Abstract
The sialylation pattern of serum α1-acid glycoprotein (AGP) in non-symptomatic cats infected by feline coronavirus (FCoV) and its possible relationship with the amount of FCoVs shed in faeces were investigated. Blood from three specific pathogen-free cats (group A) and from 10 non-symptomatic FCoV-positive cats from catteries with low (group B, three cats) or high (group C, seven cats) levels of faecal shedding were collected monthly. AGP was purified from serum and Western blotting followed by lectin-staining of α(2,3)-linked and α(2,6)-linked sialic acid. Faecal shedding was quantified in group C by quantitative polymerase chain reaction. Variations of AGP sialylation were recorded only in cats from group C, on which viral shedding peaked before the occurrence of feline infectious peritonitis (FIP) in the cattery, and decreased 1 month later, when serum AGP had an increase of α(2,3)-linked sialic acid. These results suggest that hypersialylation of AGP may be involved in host–virus interactions.
The acute phase protein α1-acid glycoprotein (AGP) increases in several inflammatory and neoplastic conditions of cats (Paltrinieri in press). In cats with feline infectious peritonitis (FIP), caused by feline coronavirus (FCoV), the increase of AGP is particularly evident (Duthie et al 1997, Paltrinieri et al 2007a) and AGP is hyposialylated (Ceciliani et al 2004). In a previous study (Paltrinieri et al 2007b) we demonstrated that AGP increased before the appearance of FIP, even in FCoV-infected cats that remain non-symptomatic, suggesting that an inflammatory response is mounted, even in those cats that do not develop FIP. In the study cited above, faecally shed FCoVs were not quantified or sequenced, therefore, hampering the ability to assess whether the increased serum AGP levels was a consequence of the higher viral load of non-mutated FCoVs or a protective response against mutant strains. However, 1 month later, the percentage of shedders decreased, suggesting a direct association between these phenomena, possibly mediated by altered sialylation of circulating AGP. Quantifying the faecal shedding of FCoV and the degree of AGP sialylation would contribute to our understanding of the host–FCoVs interactions.
The aims of the present study are as follows: (1) to assess whether the fluctuations of AGP concentration are associated with changes in its sialylation pattern; (2) to assess the possible relationship between AGP concentration or sialylation and the intensity of FCoVs shedding.
The study was performed on serum and faecal samples included in the previous study (Paltrinieri et al 2007b) and stored at −30°C. Only samples with sufficient volumes remaining to proceed with AGP purification (and the corresponding faecal samples) were included in the present study. Cats were grouped as follows:
Specific pathogen-free (SPF) cats: six sera (provided by Prof Hans Lutz and Dr Marina Meli, Zurich University) from three domestic shorthair (DSH) SPF cats (sampled twice at a 60-day interval), whose serum AGP level was low and stable over the time (Table 1).
-
(B)
Non-symptomatic FCoV-infected cats from a cattery with low prevalence of FIP. This group corresponds to group B3 in the previous study, which recorded only one case of FIP in the past 5 years. The five cats (DSH) of this cattery were sampled monthly for 4 months. Faecal polymerase chain reaction (PCR) of these cats (performed using the same method employed in the former study) was only occasionally positive, and serum antibody titres (immunofluorescence test) were low (median 1:25; range: 0–1:50). Based on these data, the cattery was classified as having a ‘low prevalence of FCoV infection’. In the present study, only samples from three cats, sampled at day 0, 30 and 90, were available. The serum AGP concentration did not show significant differences over time (Table 1).
-
(C)
Non-symptomatic FCoV-infected cats from a cattery with high prevalence of FIP. This group corresponds to group B1 of the previous study. In that study, 10 Persian cats from a cattery that recorded between one and four cases of FIP per year were sampled monthly for 7 months. Both the percentage of shedders (50–100%) and the FCoV antibody titres (median 1:200; range: 1:50–1:400) were variable. Based on these data, the cattery was classified as having a ‘high prevalence of FCoV infection’. Samples from seven cats were included in the present study. Their AGP levels varied over time, with high concentrations seen at sample 5 (Table 1).
Table 1.
