Abstract
Multiple sclerosis (MS) is a serious chronic neurological disorder in which demyelination and inflammation occur in the white matter of the CNS. The findings of many epidemiological studies and a discordance of MS in monozygotic twins suggest that the disorder is acquired. The most likely cause is a virus because more than 90% of patients with MS have high concentrations of IgG, manifest as oligoclonal bands, in the brain and CSF. Most chronic inflammatory CNS disorders are infectious. More indirect evidence that MS is caused by a virus is the association of several viruses with demyelinating encephalomyelitis in human beings, and the induction of demyelination in animals infected with viruses in research. Nevertheless, no virus has been isolated from the brains of patients who had MS. Molecular analysis of IgG gene specificity in the brain and CSF of those with MS has shown features of an antigen-driven response: clonal amplification and extensive somatic mutations. A viral antigen against which the IgG in MS brain and CSF is directed might be identified.
Multiple sclerosis (MS) is the most common demyelinating disease of human beings in more developed countries. In the USA, for example, there are 250 000–350 000 people with MS, resulting in more than 3000 deaths per year, with an estimated annual morbidity cost of over US$2·5 billion. Most patients with MS are young. A relapsing-remitting course that begins between the ages of 15 years and 50 years is common, although a substantial proportion of patients eventually develop chronic progressive disorder. The plaque, an area of white-matter demyelination, is the pathological hallmark of MS; early in the course of the disorder it is accompanied by inflammation and later by astrogliosis. Active plaques consist of mononuclear inflammatory cell infiltrates concentrated in perivascular spaces. The inflammatory infiltrates have features consistent with an active infection: T lymphocytes, B lymphocytes, plasma cells, and macrophages or microglia. IgG is found primarily at the periphery of plaques.1 Although inflammation is generally believed to be a primary feature of demyelination in MS, myelin destruction has recently been reported to occur before inflammation.2 Thus, endogenous glia, such as microglia or astrocytes, might be a source of injury mediators.3
Infection and chronic neurological diseases
Various studies in the 1960s found that persistent virus infections caused chronic neurological disease. For example, paramyxovirus nucleocapsids were found in brains of patients with subacute sclerosing panencephalitis, a chronic inflammatory disease of both grey and white matter.4 Shortly after this study, high concentrations of antibody to measles virus were found in the serum and CSF of patients with subacute sclerosing panencephalitis.5 Within a few years, measles virus was isolated in tissue culture from subacute sclerosing panencephalitis brain explants.6
Another important discovery was that progressive multifocal leucoencephalopathy (PML), a fatal human demyelinating disease characterised by rapidly progressive dementia and motor deficit, was also caused by a virus. Human papovavirus (JC virus) was found in the oligodendrocytes of a patient with PML. JC virus was isolated from PML brain by cocultivation of explanted brain cells with normal human fetal brain.7 However, infection of rodents with JC virus produced tumours instead of demyelination. PML is the only human demyelinating disease for which a viral cause is known.
Rationale for an infectious cause of MS
Leading theories are that MS has an infectious or virus-triggered immunopathology, and possibly an autoimmune component. A virus might reactivate after years of latency and lyse oligodendrocytes, as occurs in PML, or could initiate immunopathology leading to demyelination, as happens in animals infected with strains of Theiler's murine encephalomyelitis virus, coronaviruses, and lentiviruses.8
Antibody in brain and CSF
The most important evidence to support infection as the cause of MS is that the brain and CSF of more than 90% of patients with the disorder have high concentrations of IgG, manifest as oligoclonal bands (figure 1 ). Few other CNS diseases are characterised by high concentrations of IgG and oligoclonal bands (table ). All those diseases that have high IgG concentrations are inflammatory and most are infectious.9 Furthermore, when the specificity of the high concentrations of IgG and oligoclonal bands in those diseases was studied, IgG was found to be antibody directed against the disease cause. For example, the oligoclonal IgG found in subacute sclerosing panencephalitis brains and CSF is directed against measles virus,10 not herpes simplex virus or mumps virus, and in cryptococcal meningitis, the IgG is directed against cryptococcus11 and not another fungus such as candida. These findings provide a rationale for the hypothesis that the oligoclonal IgG in MS brain and CSF is antibody directed against the cause of MS.
Figure 1.
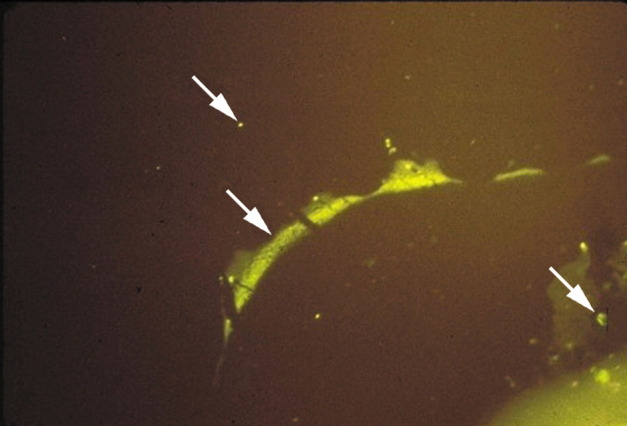
MS brain plaque–periplaque white matter
Direct immunofluorescence with a 1 to 20 dilution of antibody to human IgG conjugated to fluorescein isothiocyanate (green fluorescence) shows IgG deposition at the junction of plaque–periplaque white matter (middle arrow), in mononuclear cells (bottom arrow), and in normal white matter (top arrow). The antigen against which the IgG in MS brain and CSF is directed is unknown.
Table.
