chung, 3 February 2020 [23] | 21 | 51 | F: 8 M: 13 | N.R. | 18 (86%) | 6 (29%) | Bilateral: 16 (76%) Unilateral: 2 (24%) Peripheral distribution: 7 (33%) | Crazy paving: 4 (19%) |
| Fibrotic streaks or linear opacities: 3 (14%) | ||||||||
| Song, 6 February 2020 [24] | 51 | 49 | F: 26 M: 25 | median 4 days (range 1 - 14) | pure GGO 39 (77%); GGO with consolidation 30 (59%) | 28 (55%) | Bilateral: 44 (86%); Lower lobes: 46 (90%); Peripheral: 44 (86%) | Crazy paving: 38 (75%) |
| Interstitial thickening or reticulation: 11 (22%) | ||||||||
| Air broncograms: 41 (80%) | ||||||||
| Lymphadenopathy: 3 (6%) | ||||||||
| Pleural Effusion: 4 (8%) | ||||||||
| Yueying Pan, 6 February 2020 [25] | 63 | 44.9 | F: 30 M: 33 | N.R. | 54 (85.7%) | 12 (19.0%) | Number of affected lobes: 3.3 | Interstitial thickening or reticulation: 11 (17.5%) |
| Dawei Wang, 7 February 2020 [26] | 138 | 56 | F: 63 M: 75 | N.R. | 138 (100%) | Bilateral: 138 (100%) | ||
| NG Ming-Yen, 13 February 2020 [27] | 21 | 56 | F: 8 M: 13 | 3 | 18 (86%) | 13 (62%) | Peripheral: 18(86%) Perihilar 1(5%) Upper zone: 3(14%) Lower zone: 8(38%) Similar upper and lower zone involvement: 8(38%) | |
| Pan, 13 February 2020 [28] | 21 | 40 | F: 15 M: 6 | 9-13 days | 15 (71%) | 19 (91%) | Single lobe: 3(14%); Bilateral Multilobe: 18(86%); Peripheral: 13 (62%); Random: 7(33%); Diffuse: 1(4.8%) | Crazy Paving: 4 (19%) |
| Rui Han, 15 February 2020 [29] | 108 | 45 | F: 70 M: 38 | 1–3 days (median, 1 day) | 65(60%); GGO with consolidation: 44(41%) | 6 (6%) | Peripheral: 97 (90%); Central: 2 (2); Peripheral and central: 9 (8%) | Crazy Paving: 43 (40%) |
| Air broncograms: 52 (48%) | ||||||||
| Yicheng Fang, 19 February 2020 [6] | 51 | 45 | F: 22 M: 29 | N.R. | 36 (72%)* | N.R. | peripherical: 36(72%), lower lobes: 36(72%) | * 36 (72%) typical CT manifestations (e.g. peripheral, subpleural ground glass opacities, often in the lower lobes); 14 (28%) atypical CT manifestations. |
| Xi Xu, 19 February 2020 [7] | 90 | 50 | F: 51 M: 39 | N.R. | 65 (72%) | 12 (13%) | Periphery: 46 (51%) Bilateral: 53 (59%) Upper lobes: 40 (44%) Lower lobes: 47 (52%) | Crazy Paving: 11 (12%) |
| Interstitial thickening or reticulation: 33 (37%) | ||||||||
| Air broncograms: 7 (8%) | ||||||||
| Fibrotic streaks or linear opacities: 55 (61%) | ||||||||
| Lymphadenopathy: 1 (1%) | ||||||||
| Pleural Effusion: 4 (4%) | ||||||||
| Pleural retraction sign / thickening: 50 (56%) | ||||||||
| Wei Zhao, 19 February 2020 [8] | 101 | 44.44 | F: 45 M: 56 | N.R. | 87 (86.1%) | 44 (43.6%) | Unilateral: 10 (9.9%); Bilateral: 83 (82.2%); Periferical: 88(87.1%); Central: 1(1.0%); Lower lobes: 55(54.5%); Upper lobes: 6 (5.9%); | Interstitial thickening or reticulation: 49 (48.5%) |
| Vascular enlargemen: 72 (71.3%) | ||||||||
| Lymphadenopathy: 1 (1.0%) | ||||||||
| Pleural Effusion: 14 (13.9%) | ||||||||
| Zhou, 19 February 2020 [9] | 62 | 52.8 | F: 23 M: 39 | 10 patients: 1–7 days (mean, 2.2 ± 1.8 days); 52 patients: 1–14 days (mean, 6.6 ± 4.0 days) | 25 (40.3%) | 21 (33.9%) | Single lesion: 10 (16.1%); Multiple lesion: 52 (83.9%); Peripheral: 48 (77,4%); Peripheral and central: 14 (22.6%) | Crazy Paving: 39 (62.9%) |
