The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), also known as the 2019 novel Coronavirus (2019-nCoV), has caused a recent outbreak of Coronavirus Disease (COVID-19) [1], [2]. To date, COVID-19 has already affected 372,757 people globally, with a 4.35% mortality rate. In Indonesia, the first case of COVID-19 was reported on March 2, 2020. Since then, the number of positive cases has risen significantly to 686 cases, according to government data on March 23, 2020.
Although most cases were mild to moderate, some patients developed severe symptoms characterized by respiratory dysfunction and/or multiple organ failure [3]. In the current situation, identification of COVID-19 disease progression mainly relies on the clinical manifestation, while no effective biomarker has been proposed. It has been suggested that one of the possible mechanisms underlying rapid disease progression is a cytokine storm [1], [2]. Previous retrospective studies indicated that an elevated level of interleukin-6 (IL-6) was associated with a high case fatality of COVID-19 infection [1]. Thus, a meta-analysis of the current scientific literature was performed to investigate whether the measurement of serum IL-6 may predict disease progression of COVID-19 patients.
A comprehensive literature search was conducted from PubMed, Scopus, Web of Science, and Google Scholar. Keywords such as “IL-6”, “coronavirus 2019/COVID-19”, “2019-nCoV”, “SARS-CoV-2” were used on their own or in combination without applying language restriction and dated up to March 25, 2020. Selection criteria were case-control or cohort studies evaluating serum IL-6 levels in COVID-19 patients with or without severe condition (i.e., the period of infection, CT score, ICU admission, troponin levels, those needing mechanical ventilation, or those who died). A meta-analysis was performed using a random-effect model to allow for heterogeneity in individual studies. Hedge's standardized mean difference (SMD) with 95% confidence interval (CI) was used to assess the IL-6 level in non-severe and severe patients. Heterogeneity was evaluated with Q-test and I2 statistics. Begg's funnel plot and Egger's regression test were applied to determine publication bias if the pooled effect size consisted of 10 or more studies. A sensitivity test was performed by sequentially omitting one study each time to evaluate the stability of the results. Data that was not expressed as mean and standard deviation was extrapolated from the sample size, median, and interquartile range (IQR). The meta-analysis was performed using RevMan version 5.3 and the value of 0.05 was indicative of statistical significance.
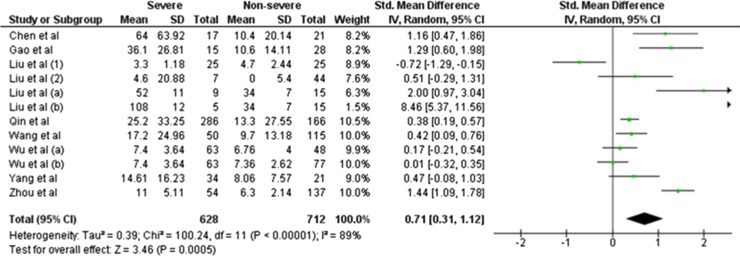
We initially identified 73 articles based on our search strategy, 64 of which were excluded after title, abstract, or full-text reading, because they were review articles, did not report IL-6 levels, or did not classify the condition of COVID-19 patients as non-severe and severe. Finally, only nine studies were included in this meta-analysis [1], [2], [3], [4], [5], [6], [7], [8], [9]. Although the heterogeneity was considerably high (I2 = 89%; P < 0.00001), we observed that IL-6 levels were significantly increased in COVID-19-infected patients with severe condition compared with those with non-severe condition (SMD = 0.71, 95%CI −0.31–1.12, P = 0.0005) (Fig. 1 ). Begg's funnel plot (data not shown) and Egger's test were performed, and no publication bias was observed (P Egger's test = 0.117). A sensitivity analysis was conducted by eliminating one individual study each time (Fig. 2 ). However, the results remained unchanged, suggesting that the findings were statistically stable and robust.
Fig. 1.
Forest plot for pooled standardized mean difference (SMD) and 95% confidence interval (95% CI) of circulating IL-6 levels in COVID-19-infected patients with severe and non-severe condition.
Fig. 2.
Sensitivity analysis of changes in IL-6 levels of COVID-19-infected patients.
Cytokines are vital in regulating immunological and inflammatory responses. Among them, IL-6 is of major importance because of its pleiotropic effects [1]. Here, we presented the evidence that circulating IL-6 levels are closely linked to the severity of COVID-19 infection. An increase in IL-6 levels has previously been observed in patients with respiratory dysfunction [2], implying a possible shared mechanism of cytokine-mediated lung damage caused by COVID-9 infection. Furthermore, it seems that the highly pathogenic SARS-CoV-2 is associated with rapid virus replication and a tendency to infect the lower respiratory tract, resulting in an elevated response of IL-6-induced severe respiratory distress. Thus, our results suggest that serial measurement of circulating IL-6 levels may be important in identifying disease progression among COVID-19-infected patients. In line with our findings, an elevated level of IL-6 has been proven to be a good biomarker for severity of hepatitis B virus (HBV) infection [10]. Therefore, it is reasonable that immediate initial evaluation of IL-6 level be performed upon hospital admission of COVID-19 patients, due to its potential benefits to assess worsening clinical features and disease progression in COVID-19.
Human and animal rights
The authors declare that the work described has not involved experimentation on humans or animals.
Informed consent and patient details
The authors declare that the work described does not involve patients or volunteers.
Funding
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng J., et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. bioRxiv. 2020 doi: 10.1101/2020.02.29.20029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Luo S., Shen Y., Li M., Zhang Z., Dong Y., et al. Multiple enzyme release, inflammation storm and hypercoagulability are prominent indicators for disease progression in COVID-19: a multi-centered, correlation study with CT imaging score. SSRN. 2020 doi: 10.2139/ssrn.3544837. [DOI] [Google Scholar]
- 3.Yang P., Ding Y., Xu Z., Pu R., Li P., Yan J., et al. Epidemiological and clinical features of COVID-19 patients with and without pneumonia in Beijing, China. bioRxiv. 2020 doi: 10.1101/2020.02.28.20028068. [DOI] [Google Scholar]
- 4.Liu J., Ouyang L., Guo P., Wu H.S., Fu P., Chen Y.L., et al. Epidemiological, clinical characteristics and outcome of medical staff infected with COVID-19 in Wuhan, China: A retrospective case series analysis. bioRxiv. 2020 doi: 10.1101/2020.03.09.20033118. [DOI] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan 4, Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [pii: S0140-6736(20)30566-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Hu X., Song J., Du C., Xu J., Yang D., et al. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) bioRxiv. 2020 doi: 10.1101/2020.02.26.20028589. [DOI] [Google Scholar]
- 7.Liu L., Gao J.Y., Hu W., Zhang X., Guo L., Liu C.Q., et al. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. bioRxiv. 2020 doi: 10.1101/2020.02.20.20025536. [DOI] [Google Scholar]
- 8.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [pii: ciaa248] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia C., Liu Y., Chen Z., Zheng M. Involvement of Interleukin 6 in Hepatitis B Viral Infection. Cell Physiol Biochem. 2015;37(2):677–686. doi: 10.1159/000430386. [DOI] [PubMed] [Google Scholar]