Diseases in which the CSF has high concentrations of IgG and oligoclonal bands
| Disease | Oligoclonal IgG |
|---|---|
| MS | Unknown |
| Subacute sclerosing panencephalitis | Measles virus |
| Chronic progressive rubella panencephalitis | Rubella virus |
| Mumps meningitis | Mumps virus |
| Neurosyphilis (active) | Treponema pallidum |
| Tuberculous meningitis | Unknown |
| CNS sarcoidosis | Unknown |
| Cryptococcal meningitis | Cryptococcus |
| Subacute carcinomatous corticocerebellar degeneration | Unknown |
Epidemiology
The notion that MS might be infectious is not new. Epidemiological studies of MS have found a geographical pattern of increasing prevalence with increasing latitude north and south of the equator. This pattern of prevalence led to the question of whether MS would be affected by migration between regions with high and low prevalence. Earlier migration studies suggested that the risk of acquiring MS is largely established before age 15 years, but more recent studies suggest that the risk may happen over many years and is not restricted to childhood and early adult life.12, 13 Analysis of the data on MS from the Faroe Islands suggested a point-source epidemic acquired over a wide age range,14 but the data have been challenged.15 Two possibilities explain the epidemiological data. The “prevalence” hypothesis of MS suggests that the disease is most common where the causative agent is most widespread. By contrast, the “polio” hypothesis suggests that acquisition of early in life (eg, maternal antibody or that produced after infection in infancy) reduces the likelihood that the agent will ever reach the CNS, but that primary infection after puberty or in adult life results in a small incidence of CNS infection, leading to MS.16
Genetic
Familial aggregation has been recognised in MS.17 Studies of identical twins have shown that when one twin has MS, the other twin of the pair develops the disease in only 30% of cases, which suggests that more than a putative susceptible genotype causes disease.18, 19 The discordance of MS in monozygotic twins is best explained by an environmental factor. Nevertheless, there is a clear association of the MHC with MS.20 Other gene regions have also been implicated, but no direct link has been shown.
Study of adopted siblings and half-siblings in a large population in Canada21 reportedly did not support a viral cause of MS. Dyment and colleagues21 incorrectly rejected the hypothesis that MS could be viral; they reasoned that, because the frequency of MS in adopted relatives was no higher than would be expected, the disorder is not caused by a virus. First, in almost all viral infections of the CNS, the ratio of cases to infections is low. For example, poliomyelitis and postinfectious measles encephalomyelitis occur in one person per 500–2000 people infected. The risk of acquiring MS in an environment shared with adopted (non-biological) relatives would be expected to be the same as in the general population (1 in 1000) because the disorder to infection ratio of MS is likely to be low, and the period over which MS is acquired is probably decades.13 Accordingly, the frequency of MS in adopted relatives would not be expected to rise above that in the general population if the disease were caused by a virus. Indeed, that is what Dyment and colleagues21 found. Thus, their interpretation that MS is not infectious because there was no difference between adoptees and their relatives is flawed. The high risk of MS in siblings and twins is most probably due to a combination of virus infection superimposed on a predisposing genetic background. Even in models of demyelination in mice produced by Theiler's murine encephalomyelitis virus, there is a crucial genetic component. For example, after infection with Theiler's murine encephalomyelitis virus caused by injection of the virus into the brain, demyelination develops in SJL/J mice, but not in C57 black mice. Therefore, there seems to be a genetic predisposition to MS, but there is no genetic disorder in which the brain and CSF contain oligoclonal bands.
Association of demyelinating disease in human beings and animals with virus
Further evidence, although indirect, that a virus may cause MS comes from the association of viruses with postinfectious encephalomyelitis. This multifocal and diffuse demyelinating disorder mostly occurs as a complication of vaccinia (smallpox) vaccination or infection by measles virus. Because smallpox vaccination has been discontinued and most people are immunised against measles virus, postinfectious encephalomyelitis is now rare. However, many viruses might produce demyelination, as suggested by the temporal association of postinfectious encephalomyelitis after smallpox vaccination, or after measles, and also varicella or rubella infection. An immune-mediated mechanism of postinfectious and postvaccinial encephalomyelitis has been hypothesised.
Additionally, various viruses can produce demyelination in animals infected for research. The most studied experimental demyelination is infection of mice with Theiler's murine encephalomyelitis virus,22 but CNS demyelination in the host occurs after infection of mice with JHM or MHV-4 (coronaviruses), dogs with canine distemper virus, and sheep and goats with Visna virus and caprine arthritis-encephalitis virus. Each of these viruses is capable of establishing a persistent infection in their host, such that there is continuous virus replication over a long period without killing the host. The replicative capacity of such viruses could be attenuated or restricted in the cells they infect to prevent elimination by the immune system. These RNA viruses are under intense study by many laboratories to elucidate the relation of virus persistence to demyelination. Figure 2 shows a possible mechanism of demyelination produced by persistence of Theiler's murine encephalomyelitis virus.23
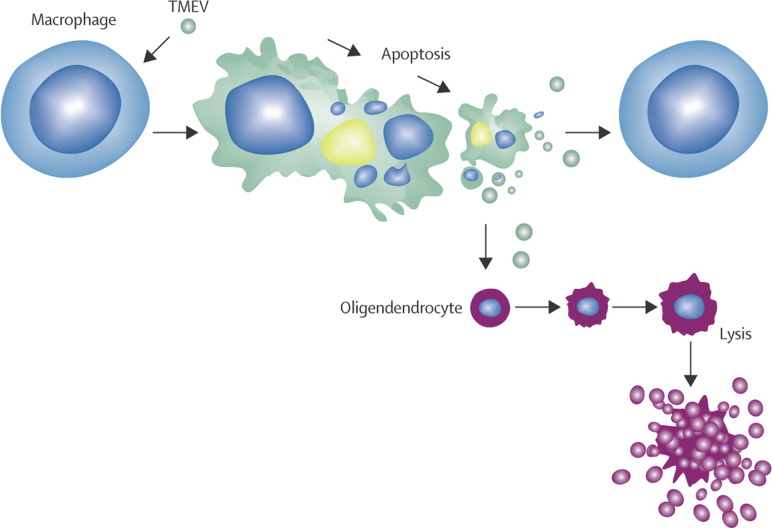
Figure 2.
A proposed mechanism of demyelination by infection with Theiler's murine encephalomyelitis virus (TMEV) in mice
TMEV is a highly cytolytic picornavirus. TMEV infection of oligodendrocytes is productive, resulting in cell lysis and liberation of more virions (bottom). By contrast, TMEV infection in macrophages is restricted, and results in apoptosis of macrophages.23 TMEV antigen is abundant in the cytoplasm of apoptotic macrophages (top centre). Small amounts of TMEV are liberated from persistently infected macrophages leading to infection of more macrophages as well as oligodendrocytes. A persistent CNS infection is established as virus spreads from macrophage to macrophage (across top). Virus released from macrophages can infect and kill more oligodendrocytes, thus adding to immunopathological destruction of myelin.