| Air broncograms: 45 (72.6%) | ||||||||
| Vascular enlargement: 28 (45.2%) | ||||||||
| Vacuolar sign: 34 (54.8%) | ||||||||
| Fibrotic streaks or linear opacities: 35 (56.5%) | ||||||||
| Pleural Effusion: 6 (9.7%) | ||||||||
| Pleural retraction sign / thickening: 35 (56.5%) | ||||||||
| Bernheim, 20 February 2020 [10] | 121 | 45.3 | F: 60 M: 61 | 36 patients: 0-2 days; 33 patients: 3-5 days; 25 patients: 6-12 days; 27 patients: unknown | 92 (76%) | 53 (43.8%) | Bilateral: 73 (60%); Right lower lobe: 79 (65%); Left lower lobe: 76 (63%); Peripheral: 63 (52%) | Crazy Paving: 6 (5%) |
| "Reversed halo" sign: 2 (2%) | ||||||||
| Fibrotic streaks or linear opacities: 9 (7%) | ||||||||
| Pleural Effusion: 1 (1%) | ||||||||
| Yu-Huan Xu, 21 February 2020 [11] | 50 | 43.9 | F: 21 M: 29 | N.R. | 21 (75.0%) | 6 (21.4%) | Peripheral: 27(96.4%); Central: 14(50.0%); Peripheral involving central: 12(42.9%); Symmetrical: 15(53.6%) | Thickened intralobular septa: 21(75.0%); Thickened interlobular septa: 20(71.4%) |
| Air broncograms: 15 (53.6%) | ||||||||
| Lymphadenopathy: 1 (3.6%) | ||||||||
| Pleural Effusion: 2 (7.1%) | ||||||||
| Yan Li, 21 February 2020 [12] | 51 | 58 | F: 24 M: 29 | N.R. | 46 (90.20%) | 31 (60.78%) | Single lobe: 3 (5.9%) Bilateral Multilobe: 32 (94,1%) Peripheral and subpleural: 49 (96.1%) | Crazy Paving: 36 (70.6%) |
| Air broncograms: 35 (68.6%) | ||||||||
| "Reversed halo" sign: 2 (3.9%) | ||||||||
| Vascular enlargement: 42 (82.4%) | ||||||||
| Pleural Effusion: 1 (2%) | ||||||||
| Wu, 21 February 2020 [13] | 80 | 44 | F: 38 M: 42 | 7 ± 4 | 73 (91%) | 50 (63%) | Subpleural: 42 (53%); Diffuse: 7 (9%); Peribronchial: 3 (4%); Mixed: 24 (30%) | Crazy Paving: 23 (29%) |
| Interstitial thickening or reticulation: 47 (59%) | ||||||||
| Spider web sign: 20 (25%) | ||||||||
| Lymphadenopathy: 3 (4%) | ||||||||
| Pleural Effusion: 5 (6%) | ||||||||
| Heshui Shi, 24 February 2020 [14] | 81 | 49,5 | F: 39 M: 42 | N.R. | 53 (65,4%) | 14 (17,3%) | Unilateral:17 (21%) Bilateral: 64 (79%) Central: 10 (12,4%); Periferical:44 (54,3%) Both central and peripheral:27 (33,3%); | Crazy paving: 8 (9,9%) |
| Interstitial thickening or reticulation: 3 (3,7%) | ||||||||
| Air broncograms: 38 (46,9%) | ||||||||
| Lymphadenopathy: 5 (6,2%) | ||||||||
| Pleural Effusion: 4 (4,9%) | ||||||||
| Pleural retraction sign / thickening: 26 (32,1%) | ||||||||
| Yang, 26 February 2020 [15] | 149 | 45.11 | F: 68 M: 81 | median 7.61 days (range 0 - 7) | reported as GGO on 287 (12.1%) segments and mixed GGO on 637 (26.8%) segments | reported as present on 170 (7.2%) segments | Peripheral 35.9%; central 2.15%; both 8.12% | Interstitial thickening or reticulation: 79 (53%) |
| Air broncograms: 81 (54%) | ||||||||
| Fibrotic streaks or linear opacities: 31 (21%) | ||||||||
| Lymphadenopathy: 7 (5%) | ||||||||
| Pleural Effusion: 10 (7%) | ||||||||
| T. Ai, 26 February 2020 [17] | 1014 | 51 | F: 547 M: 467 | N.R. | 409 (40%) | 447 (44%) | Interstitial thickening or reticulation: 8 (8%) | |
| Kunhua Li, 29 february 2020 [18] | 83 | 45.5 | F: 39 M: 44 | 58 patients: median 6 days (range: 3 - 8.5 days); 25 patients: median 8 days (range: 6 - 12 days) | 81 (97.6%) | 53 (63.9%) | Bilateral: 79 (95.2%); lower lobe: 80 (96.4%); right lower lobe: 78 (94%); left lower lobe: 80 (96.4%) | Crazy Paving: 30 (36.1%) |
