Respiratory virus infections (RVI) cause significant morbidity and mortality in transplant patients. A recent study reported similar sensitivity of nasal and saliva specimens in diagnosing RVI, but samples were collected by a trained provider in a health-care setting, from immunocompetent patients, and at one time point [1]. An accurate and patient-acceptable method of sample self-collection could allow for home testing, facilitate community-based assessment of RVI epidemiology, and enable longitudinal monitoring of RVI. We previously reported the use of self-collected foam nasal swabs (NS) for longitudinal monitoring of RVI; in this study, we compared self-collected NS and oral wash (OW) specimens for monitoring RVI in a cohort of lung transplant recipients (LTR).
We prospectively assessed semi-quantitative viral load (VL) in sequential self-collected NS and OW specimens in LTR with symptomatic RVI. Between 11/2015 and 3/2016 we enrolled six consecutive LTR with RVI clinically diagnosed by a provider-obtained nasopharyngeal swab. After verbal and written instructions, patients self-collected concurrent NS and OW samples daily for 14 days. Samples were either given directly to study coordinators (inpatient, n = 26 paired samples) or mailed to the laboratory via FedEx envelopes (outpatient, n = 51 paired samples). NS were obtained as previously described [2], [3], [4], and OW were performed by gargling 10 mL saline for 10 s and then expectorating into a sterile collection cup. Specimens were tested via a validated, lab-developed real-time PCR for 12 viruses (rhinovirus [RHV], coronavirus [CoV], parainfluenza 1-4, influenza A-B, respiratory syncytial virus, adenovirus, bocavirus, metapneumovirus) as previously published [2], [3], [4]. Results were expressed semi-quantitatively as cycle threshold (Ct) values, where Ct values <40 were considered positive and lower values represented higher viral load. Descriptive statistics and the chi-squared test were used to compare the sensitivity of each method. All patients provided written informed consent.
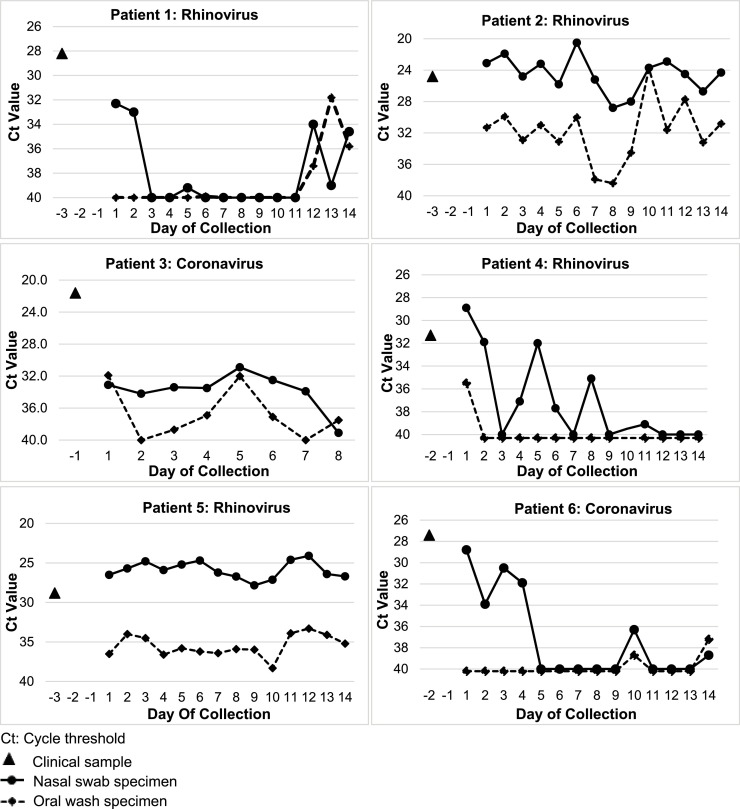
Among the six patients (4 RHV, 2 CoV), the median Ct value at baseline was 27.5 (range: 24.8–31.3) (Fig. 1 ). Of the possible 84 paired nasal-oral samples, 77 (92%) were collected (26/26 of inpatient and 51/58 [88%] outpatient). At least 13 of 14 paired samples were obtained in 5/6 (83%) of patients, and viral RNA was detected on the last day of sampling by at least one method in 5/6 (83%) patients. Overall, 70% (54/77) of NP and 52% (40/77) of OW were positive (p = 0.02). When either paired sample method was positive (n = 54), 100% of NS vs. 74% of OW were positive (p < 0.001). When both paired samples were positive (n = 40), 90% of NS samples had higher VL. Patterns of change in VL over time were similar for both methods. Patients reported both methods as equally easy and comfortable to collect.
Fig. 1.
Cycle threshold values of paired patient self-collected nasal swabs and oral washes in lung transplant recipients with respiratory virus infection.
In our prospective study of sequential self-collected samples in LTR with RVI, we found that both methods were acceptable by patients, but that NS were more frequently positive and had higher VL compared to concurrently collected OW samples. Limitations of this study include a small study size at a single center and the detection of only RHV and CoV, which restrict our ability to more broadly generalize these findings. Additionally, NS sample may be relatively higher in viral load compared to OW samples due to the preferred anatomic location of certain viruses, and this study was not designed to address this issue. Although larger studies are needed, these results support the use of self-collected NS over OW for community-based studies for both detection and longitudinal monitoring of the course of RVI in immunocompromised patients.
Funding
None.
Competing interests
None declared.
Ethical approval
University of Washington IRB (IRB #44580).
Acknowledgment
None.
References
- 1.Kim Y.G., Yun S.G., Kim M.Y., Park K., Cho C.H., Yoon S.Y., Nam M.H., Lee C.K., Cho Y.J., Lim C.S. Comparison between saliva and nasopharyngeal swab specimens for detection of respiratory viruses by multiplex reverse transcription-PCR. J. Clin. Microbiol. 2017;55:226–233. doi: 10.1128/JCM.01704-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preiksaitis C.M., Kuypers J.M., Fisher C.E., Campbell A.P., Jerome K.R., Huang M.L., Boeckh M. A patient self-collection method for longitudinal monitoring of respiratory virus infection in solid organ transplant recipients. J. Clin. Virol. 2015;62:98–102. doi: 10.1016/j.jcv.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell A.P., Kuypers J., Englund J.A., Guthrie K.A., Corey L., Boeckh M. Self-collection of foam nasal swabs for respiratory virus detection by PCR among immunocompetent subjects and hematopoietic cell transplant recipients. J. Clin. Microbiol. 2013;51:324–327. doi: 10.1128/JCM.02871-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuypers J., Wright N., Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J. Clin. Virol. 2004;31:123–129. doi: 10.1016/j.jcv.2004.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]