Data regarding serum AGP concentration (α), in mg/ml and antibody titres (a) recorded in the different samplings (S1–S7) from cats included in the study (from Paltrinieri et al 2007b) and percentage of sialylation recorded in individual sera after staining with the lectins SNAI (S) and MAA (M)
| Group | Cat number | Test | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|---|---|
| A | 1 | α/a | 0.24/0 | 0.22/0 | |||||
| S | 117 | 95 | |||||||
| M | 102 | 103 | |||||||
| 2 | α/a | 0.23/0 | 0.25/0 | ||||||
| S | 106 | 97 | |||||||
| M | 112 | 89 | |||||||
| 3 | α/a | 0.25/0 | 0.21/0 | ||||||
| S | 102 | 83 | |||||||
| M | 91 | 103 | |||||||
| Mean±SD (S) | 108±8 | 92±8 | |||||||
| Mean±SD (M) | 102±11 | 98±8 | |||||||
| B | 1 | α/a | 0.44/1:50 | 0.52/1:25 | 0.44/1:50 | ||||
| S | 79 | 74 | 75 | ||||||
| M | 86 | 92 | 85 | ||||||
| 2 | α/a | 0.3/0 | 0.35/0 | 0.28/0 | |||||
| S | 103 | 91 | 118 | ||||||
| M | 101 | 101 | 111 | ||||||
| 3 | α/a | 0.33/0 | 0.31/0 | 0.38/0 | |||||
| S | 106 | 86 | 76 | ||||||
| M | 93 | 89 | 89 | ||||||
| Mean±SD (S) | 96±15 | 84±9 | 90±25 | ||||||
| Mean±SD (M) | 93±8 | 94±6 | 95±14 | ||||||
| C | 1 | α/a | 0.40/1:50 | 0.31/1:400 | 0.421:100 | 0.27/1:200 | 0.77/1:3,200 | 0.34/1:400 | 0.39/1:800 |
| S | 96 | 115 | 133 | 113 | 117 | 90 | 113 | ||
| M | 80 | 84 | 71 | 68 | 89 | 116 | 125 | ||
| 2 | α/a | 0.39/1:100 | 0.34/1:800 | 0.40/1:25 | 0.40/1:200 | 0.39/1:400 | 0.26/1:400 | 0.31/1:3,200 | |
| S | 73 | 127 | 144 | 115 | 116 | 135 | 103 | ||
| M | 140 | 105 | 60 | 112 | 77 | 150 | 138 | ||
| 3 | α/a | 0.39/1:100 | 0.34/1:1,600 | 0.30/1:200 | 0.37/1:1,600 | 0.28/1:800 | 0.30/1:800 | 0.31/1:400 | |
| S | 84 | 131 | 55 | 67 | 86 | 73 | 104 | ||
| M | 75 | 112 | 79 | 95 | 92 | 80 | 131 | ||
| 4 | α/a | 0.30/1:100 | 0.28/0 | 0.39/1:50 | 0.22/1:25 | 0.22/1:400 | 0.39/1:100 | 0.25/1:200 | |
| S | 81 | 128 | 77 | 67 | 96 | 108 | 74 | ||
| M | 94 | 108 | 145 | 81 | 77 | 109 | 113 | ||
| 5 | α/a | 0.30/1:200 | 0.35/1:25 | 0.42/1:25 | 0.40/1:25 | 0.44/1:50 | 0.34/1:100 | 0.29/1:100 | |
| S | 72 | 66 | 122 | 74 | 86 | 121 | 154 | ||
| M | 107 | 66 | 103 | 74 | 102 | 125 | 139 | ||
| 6 | α/a | 0.34/1:50 | 0.37/1:200 | 0.37/1:50 | 0.37/1:50 | 0.88/1:200 | 0.30/1:200 | 0.38/1:400 | |
| S | 85 | 93 | 59 | 96 | 97 | 39 | 69 | ||
| M | 111 | 84 | 83 | 59 | 66 | 123 | 97 | ||
| 7 | α/a | 0.35/1:100 | 0.31/1:100 | 0.44/1:200 | 0.24/1:50 | 0.88/1:100 | 0.39/1:200 | 0.35/1:100 | |
| S | 107 | 99 | 98 | 111 | 99 | 74 | 102 | ||
| M | 94 | 97 | 146 | 74 | 97 | 95 | 77 | ||
| Mean±SD (S) | 86±12 | 108±24 | 98±36 | 92±22 | 100±13 | 91±33 | 103±28 | ||
| Mean±SD (M) | 100±22 | 94±17 | 98±35 | 80±18 | 86±13 | 114±23 * | 117±23 * | ||
For each group (A=SPF; B=FCoV ‘low prevalence’; C=FCoV ‘high prevalence’) mean±SD values of SNAI and MAA staining are also reported.
P=0.05 vs S4.