Some researchers have argued that more than one infectious agent might cause MS because there are various degrees of inflammation and demyelination. However, some chronic infectious disorders of the CNS that are caused by a single agent also have different pathologies. For example, in neurosyphilis, patients can have diffuse parenchymatous disease (general paresis), infection and inflammation restricted to cerebral arteries (meningovascular syphilis), isolated involvement of the optic nerve (syphilitic optic atrophy), lesions in only the posterior roots and columns (tabes dorsalis), or a solitary mass lesion (a syphilitic gumma). The same organism, Treponema pallidum, causes all these forms of neurosyphilis. Similarly, in some patients with MS perivascular inflammation is prominent; others have little more than optic atrophy, or may even develop a solitary demyelinating lesion that mimics tumour. Besides T pallidum, other organisms such as Mycobacterium tuberculosis and various fungi can produce chronic CNS disease with differing pathologies. Thus, a more reasonable hypothesis is that a single infectious agent causes MS.
If MS is infectious, it might result from a virus-induced or autoimmune immunopathology. To investigate autoimmunity, we have in our laboratory repeatedly attempted, unsuccessfully, to bind IgG extracted from the brains and CSF of patients with MS to brain from healthy people and those with MS. The lack of MS-brain IgG specificity for abnormal or healthy brain seems inconsistent with autoantigen involvement in the pathogenesis of this disorder. Although the viral hypothesis is not supported by binding inability, the putative MS virus might be latent and of low abundance in the brain. During virus latency in the nervous system, infectious virus is not produced, and viral gene expression is limited. For example, herpes simplex virus and varicella zoster virus are both latent in human ganglia, and antibody against either virus does not bind to human ganglia. However, latent virus can produce clinical disease on reactivation by unknown stimuli. Even with low-grade persistent infections and continuous virus replication, such as in models of virus-induced demyelination, the signs of a virus cannot be found without a known identity—eg, having specific antibodies to detect virus antigen.
Analysis of MS brain cells for an infectious agent
Researchers have assessed whether MS is transmissible and attempted to find an infectious agent in the brain in the disorder. Both measles and papovavirus had been identified from subacute sclerosing panencephalitis and PML brain, and the transmissibility of two degenerative neurological diseases (kuru and Creutzfeldt-Jakob disease) have been shown. Brain cells from patients with MS were propagated in tissue culture by the same techniques as are used to grow ganglia and to rescue herpes simplex virus from human trigeminal,24 nodose, and vagus ganglia.25 Although only glial cells and brain fibroblasts, but not neurons, in tissue culture survived subcultivation,26, 27 sufficient quantities of brain cells from patients with MS were generated. Initially, these cells were mixed with indicator cells (lung fibroblasts and kidney cells) in tissue culture, because the latter cells allow many viruses and other microorganisms to propagate readily and might permit replication and eventual isolation even if replication of an infectious agent in MS brain cells in tissue culture is restricted. However, cocultivation of MS brain cells in tissue culture with indicator cells did not lead to a cytopathic effect characteristic of any infectious agent.
Cell fusion provides even more contact between MS brain cells and indicator cells than cocultivation of these cells. In cell fusion, a fusing agent brings the membranes of two cells together to produce a heterokaryon (a cell containing different nuclei inside a common cytoplasm). Use of inactivated Sendai virus (a parainfluenza virus) as the fusing agent and pH 7·0, which had been shown to be optimum for heterokaryon production, led to the isolation of a parainfluenza virus (6/94 virus) from brains from patients with MS.28 However, 6/94 virus did not prove to be the cause of MS. Furthermore, because Sendai virus is a parainfluenza virus, it might not have been completely inactivated. Lysolecithin and eventually polyethylene glycol were tried as the fusing agent. Cells from the brains of many patients with MS were fused with indicator cells. The explanted MS brain cells were assessed before and after cell fusion, not only for a spontaneous cytopathic effect, but also for binding to measles virus, herpesviruses (herpes simplex virus, varicella zoster virus, and cytomegalovirus), coxsackie and ECHO viruses, rubella, influenza A and B, and coronaviruses by indirect immunofluorescence with various antisera. Explanted and fused MS brain cells were also inoculated into embryonated hen eggs, in which influenza and parainfluenza viruses are readily propagated, and into rodent species and chimpanzees in an attempt to produce neurological disease. Although no agent specific for MS was found, an endogenous mouse picornavirus (the WW strain of Theiler's murine encephalomyelitis virus) that produces demyelination was isolated.27 In addition, one chimpanzee inoculated intracerebrally with MS brain cells developed demyelinating disease, and cytomegalovirus was subsequently isolated from the brain of this animal;29 the virus was identified as a chimpanzee strain of cytomegalovirus.30 Inoculation of both chimpanzee and mouse with brain xenografts (MS cells) induced reactivation of an endogenous virus that can cause demyelination. Overall, virus was not found in the brain of any of the patients analysed.31
A pseudotype virus might be latent in the brain in MS. Weiss and co-workers32 showed that cells in tissue culture containing an enveloped virus and superinfected with vesicular stomatitis virus (varicella zoster virus) produced a pseudotype virus. The pseudotype or “transvestite” virus contained the genome of one virus and the protein coat of the second. The pseudotype virus was shown by a small virus fraction resistant to antibody against varicella zoster virus that was completely neutralised by antiserum to the second virus. To investigate the possibility of a latent enveloped virus in the brain in MS, brain cells from patients with MS in culture were infected with varicella zoster virus. When a cytopathic effect appeared, the cells were harvested and assayed for a varicella zoster virus non-neutralisable fraction, however, no enveloped virus latent in MS brain cells was found.