| Interstitial thickening or reticulation: 52 (62.7%) | ||||||||
| Fibrotic streaks or linear opacities: 54 (65.1%) | ||||||||
| Spider web sign: 21 (25.3%) | ||||||||
| Pleural Effusion: 7 (8.4%) | ||||||||
| Pleural retraction sign / thickening: 7 (8.4%) | ||||||||
| Xiong, 3 March 2020 [19] | 42 | 49.5 | F: 17 M: 25 | mean 4.5 days (range 1-11 days) | n of lobes with opacification 158/210 (75%) | 23 (55%) | Single lobe: 10 (24%); Bilateral Multilobe: 32 (76%); Central: 5 (12%); Peripheral: 12 (29%); Both central and peripheral: 25 (59%) | Interstitial thickening or reticulation: 17 (41%) |
| Air broncograms: 14 (33%) | ||||||||
| Fibrotic streaks or linear opacities: 15 (36%) | ||||||||
| Lymphadenopathy: 12 (29%) | ||||||||
| Pleural Effusion: 5 (12%) | ||||||||
| W. Guan, 6 March 2020 [2] | 1099 | 47.0 | F: 459 M: 640 | N.R. | 550 (56.4%) | 409 (41.9%) | Bilateral: 505 (51.8%) | Interstitial thickening or reticulation: 143 (14.7%) |
| Harrison X. Bai, 10 March 2020 [20] | 219 | 44.8 | F: 100 M: 119 | 4.9 | 200 (91%) | 150 (69%) | Unilateral: 41 (19%) Bilateral: 165 (75%) central: 3 (1%) periferical: 176 (80%) Central + Peripheral: 31 (14%) | Crazy paving: 11 (5%) |
| Interstitial thickening or reticulation: 123 (56%) | ||||||||
| Air broncograms: 30 (14%) | ||||||||
| "Reversed halo" sign: 11 (5%) | ||||||||
| Fibrotic streaks or linear opacities: 111 (51%) | ||||||||
| Lymphadenopathy: 6 (3%) | ||||||||
| Pleural Effusion: 9 (4%) | ||||||||
| Pleural retraction sign / thickening: 32 (15%) | ||||||||
| Dahai Zhao, 13 March 2020 [21] | 19 | 48 | F: 8 M: 11 | 5 | 17 (89.47%) | N.R. | Single lobe: 4 (21.05%) Bilateral: 15 (78.95%) | |
| Cheng, 14 March 2020 [22] | 11 | 50.36 | F: 3 M: 8 | N.R. | GGO 11 (100%); Mixed GGO 7 (63.6%) | 6 (54.5%) | Peripheral: 11 (100%); right lower lobe: 8 (72.7%); left lower lobe: 7 (63.6%) | Interstitial thickening or reticulation: 9 (81.8%) |
| Air broncograms: 8 (72.7%) | ||||||||
| Fibrotic streaks or linear opacities: 2 (18.2%) | ||||||||
GGO: Ground-Glass Opacity; N.R.: Not Reported; CT: Computed Tomography
4. Discussion
Since December 2019, the COVID-19 started in Wuhan, China and rapidly spread outside China with cases confirmed in several countries being officially declared as pandemic by the World Health Organization (WHO) on March 11, 2020 [32]. At present, Italy is the second most affected SARS-CoV-2 country in the world with 35713 total confirmed cases up to 19 March 2020 [33]. In a setting of high clinical suspicion, due to typical clinical symptoms and previous exposure to other individuals with SARS-CoV-2, a combination of chest imaging elements and repeat laboratory RT-PCR may help to increase the COVID-19 diagnosis. For this reason, it is of crucial importance to define imaging patterns of COVID-19 pneumonia in order to promptly diagnose the viral infection in acute stages and to lead to the correct work-up. We retrospectively evaluated multi-modality imaging hallmarks of 36 patients with laboratory-confirmed COVID-19, during the early phases of the disease.