AGP was isolated by conventional high pressure liquid chormoatography (HPLC) ion exchange chromatography, and purified AGP was subjected to sodium dodecyl sulphate – polyacrylamide gel electrophoresis (SDS-PAGE), Western blotting and lectin-staining, as previously described (Ceciliani et al 2004). Specifically, the digoxigenin-conjugated lectins Sambucus nigra agglutinin (SNAI) (Roche Diagnostic, Penzberg, Germany), specific for sialic acid α(2,6)-linked to galactose, and Maackia amurensis agglutinin (MAA) (Roche), specific for sialic acid α(2,3)-linked to galactose were employed. Positivity was detected with the Glycan differentiation kit (Roche), using an alkaline phosphatase conjugated sheep anti-digoxigenin immunoglobulin. Densitometric analysis was performed using the Imagemaster 1D software (Amersham Biosciences, Piscataway, USA). In order to relatively quantify sialic acid, part of the AGP purified from SPF cats was pooled and used as a reference material. The amount of sialic acid was expressed as a percentage of intensity compared to the reference material (arbitrarily considered as 100%). Within each group, the results of sequential time-samplings were compared to each other using the non-parametric Friedman's analysis of variance (ANOVA) followed by the Bonferroni test.
For both the lectins (Table 1), the results of SPF cats were closely distributed around the value of the pooled serum, while groups B and C showed a high individual variability. This variability, together with the low number of animals examined, was likely responsible for the lack of significant differences in spite of evident variations in mean values among sequential samplings. Nevertheless, in group C the sialic acid α(2,6)-linked to galactose tended to decrease at samplings 4 and 5, and significantly increased at samplings 6 and 7. The only difference between group C and other groups was the breed composition and the level of FCoV contamination. Breed-related differences of AGP sialylation are unlikely, based on the results of tests performed on cats of different breeds (data not included in this study). The difference recorded is, thus, likely depending on FCoV infection. Interestingly, in the previous study both the antibody titres and the concentration of AGP increased at sampling 5, 2 weeks before the occurrence of episodes of FIP in the cattery. The increased sialylation recorded here could thus be interpreted as a protective response against the virus, possibly mediated by an interaction between FCoVs and sialylated AGP. Moreover, hypersialylation elongates the half-life of circulating proteins (Gregoriadis et al 2005) which may partly explain why the concentration of serum AGP did not show, at sampling 7, the same increase recorded at sampling 5 (both these samplings were followed by outbreaks of FIP). In humans, AGP has immunomodulatory functions (Vasson et al 1994) and its degree of glycosylation influences susceptibility or resistance to viruses (Rabehi et al 1995). Some species of coronavirus have a high affinity for sialylated molecules (Schwegmann-Wessels and Herrler 2006), and, although the affinity of FCoVs for sialic acid has not yet been investigated, the results presented here stimulate future research on this topic.
The Spearmann correlation test showed that the levels of sialylation in position α(2,3) and α(2,6) were not statistically correlated with each other or with serum concentration of AGP, suggesting that AGP production and sialylation are driven by independent mechanisms.
As regards faecal shedding in group C, the cDNAs synthesised in the former study and stored at −80°C were used in the current study for quantitative PCR, which was performed as previously described (Battilani et al 2006) on the Corbett Research Rotor-Gene Real Time Amplification system (RG-3000, Corbett Research, Mortlake, NSW, Australia) using the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) and the forward and reverse primers FCoV1128f and FCoV1229r (Gut et al 1999). The number of copies of RNA from each sampling was compared to each other using the non-parametric tests mentioned above.
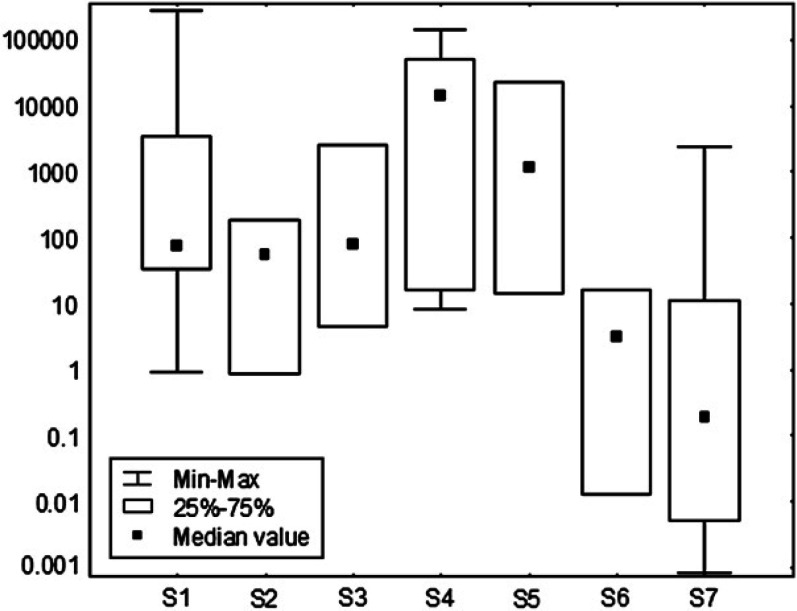
The samples that in the former study were negative to traditional PCR had no or few (<4) copies of RNA. Among the samples that were positive with traditional PCR, four were negative, seven had less than 10 copies of RNA and 26 had 20 to 2.77×105 copies of RNA. With rare exception, the median RNA excretion was moderate at sampling 1 (75 copies of RNA), 2 (55), and 3 (77), it significantly increased (P<0.01) at samplings 4 (14.3×103) and 5 (1.18×103), then significantly decreased at samplings 6 (three) and 7 (zero) (Fig 1). No significant correlation between AGP concentration or sialylation and faecal shedding was found, likely due to the extreme variability of data from faeces.