Association of various microorganisms with MS
Chlamydia pneumoniae
One of the organisms most recently implicated in MS is C pneumoniae, a gram-negative bacterium. Since the original detection of C pneumoniae DNA and antibody in CSF of some patients with MS,33 many laboratories have attempted replication. In an analysis of the humoral immune responses to C pneumoniae in paired serum and CSF samples of patients with definite MS and other inflammatory and non-inflammatory neurological disorders, no difference in seropositivity was found between the groups, although titres of IgG specific for C pneumoniae were substantially higher in the CSF of patients with MS than in controls. 16 (31%) of 52 patients with MS who were seropositive showed intrathecal synthesis of IgG specific for C pneumoniae compared with only one (2%) of 43 seropositive controls, and was strongly associated with intrathecal synthesis of polyclonal-specific IgG directed against C pneumoniae in 13 of 16 patients with MS. However, high titres of antibody to C pneumoniae in the CSF of patients with MS were not significantly correlated with disease duration, disease course, clinical or MRI disease activity, disability, or presence of oligoclonal IgG.34 Overall, many studies have assessed a possible relation between C pneumoniae and MS but none have found one.35 Further, an organism larger than a virus (eg, a rickettsial agent or a bacterium) is unlikely to have been missed by the many electron microscopists who have assessed MS plaques ultrastructurally.
Herpesviruses
In the past decade, two human herpesviruses have been associated with MS: human herpesvirus 6 (HHV-6), the cause of roseola, and Epstein-Barr virus (EBV), the cause of infectious mononucleosis. The detection of these ubiquitous viruses, known to be latent in blood B cells (EBV) or T cells (HHV-6), is intriguing because seroconversion to both viruses happens from before or during puberty into adult life—matching epidemiological evidence for the time of exposure to the disease-causing agent of MS.
HHV-6
HHV-6 DNA and antibody to the virus were detected in blood samples from patients with MS but were not associated with clinical disease.36 Studies assessing patients over time had mixed findings: serum only rarely contained HHV-6 DNA;37 HHV-6 DNA was more common during exacerbations;38 and HHV-6 was not detected.39 Increased concentrations of IgG to HHV-6 were found in blood samples from patients with relapsing-remitting MS than in those with chronic-progressive MS, other neurological diseases, and healthy controls.40 Titres of IgM to HHV-6 were high in 21 of 25 (80%) patients with MS, in three of 19 (16%) controls without MS, in two of 14 (14%) patients with autoimmune diseases,41 and active HHV-6A DNA was found in 15 of 103 (15%) patients with MS.42
A nucleotide fragment that was more than 99% identical to the major DNA-binding protein gene of HHV-6B was found in 25 (78%) of 32 brain specimens from patients with MS and 40 (74%) of 54 controls. HHV-6 antigen was found in oligodendrocytes in 12 (80%) of 15 brain specimens from patients with MS, more in cells associated with plaques than in normal white matter, and in none of 45 brain specimens from controls, which suggests that virus was actively replicating. Other cells (neurons, astrocytes, macrophages, ependymal cells, choroid plexus, and endothelial cells) were also positive in brains from patients and controls; antigen staining was prominent in brains from patients with CNS inflammatory disease, and antigen-positive cells were primarily macrophages.43 HHV-6 DNA has been found not only in the brains and CSF of patients with MS, but also in neoplastic and normal brains.44
However, other PCR studies did not detect HHV-6 DNA in CSF from any person in groups of 32 and 23 patients with MS,45, 46 and in another study, HHV-6 was found only in 11% of patients with MS compared with 7% of patients with HIV infection and no controls with other neurological disorders. Interestingly, when cellular, instead of cell-free CSF was analysed, HHV-6 DNA was detected in 29% of controls with other neurological diseases, 41% of patients with HIV infection, and 39% of patients with MS.36 This finding suggests that any association between HHV-6 and MS is attributable to virus in cells that were part of the inflammatory infiltrate. Support for this notion comes from analyses of 36 patients with MS, 27 with AIDS-related neurological disease, and 24 with non-inflammatory disease, which found HHV-6 DNA in 30–40% of CSF in all groups in which pleiocytosis was present, but no difference in HHV-6 antibody titres between patients with MS (including those in different disease stages) and controls.47
Further studies applied in-situ hybridisation combined with PCR to tissue sections fixed with formalin. HHV-6 DNA, but not antigen-positive cells, was found in 11 of 13 sections from eight brain specimens, mostly in oligodendrocytes.48 Laser microdissection was used to isolate tissue from plaques and normal-appearing white matter from the brains of 13 patients with MS, after which nested PCR amplified the HHV-6 major capsid protein gene. The HHV genome was detected in 16–27% of normal-appearing white matter from patients with MS, samples from healthy people, and samples from patients with other neurological disorders, whereas the HHV-6 genome was detected in 57% of plaques from patients with MS, a difference that was highly significant. There were no statistically significant differences among the groups when only HHV-6-positive patients were included.49
Overall, HHV-6 DNA and increased concentrations of antibody to HHV-6 in blood and CSF have been found in only a minority of patients with MS. Furthermore, HHV-6 DNA and increased concentrations of HHV-6 antibody occur in patients with other neurological diseases. Detection of HHV-6 DNA and antigen in brain might reflect HHV-6 reactivation from latency in blood T cells trafficking through the brains of patients with inflammatory CNS disease.