The salient manifestations on CXR were consolidations in fifteen patients and hazy increased opacities in twelve patients with bilateral lower lobe predominant distribution; as reported in the literature review, CXR lacks sensitivity in the early stages of lung disease [16,27].
Lung US was performed on twenty-two patients and showed thickened pleural lines, B lines organized in different patterns and patchy consolidations; among these patients, seventeen had US along with CT scans, demonstrating an association with CT findings such as GGO and consolidations. These findings were consistent with the experience provided by Peng et al and Poggiali et al. [30,31], confirming the important role of lung US on the management of patients with SARS-CoV-2 allowing to rapidly diagnose and monitor COVID-19 pneumonia and its evolution toward ARDS, in critically ill patients.
Chest CT key findings in our case series were GGO with or without consolidations and crazy-paving appearances composed by GGO with reticular and/or interlobular septal thickening with predominant peripheral distribution; it was interesting to note in some cases the simultaneous presence of these patterns in the same patient suggesting a progression of the pulmonary involvement. Our findings were consistent with the collected data described in recent studies. Regarding lesions distribution, we confirmed the bilateral and multifocal lung involvement, concordant with data of previous works. In six cases we found lymphadenopathy and in three cases pleural effusion considered uncommon findings based on recent literature. Furthermore, we noted the presence of perilesional vascular thickening in ten patients (23.8%), representing a peculiar CT manifestation of COVID-19; this sign was observed in the studies of Yan Li 82.4% [12], Zhao 71.3% [8] and Zhou 45.2% [9], and it has been correlated to the hyperemia induced by an acute inflammatory response, expression of temporal changes.
Accordingly, different CT manifestations have been associated with the disease progression, based on the physiopathology of the acute lung injury induced by viral pneumonia; of note Jin et al [34] recognized five temporal stages classified as ultra-early, early, rapid progression, consolidation, and dissipation stages. In details, in the ultra-early stage, CT elements may be single or multiple focal GGO, nodules enclosed by GGO, patchy consolidations, and air-bronchogram signs; the early stage is characterized by multiple GGO, patchy consolidations, or a combination of GGO and interlobular septal thickening expression of increased interlobular interstitial edema, alveolar capillary congestion and exudate fluid in alveolar sac [34]. During the rapid progression, there is a worsening of the inflammatory damage corresponding to CT features such as large consolidative opacities and contextual air bronchograms; these findings gradually may decrease in size and density during the consolidation stage. Finally, in the dissipation stage, lesions may be reduced of number and extension, with remaining small ill-defined consolidative opacities, reticular opacities and interlobular septal thickening [34].