Fig 1.
Copies of viral RNA detected in faecal samples, distributed in a log-decimal scale.
Despite the lack of significant correlations, the different parameters showed some common trends. At sampling 4, the amount of shedding increased, followed by a decrease at sampling 5 when both the antibody titre and the serum AGP concentration increased. Two weeks later two episodes of FIP occurred in the cattery. At sampling 6, antibody titres returned to normal levels and the percentage of shedders decreased. Simultaneously, serum concentration of AGP decreased but increased its α(2,6)-sialylation. One month later, the percentage of shedders and the antibody titre increased and AGP was hypersialylated in spite of a normal serum level. These findings support the hypothesis that hypersialylation of AGP may be one of the factors that could explain why FCoV-infected cats do not develop FIP in spite of the presence of large amount of viral RNA shed in the environment.
In conclusion, sialylation of serum AGP fluctuated in clinically healthy cats from a cattery with high prevalence of FCoV infection. These fluctuations were not statistically correlated to the amount of AGP or to the ‘FCoV status’ of the cats, but they were noticed after the occurrence of FIP in the cattery and were associated with a decrease in viral shedding. This supports the hypothesis that hypersialylated AGP might interact with FCoVs, possibly conferring protection from the development of FIP, and stimulates further research into the possible role of AGP in conferring resistance/susceptibility to FCoV infection.
Acknowledgements
This work was co-funded by the Winn Feline Foundation and by the Italian Government (grant: COFIN 2005).
References
- Battilani M, Balboni A, Bassani M, Prosperi S. (2006) Quantification of feline coronaviruses (FCoVs) by Real Time PCR. In: VI National Congress of the Italian Society of Virology, Orvieto (TR) Italy, September 18–20, 2006, p. 26.
- Ceciliani F., Grossi C., Giordano A., Pocacqua V., Paltrinieri S. Decreased sialylation of the acute phase protein α1-acid glycoprotein in feline infectious peritonitis (FIP), Veterinary Immunology and Immunopathology 99, 2004, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie S., Eckersall P.D., Addie D.D., Lawrence C.E., Jarrett O. Value of alpha-1-acid glycoprotein in the diagnosis of feline infectious peritonitis, Veterinary Record 141, 1997, 299–303. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Jain S., Papaioannou I., Laing P. Improving the therapeutic efficacy of peptides and proteins: a role for polysialic acids, International Journal of Pharmaceutics 300, 2005, 125–130. [DOI] [PubMed] [Google Scholar]
- Gut M., Leutenegger C.M., Huder J.B., Pedersen N.C., Lutz H. One-tube fluorogenic reverse transcription-polymerase chain reaction for the quantitation of feline coronaviruses, Journal of Virological Methods 77, 1999, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrinieri S. The feline acute phase reaction. Veterinary Journal, in press, doi:10.1016/j.tvjl.2007.06.005. [DOI] [PMC free article] [PubMed]
- Paltrinieri S., Giordano A., Tranquillo V., Guazzetti S. Critical assessment of the diagnostic value of feline α1-acid glycoprotein for feline infectious peritonitis using likelihood ratios approach, Journal of Veterinary Diagnostic Investigation 19, 2007a, 266–272. [DOI] [PubMed] [Google Scholar]
- Paltrinieri S., Metzger C., Battilani M., Pocacqua V., Gelain M.E., Giordano A. Serum α1-acid glycoprotein (AGP) concentration in non-symptomatic cats with feline coronavirus (FCoV) infection, Journal of Feline Medicine and Surgery 9, 2007b, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabehi L., Ferriere F., Saffar L., Gattegno L. Alpha 1-acid glycoprotein binds human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein via N-linked glycans, Glycoconjugate Journal 12, 1995, 7–16. [DOI] [PubMed] [Google Scholar]
- Schwegmann-Wessels C., Herrler G. Sialic acids as receptor determinants for coronaviruses, Glycoconjugate Journal 23, 2006, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasson M.P., Roch-Arveiller M., Couderc R., Baguet J.C., Raichvarg D. Effects of alpha-1 acid glycoprotein on human polymorphonuclear neutrophils: influence of glycan microheterogeneity, Clinica Chimica Acta 224, 1994, 65–71. [DOI] [PubMed] [Google Scholar]