EBV
All patients with MS have antibody against EBV, compared with 86–95% of controls. Whether infection with EBV is a prerequisite for the development of MS or whether 100% seropositivity for EBV is a consequence of MS is not known.50 A meta-analysis of EBV in MS identified eight studies that included a total of 1005 patients with MS and 1060 controls. The summary odds ratio for patients with MS comparing EBV-seropositive with EBV-seronegative individuals was 13·5 (95% CI 6·3–31·4), a finding that the investigators believed supported a role for EBV in the cause of MS.51 Bray and colleagues52 found antibodies to the Epstein-Barr nuclear antigen in 85% of patients with MS compared with 13% of EBV-seropositive controls. Furthermore, a prospective serological study of 62 439 women53 found substantial increases in serum titres of anti-EBV before the onset of MS, particularly antibody to the Epstein-Barr nuclear antigen 2. An unexpected finding was the late onset of disease in most of the women who developed MS (median age 52 years). Unfortunately, no CSF data were available; such data are important because the IgG in MS brain and CSF is synthesised intrathecally and might reflect the immune response at the site of disease. The strongest predictors of MS were serum concentrations of IgG antibodies to the EBV viral capsid antigen or the Epstein-Barr nuclear antigen complex.54 Finally, because strong associations between EBV antibodies and the risk of MS were already apparent from samples collected from patients with MS 5 years or more before onset of disease, late EBV infection could have a role in MS.55
Overall, the neurotropism of EBV and its ability to produce serious neurological disease at all parts of the human nervous system have been documented.56 However, no studies have attempted to show that the oligoclonal IgG in brain and CSF in MS is directed against EBV, and in-situ hybridisation did not find EBV-specific RNA in brains from ten individuals with MS.57
Retroviruses
The possibility that MS is infectious has been extended to the idea that the disease could be sexually transmitted. A relation between sexual permissiveness and MS prevalence has been proposed, and infection with HTLV-1, a disorder with some features in common with MS, can be transmitted sexually. The sexual transmission hypothesis could be assessed with a case-control study of patients with MS and their partners, and by studies of MS in social groups adhering to a strict moral code, such as Mormons or nuns.58
Many researchers have searched for retrovirus sequences in MS brain. Jocher and co-workers59 found no HTLV-1 sequences in peripheral-blood mononuclear cells or brain of patients with MS, and a large masked PCR study of several populations did not find HTLV-1.60 Concentrations of antibody to reverse transcriptase of human retroviruses have been found to differ substantially between patients with MS and controls,61 but Rozenberg and colleagues62 used three sets of oligonucleotides, which detect all known human oncoretroviruses or lentiviruses, and found none in patients with MS or controls. Further studies did not find retrovirus in serum, CSF, or blood mononuclear cells of patients with MS.63 However, Rasmussen and co-workers64 compared peripheral-blood mononuclear cells from 22 patients with MS with those of matched healthy donors and five patients with other CNS disease; samples of brain were also studied. Several endogenous retrovirus sequences were transcribed in peripheral blood mononuclear cells and brain from patients with MS and controls. The significance of these findings is unknown. At the same time, Perron and co-workers65 repeatedly isolated a novel retrovirus (LM7) from the leptomeninges, choroid plexus, and EBV-immortalised B cells of patients with MS. The same sequences were also detected in non-cellular RNA from plasma of patients with MS and in CSF from untreated patients with MS. Further epidemiological studies are needed to elucidate the apparent association of retroviruses with MS.
Coronaviruses
The ability of coronaviruses to produce demyelination in experimentally infected mice has led to a few searches for human coronaviruses in MS brain. By use of in-situ hybridisation, Murray and colleagues66 detected coronavirus RNA in brains of 12 of 22 patients with MS, including coronavirus antigen in two patients with rapidly progressive disease; control brains were negative. Stewart and colleagues67 detected human coronavirus 229E RNA in four of 11 patients with MS, but not in brains of six patients with neurological disease or in the brains of five healthy people; coronavirus OC43 was not detected in any specimens. By contrast, PCR done with primers specific for two human coronaviruses (229E and OC43) showed no evidence of coronavirus infection in demyelinating MS brain.68
JC virus
Polyoma JC virus is the cause of PML, the only human demyelinating disease with a proven viral cause. The kidney is the only known site of latent infection. JC virus was not found in the urine of 53 patients with clinically definite MS or 53 controls matched for age and sex.69 In a study of 37 patients with MS who were taking ciclosporin,70 PCR showed DNA of JC virus in the urine of 30 (81%). However, this virus is intermittently excreted in urine by 40% of the general population.71 JC virus DNA was detected in the CSF of 9% of patients with MS but not in any patients with other neurological diseases or in other controls.72
New methods of detecting virus in MS
Developments in techniques from molecular biology enable studies of virus latency that were not possible 20 years ago. We know that DNA is transcribed into RNA, and that RNA becomes protein. The continuous production of increased amounts of IgG in the brain (figure 1) and CSF in MS suggest the presence of an antigen (probably a protein) against which the IgG is directed. The RNA from which this protein was translated could be used to identify the putative unique MS antigen. RNA can be readily extracted from the brain of patients with MS. Although RNA degrades quickly, it can be reverse-transcribed into cDNA, which are stable molecules. There are many methods by which cDNA libraries of a brain, containing all genes expressed in that brain, can be used to identify genes and RNA unique to disease. For example, the ability to prepare and characterise libraries of genes from human tissue has allowed the isolation of cDNA clones derived from hepatitis C virus (HCV) without prior knowledge of the virus, the viral genome, or the presence of circulating viral antibodies.73 Abundance of HCV-specific RNA in total liver RNA of infected animals is 0·00001%.73 Combined cloning in expression vectors (even without subtraction hybridisation) and immunological screening of about a million recombinant phages (prepared from a cDNA library derived from infectious material and constructed in bacteriophage) led to the identification of an HCV-specific antigen.
Some of these strategies have been applied in MS research. Cloning of IgG in brain and CSF in MS has shown over-represented (identical) heavy-chain sequences expressed at many plaque sites.74 Normally, the sequence of every heavy-chain and light-chain antibody is different. Alignment of the heavy-chain sequences to their closest germline counterparts showed clonal amplification and extensive somatic mutation—features of an antigen-driven response.74, 75 Comparison of the IgG heavy-chain sequences in MS and subacute sclerosing panencephalitis again showed features of an antigen-driven response in both diseases. Because the antigen in subacute sclerosing panencephalitis is known to be measles virus, the parallel findings in MS suggest an antigen-driven immune response76 rather than a non conventional mechanism of B-cell activation. Furthermore, the antigen-driven clonal B-lymphocyte and plasma-cell response has been found after a single clinically isolated syndrome,77, 78 which suggests that detection of the disease-relevant antigens in early CNS demyelination may bear on the inciting antigens in MS. Further analysis of the specificity of IgG in brain and CSF has the potential to identify an infectious agent in MS.
Drugs of the interferon beta family are among the most widely used to treat MS. These drugs are presumed to have immunomodulatory effects, but they could also be antiviral because interferons are produced by cells hours after virus infection.