This proteiform spectrum of CT imaging elements could overlap with other viral pneumonia especially within the same Coronaviridae family because of the similar pathogenesis resulting in diffuse interstitial and alveolar damage [35]; nevertheless, bilateral and multilobar lung involvement of SARS-CoV-2 depicted in our study and prior works differs from unifocal lung disease of patients with SARS and patients with MERS [36]. Furthermore, among the associated COVID-19 pneumonia signs, the reversed halo-sign and pulmonary nodules with a halo sign observed in recent studies [9,10] were not described in the literature regarding SARS and MERS. In addition, the described chest CT appearance of COVID-19 pneumonia of bilateral peripheral GGO could resemble other processes such as influenza pneumonia and organizing pneumonia, either idiopathic or secondary to drug toxicity and connective tissue disease [20,37].
Limitations of our study were related to the retrospective design, the small size sample and the lack of follow-up studies. Indeed, we included in our analysis solely imaging examinations performed at admission to the hospital, without assessing the imaging changes of the disease along with temporal phases.
In conclusion, we provided an overview of multi-modality lung imaging lesions encountered in patients with COVID-19 pneumonia supplemented by an exhaustive review of the current literature; hence, we highlighted the typical CT features observed at admission to delineate an informative picture helping radiologists to early recognize this emerging severe infectious disease.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
CRediT authorship contribution statement
Pascal Lomoro: Conceptualization, Writing - original draft, Investigation. Francesco Verde: Writing - original draft, Writing - review & editing, Data curation. Filippo Zerboni: Visualization, Investigation. Igino Simonetti: Writing - review & editing, Methodology, Data curation, Supervision. Claudia Borghi: Methodology. Camilla Fachinetti: Visualization. Anna Natalizi: Investigation. Alberto Martegani: Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We thank all the following colleagues for their valuable help: Azzaretti Andrea, Beneggi Irene Maria, Borelli Anna, Catania Roberta, Galluzzi Pietro Antonio, Luciani Antongiulio, Mombelloni Sara, Togni Giorgio, Tufarulo Loredana. We also thank the clinical staff of the emergency department for their frontline tireless work in facing such a critical public health condition.
Contributor Information
Pascal Lomoro, Email: lmr.pcl89@gmail.com.
Francesco Verde, Email: francescoverde87@gmail.com.
Filippo Zerboni, Email: fzerboni@valduce.it.
Igino Simonetti, Email: igino.simonetti@gmail.com.
Claudia Borghi, Email: clborghi@libero.it.
Camilla Fachinetti, Email: camifachinetti@gmail.com.
Anna Natalizi, Email: an.natalizi@gmail.com.
Alberto Martegani, Email: amartegani@valduce.it.
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. China Medical Treatment Expert Group for Covid-19, Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zu Z.Y., Di Jiang M., Xu P.P., Chen W., Ni Q.Q., Lu G.M., Zhang L.J. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020:200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., Ji W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D., Chen B., Zhang Z., Guan W., Ling Z., Jiang R., Hu T., Ding Y., Lin L., Gan Q., Luo L., Tang X., Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020 doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR. Am. J. Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S., Wang Y., Zhu T., Xia L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China. Am. J. Roentgenol. 2020:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 10.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Diao K., Lin B., Zhu X., Li K., Li S., Shan H., Jacobi A., Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y.-H., Dong J.-H., An W.-M., Lv X.-Y., Yin X.-P., Zhang J.-Z., Dong L., Ma X., Zhang H.-J., Gao B.-L. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Xia L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. Am. J. Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 13.Wu J., Wu X., Zeng W., Guo D., Fang Z., Chen L., Huang H., Li C. Chest CT Findings in Patients with Corona Virus Disease 2019 and its Relationship with Clinical Features. Invest. Radiol. 2020:1. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A., Dai J., Sun Q., Zhao F., Qu J., Yan F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K., Wu J., Wu F., Guo D., Chen L., Fang Z., Li C. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Invest. Radiol. 2020 doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Y., Sun D., Liu Y., Fan Y., Zhao L., Li X., Zhu W. Clinical and High-Resolution CT Features of the COVID-19 Infection: Comparison of the Initial and Follow-up Changes. Invest. Radiol. 2020 doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai H.X., Hsieh B., Xiong Z., Halsey K., Choi J.W., Tran T.M.L., Pan I., Shi L.-B., Wang D.-C., Mei J., Jiang X.-L., Zeng Q.-H., Egglin T.K., Hu P.-F., Agarwal S., Xie F., Li S., Healey T., Atalay M.K., Liao W.-H. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao D., Yao F., Wang L., Zheng L., Gao Y., Ye J., Guo F., Zhao H., Gao R. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Z., Lu Y., Cao Q., Qin L., Pan Z., Yan F., Yang W. Clinical Features and Chest CT Manifestations of Coronavirus Disease 2019 (COVID-19) in a Single-Center Study in Shanghai, China. Am. J. Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22959. [DOI] [PubMed] [Google Scholar]
- 23.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., Cui J., Xu W., Yang Y., Fayad Z.A., Jacobi A., Li K., Li S., Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H., Ling Y., Jiang Y., Shi Y. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T., Hu Q., Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng M.-Y., Lee E.Y., Yang J., Yang F., Li X., Wang H., Lui M.M., Lo C.S.-Y., Leung B., Khong P.-L., Hui C.K.-M., Yuen K., Kuo M.D. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol. Cardiothorac. Imaging. 2020;2 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., Zheng D., Wang J., Hesketh R.L., Yang L., Zheng C. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology. 2020:200370. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han R., Huang L., Jiang H., Dong J., Peng H., Zhang D. Early Clinical and CT Manifestations of Coronavirus Disease 2019 (COVID-19) Pneumonia. Am. J. Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22961. [DOI] [PubMed] [Google Scholar]
- 30.Poggiali E., Dacrema A., Bastoni D., Tinelli V., Demichele E., Mateo Ramos P., Marcianò T., Silva M., Vercelli A., Magnacavallo A. Can Lung US Help Critical Care Clinicians in the Early Diagnosis of Novel Coronavirus (COVID-19) Pneumonia? Radiology. 2020:200847. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng Q.Y., Wang X.T., Zhang L.N., Critical C., Ultrasound C., Group S. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019 – 2020 epidemic. Intensive Care Med. 2020:6–7. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coronavirus (COVID-19) events as they happen (n.d.). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (Accessed 20 March 2020).
- 33.Situation Report-59 HIGHLIGHTS (n.d.) https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200319-sitrep-59-covid-19.pdf?sfvrsn=c3dcdef9_2 (Accessed 20 March 2020).
- 34.Jin Y.-H., Cai L., Cheng Z.-S., Cheng H., Deng T., Fan Y.-P., Fang C., Huang D., Huang L.-Q., Huang Q., Han Y., Hu B., Hu F., Li B.-H., Li Y.-R., Liang K., Lin L.-K., Luo L.-S., Ma J., Ma L.-L., Peng Z.-Y., Pan Y.-B., Pan Z.-Y., Ren X.-Q., Sun H.-M., Wang Y., Wang Y.-Y., Weng H., Wei C.-J., Wu D.-F., Xia J., Xiong Y., Xu H.-B., Yao X.-M., Yuan Y.-F., Ye T.-S., Zhang X.-C., Zhang Y.-W., Zhang Y.-G., Zhang H.-M., Zhao Y., Zhao M.-J., Zi H., Zeng X.-T., Wang Y.-Y., Wang X.-H. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo H.J., Lim S., Choe J., Choi S.-H., Sung H., Do K.-H. Radiographic and CT Features of Viral Pneumonia. RadioGraphics. 2018;38:719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 36.Das K.M., Lee E.Y., Langer R.D., Larsson S.G. Middle East Respiratory Syndrome Coronavirus: What Does a Radiologist Need to Know? Am. J. Roentgenol. 2016;206:1193–1201. doi: 10.2214/AJR.15.15363. [DOI] [PubMed] [Google Scholar]
- 37.Obadina E.T., Torrealba J.M., Kanne J.P. Acute pulmonary injury: High-resolution CT and histopathological spectrum. Br. J. Radiol. 2013 doi: 10.1259/bjr.20120614. [DOI] [PMC free article] [PubMed] [Google Scholar]