Conclusion
Research has not isolated or directly linked a virus with MS. Many researchers think that MS is an immune-mediated disease, probably triggered by an infectious agent. Molecular biological and immunological strategies and techniques now enable studies of virus latency that were not possible previously. In particular, molecular analysis of the specificity of IgG in brain and CSF has the potential to identify an infectious agent in MS. Application of these techniques to single B cells and plasma cells in the CSF of patients with clinically isolated syndromes that commonly herald the onset of MS (eg, optic neuritis) has already shown features of an antigen-driven response. The search for a viral cause of MS must be continued.
Acknowledgments
Acknowledgments
I thank Howard Lipton for a critical review and for providing figure 2, Marina Hoffman for editorial review, and Cathy Allen for preparing the paper.
Conflicts of interest
I have no conflicts of interest.
Role of the funding source
No funding source had a role in the preparation of this paper or the decision to submit it for publication.
References
- 1.Lumsden CE. The immunogenesis of the multiple sclerosis plaque. Brain Res. 1971;28:365–390. doi: 10.1016/0006-8993(71)90052-7. [DOI] [PubMed] [Google Scholar]
- 2.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly formed lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 3.Lassmann H, Raine CS, Antel J, Prineas JW. Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J Neuroimmunol. 1998;86:213–217. doi: 10.1016/s0165-5728(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 4.Bouteille M, Fontaine C, Vedrenne C, Delarue J. Sur un cas d'encéphalite subaiguë à inclusions: étude anatomo-clinique et ultrastructurale. Rev Neurol (Paris) 1965;113:454–458. [Google Scholar]
- 5.Connolly JH, Allen IV, Hurwitz LJ, Miller JD. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967;1:542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- 6.Payne FE, Baublis JV, Itabashi HH. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969;281:585–616. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- 7.Padgett BL, Walker DL, ZuRhein GM. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 8.Buchmeier MJ, Lane TE. Viral-induced neurodegenerative disease. Curr Opin Microbiol. 1999;2:398–402. doi: 10.1016/S1369-5274(99)80070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilden DH, Devlin ME, Burgoon MP, Owens GP. The search for virus in multiple sclerosis brain. Mult Scler. 1996;2:179–183. doi: 10.1177/135245859600200403. [DOI] [PubMed] [Google Scholar]
- 10.Vandvik B, Norrby E, Nordal HJ, Derge M. Oligoclonal measles virus-specific IgG antibodies isolated from cerebrospinal fluids, brain extracts, and sera from patients with subacute sclerosing panencephalitis and multiple sclerosis. Scand J Immunol. 1976;5:979–992. doi: 10.1111/j.1365-3083.1976.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 11.Porter KG, Sinnamon DG, Gillies RR. Cryptococcus neoformans-specific oligoclonal immunoglobulins in cerebrospinal fluid in cryptococcal meningitis. Lancet. 1977;1:1262. doi: 10.1016/s0140-6736(77)92473-4. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzke JF, Delasnerie-Laupretre N, Wallin MT. Multiple sclerosis in North African migrants to France. Acta Neurol Scand. 1998;98:302–309. doi: 10.1111/j.1600-0404.1998.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 13.Hammond SR, English DR, McLeod JG. The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain. 2000;123:968–974. doi: 10.1093/brain/123.5.968. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzke JF, Hyllested K. Mutliple sclerosis in the Faroe Islands: I—clinical and epidemiological features. Ann Neurol. 1979;5:6–21. doi: 10.1002/ana.410050104. [DOI] [PubMed] [Google Scholar]
- 15.Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004;3:709–718. doi: 10.1016/S1474-4422(04)00933-0. [DOI] [PubMed] [Google Scholar]
- 16.Nathanson N, Miller A. Epidemiology of multiple sclerosis: critique of the evidence for a viral etiology. Am J Epidemiol. 1978;107:451–461. doi: 10.1093/oxfordjournals.aje.a112564. [DOI] [PubMed] [Google Scholar]
- 17.Mackay RP. The familial occurrence of multiple sclerosis and its implications. Ann Intern Med. 1950;33:298–320. doi: 10.7326/0003-4819-33-2-298. [DOI] [PubMed] [Google Scholar]
- 18.Spielman RS, Nathanson N. The genetics of susceptibility to multiple sclerosis. Epidemiol Rev. 1982;4:45–65. doi: 10.1093/oxfordjournals.epirev.a036251. [DOI] [PubMed] [Google Scholar]
- 19.Willer CJ, Dyment DA, Risch NJ. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci USA. 2003;100:12877–12882. doi: 10.1073/pnas.1932604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotgiu S, Rosati G, Saana A. Multiple sclerosis complexity in selected populations: the challenge of Sardinia, insular Italy. Eur J Neurol. 2002;9:1–13. doi: 10.1046/j.1468-1331.2002.00412.x. [DOI] [PubMed] [Google Scholar]
- 21.Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004;3:104–110. doi: 10.1016/s1474-4422(03)00663-x. [DOI] [PubMed] [Google Scholar]
- 22.Lipton HL, Dal Canto MC. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976;192:62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- 23.Schlitt BP, Felrice M, Jelachich ML, Lipton HL. Apoptotic cells, including macrophages, are prominent in Theiler's virus-induced inflammatory, demyelinating lesions. J Virol. 2003;77:4383–4388. doi: 10.1128/JVI.77.7.4383-4388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren KG, Devlin M, Gilden DH. Isolation of herpes simplex virus from human trigeminal ganglia, including ganglia from one patient with multiple sclerosis. Lancet. 1977;2:637–639. doi: 10.1016/s0140-6736(77)92501-6. [DOI] [PubMed] [Google Scholar]
- 25.Warren KG, Brown SM, Wroblewska Z. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of humans. N Engl J Med. 1978;298:1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- 26.Gilden DH, Wroblewska Z, Chesler M. Experimental panencephalitis induced in suckling mice by parainfluenza type I (6/94) virus II: virologic studies. J Neuropathol Exp Neurol. 1976;35:59–270. doi: 10.1097/00005072-197605000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Wroblewska Z, Gilden DH, Wellish M. Virus-specific intracytoplasmic inclusions in mouse brain produced by a newly isolated strain of Theiler virus: I—virologic and morphologic studies. Lab Invest. 1977;37:595–602. [PubMed] [Google Scholar]
- 28.ter Meulen V, Koprowski H, Iwasaki Y. Fusion of cultured multiple-sclerosis brain cells with indicator cells: presence of nucleocapsids and virions and isolation of parainfluenza-type virus. Lancet. 1972;2:1–5. doi: 10.1016/s0140-6736(72)91273-1. [DOI] [PubMed] [Google Scholar]
- 29.Rorke LB, Iwasaki Y, Koprowski H. Acute demyelinating disease in a chimpanzee three years after inoculation of brain cells from a patient with MS. Ann Neurol. 1979;5:89–94. doi: 10.1002/ana.410050113. [DOI] [PubMed] [Google Scholar]
- 30.Wroblewska Z, Gilden DH, Devlin M. Cytomegalovirus isolation from a chimpanzee with acute demyelinating disease after inoculation of MS brain cells. Infect Immun. 1979;25:1008–1015. doi: 10.1128/iai.25.3.1008-1015.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilden DH. A search for virus in multiple sclerosis. Hybrid Hybridomics. 2002;21:93–97. doi: 10.1089/153685902317401672. [DOI] [PubMed] [Google Scholar]
- 32.Weiss RA, Boettiger D, Love DN. Phenotypic mixing between vesicular stomatitis virus and avian RNA tumor viruses. Cold Spring Harbor Symp. 1975;39:913–918. doi: 10.1101/sqb.1974.039.01.106. [DOI] [PubMed] [Google Scholar]
- 33.Sriram S, Stratton CW, Yao S. Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis. Ann Neurol. 1999;46:6–14. [PubMed] [Google Scholar]
- 34.Krametter D, Niederwieser G, Berghold A. Chlamydia pneumoniae in multiple sclerosis: humoral immune responses in serum and cerebrospinal fluid and correlation with disease activity marker. Mult Scler. 2001;7:13–18. doi: 10.1177/135245850100700103. [DOI] [PubMed] [Google Scholar]
- 35.Tsai JC, Gilden DH. Chlamydia pneumoniae and multiple sclerosis: no significant association. Trends Microbiol. 2001;9:152–154. doi: 10.1016/s0966-842x(01)01985-0. [DOI] [PubMed] [Google Scholar]
- 36.Liedtke W, Malessa R, Faustmann PM, Eis-Hubinger AM. Human herpesvirus 6 polymerase chain reaction findings in human immunodeficiency virus associated neurological disease and multiple sclerosis. J Neurovirol. 1995;1:253–258. doi: 10.3109/13550289509114021. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg SH, Albright AV, Lisak RP, Gonzalez-Scarano F. Polymerase chain reaction analysis of human herpesvirus-6 sequences in the sera and cerebrospinal fluid of patients with multiple sclerosis. J Neurovirol. 1999;5:134–139. doi: 10.3109/13550289909021995. [DOI] [PubMed] [Google Scholar]
- 38.Berti R, Brennan MB, Soldan SS. Increased detection of serum HHV-6 DNA sequences during multiple sclerosis (MS) exacerbations and correlation with parameters of MS disease progression. J Neurovirol. 2002;8:250–256. doi: 10.1080/13550280290049615-1. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez J, Vergara MJ, Guerrero M. Multiple sclerosis and human herpesvirus 6. Infection. 2002;30:145–149. doi: 10.1007/s15010-002-2056-7. [DOI] [PubMed] [Google Scholar]
- 40.Soldan SS, Berti R, Salem N. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 41.Friedman JE, Lyons MJ, Ablashi DV. The association of the human herpesvirus-6 and MS. Mult Scler. 1999;5:355–362. doi: 10.1177/135245859900500509. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez-Lafuente R, Martin-Estefania C, de Las Heras V. Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch Neurol. 2002;59:929–933. doi: 10.1001/archneur.59.6.929. [DOI] [PubMed] [Google Scholar]
- 43.Challoner PB, Smith KT, Parker JD. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:7740–7744. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuomo L, Trivedi P, Cardillo MR. Human herpesvirus 6 infection in neoplastic and normal brain tissue. J Med Virol. 2001;63:45–51. [PubMed] [Google Scholar]
- 45.Mirandola P, Stefan A, Brambilla E. Absence of human herpes virus 6 and 7 from spinal fluid and serum of multiple sclerosis patients. Neurology. 1999;53:1367–1368. doi: 10.1212/wnl.53.6.1367-a. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez Carnero S, Martinez-Vazquez C, Potel Alvarellos C. Lack of human herpesvirus type 6 DNA in CSF by nested PCR among patients with multiple sclerosis. Rev Clin Esp. 2002;202:588–591. [PubMed] [Google Scholar]
- 47.Nielsen L, Larsen AM, Munk M, Vestergaard BF. Human herpesvirus-6 immunoglobulin G antibodies in patients with multiple sclerosis. Acta Neurol Scan Suppl. 1997;169:76–78. doi: 10.1111/j.1600-0404.1997.tb08154.x. [DOI] [PubMed] [Google Scholar]
- 48.Blumberg BM, Mock DJ, Powers JM. The HHV6 paradox: ubiquitous commensal or insidious pathogen? A two-step in situ PCR approach. J Clin Virol. 2000;16:159–178. doi: 10.1016/s1386-6532(99)00084-0. [DOI] [PubMed] [Google Scholar]
- 49.Cermelli C, Bert R, Soldan SS. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by aster microdissection. J Infect Dis. 2003;187:1377–1387. doi: 10.1086/368166. [DOI] [PubMed] [Google Scholar]
- 50.Wandinger K, Jabs W, Siekhaus A. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 2000;55:178–184. doi: 10.1212/wnl.55.2.178. [DOI] [PubMed] [Google Scholar]
- 51.Ascherio A, Munch M. Epstein-Barr virus and multiple sclerosis. Epidemiology. 2000;11:220–304. doi: 10.1097/00001648-200003000-00023. [DOI] [PubMed] [Google Scholar]
- 52.Bray PF, Luka J, Bray PF. Antibodies against Epstein-Barr nuclear antigen (EBNA) in multiple sclerosis CSF, and two pentapeptide sequence identities between EBNA and myelin basic protein. Neurology. 1992;42:1798–1804. doi: 10.1212/wnl.42.9.1798. [DOI] [PubMed] [Google Scholar]
- 53.Ascherio A, Gorham KL, Lennette ET. Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA. 2002;286:3083–3088. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- 54.Lewin LI, Munger KL, Rubertone MV. Multiple sclerosis and Epstein-Barr virus. JAMA. 2003;289:1533–1536. doi: 10.1001/jama.289.12.1533. [DOI] [PubMed] [Google Scholar]
- 55.Haahr S, Plesner AM, Vestergaard BF, Hollsberg P. A role of late Epstein-Barr virus infection in multiple sclerosis. Acta Neurol Scand. 2004;109:270–275. doi: 10.1046/j.1600-0404.2003.00221.x. [DOI] [PubMed] [Google Scholar]
- 56.Majid A, Galetta SL, Sweeney CJ. Epstein-Barr virus myeloradiculitis and encephalomyeloradiculitis. Brain. 2002;125:1–7. doi: 10.1093/brain/awf010. [DOI] [PubMed] [Google Scholar]
- 57.Hilton DA, Love S, Fletcher A, Pringle JH. Absence of Epstein-Barr virus in multiple sclerosis as assessed by in situ hybridisation. J Neurol Neurosurg Psychiatry. 1994;57:975–976. doi: 10.1136/jnnp.57.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawkes CH. Is multiple sclerosis a sexually transmitted infection? J Neurol Neurosurg Psychiatry. 2002;73:439–443. doi: 10.1136/jnnp.73.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jocher R, Rethwilm A, Kappos L, ter Meulen V. Search for retroviral sequences in peripheral blood mononuclear cells and brain tissue of multiple sclerosis patients. J Neurol. 1990;237:352–355. doi: 10.1007/BF00315658. [DOI] [PubMed] [Google Scholar]
- 60.Ehrlich GD, Glaser JB, Bryz-Gornia V. Multiple sclerosis, retroviruses, and PCR. Neurology. 1991;41:335–343. doi: 10.1212/wnl.41.3.335. [DOI] [PubMed] [Google Scholar]
- 61.Perron H, Geny C, Genoulaz O. Antibody to reverse transcriptase of human retroviruses in multiple sclerosis. Acta Neurol Scand. 1991;84:507–513. doi: 10.1111/j.1600-0404.1991.tb05004.x. [DOI] [PubMed] [Google Scholar]
- 62.Rozenberg F, Lefebvre S, Lubetzki C. Analysis of retroviral sequences in the spinal form of multiple sclerosis. Ann Neurol. 1991;29:333–336. doi: 10.1002/ana.410290317. [DOI] [PubMed] [Google Scholar]
- 63.Hackett J, Jr, Swanson P, Leahy D. Search for retrovirus in patients with multiple sclerosis. Ann Neurol. 1996;40:805–809. doi: 10.1002/ana.410400520. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen HB, Geny C, Deforges L. Expression of endogenous retroviruses in blood mononuclear cells and brain tissue from multiple sclerosis patients. Acta Neurol Scand Suppl. 1997;169:38–44. doi: 10.1111/j.1600-0404.1997.tb08148.x. [DOI] [PubMed] [Google Scholar]
- 65.Perron H, Garson JA, Bedin F. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. Proc Natl Acad Sci USA. 1997;94:7583–7588. doi: 10.1073/pnas.94.14.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray RS, Brown B, Brain D, Cabirac GF. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart JN, Mounir S, Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191:502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dessau RB, Lisby G, Frederiksen JL. Coronaviruses in brain tissue from patients with multiple sclerosis. Acta Neuropathol (Berl) 2001;101:601–604. doi: 10.1007/s004010000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boerman RH, Bax JJ, Beekhuis-Brussee JA. JC virus and multiple sclerosis: a refutation? Acta Neurol Scand. 1993;87:353–355. doi: 10.1111/j.1600-0404.1993.tb04116.x. [DOI] [PubMed] [Google Scholar]
- 70.Stoner GL, Agostini HT, Ryschkewitsch CF. Characterization of JC virus DNA amplified from urine of chronic progressive multiple sclerosis patients. Mult Scler. 1996;1:193–199. [PubMed] [Google Scholar]
- 71.Agostini HT, Ryschkewitsch CF, Baumhefner RW. Influence of JC virus coding region genotype on risk of multiple sclerosis and progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:S101–S108. [PubMed] [Google Scholar]
- 72.Ferrante P, Omodeo-Zorini E, Caldarelli-Stefano R. Detection of JC virus DNA in cerebrospinal fluid from multiple sclerosis patients. Mult Scler. 1998;4:49–54. doi: 10.1177/135245859800400202. [DOI] [PubMed] [Google Scholar]
- 73.Choo Q-L, Kuo G, Weiner A. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 74.Owens GP, Kraus H, Burgoon MP. Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann Neurol. 1998;43:236–243. doi: 10.1002/ana.410430214. [DOI] [PubMed] [Google Scholar]
- 75.Owens GP, Ritchie A, Burgoon MP. Single-cell repertoire analysis demonstrates that clonal expansion is a prominent feature of the B cell response in multiple sclerosis cerebrospinal fluid. J Immunol. 2003;171:2725–2733. doi: 10.4049/jimmunol.171.5.2725. [DOI] [PubMed] [Google Scholar]
- 76.Smith-Jensen T, Burgoon MP, Anthony J. Comparison of IgG heavy chain sequences in MS and SSPE brains reveals an antigen-driven response. Neurology. 2000;54:1227–1232. doi: 10.1212/wnl.54.6.1227. [DOI] [PubMed] [Google Scholar]
- 77.Haubold K, Owens GP, Kaur P. B-lymphocyte and plasma cell clonal expansion in monosymptomatic optic neuritis cerebrospinal fluid. Ann Neurol. 2004;56:97–107. doi: 10.1002/ana.20152. [DOI] [PubMed] [Google Scholar]
- 78.Ritchie AM, Gilden DH, Williamson RA. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol. 2004;173:649–656. doi: 10.4049/jimmunol.173.1.649. [DOI] [PubMed] [Google Scholar]