Highlights
-
•
IL-5 levels were higher in viral SCAP compared to other etiologies.
-
•
Mixed etiology SCAP had the highest but not statistically significant IP-10 values.
-
•
There were no significant differences in TNF-α between groups.
-
•
Procalcitonin levels were highest in SCAP with mixed etiology.
Keywords: Severe community-acquired pneumonia, Pneumonia, Cytokine, IL-5, IL-6, IP-10, Pneumonia etiology
Abstract
Background
The serum cytokine levels among 45 mechanically ventilated, intensive care unit (ICU)-treated severe community-acquired pneumonia (SCAP) patients with known microbial etiology in three different etiology groups were assessed.
Methods
Blood samples for C-reactive protein (CRP), procalcitonin (PCT), interleukin (IL)-5, IL-6, IL-10, human interferon gamma induced protein (IP)-10, and TNF-α (tumor necrosis factor alpha) were collected at time points 0, 12, 24, 48, 72 and 96 h after study inclusion.
Results
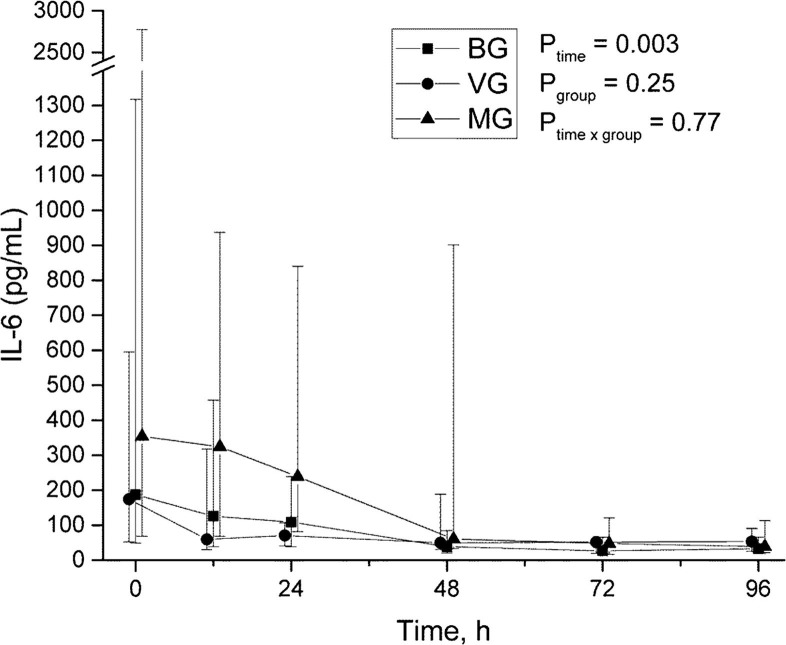
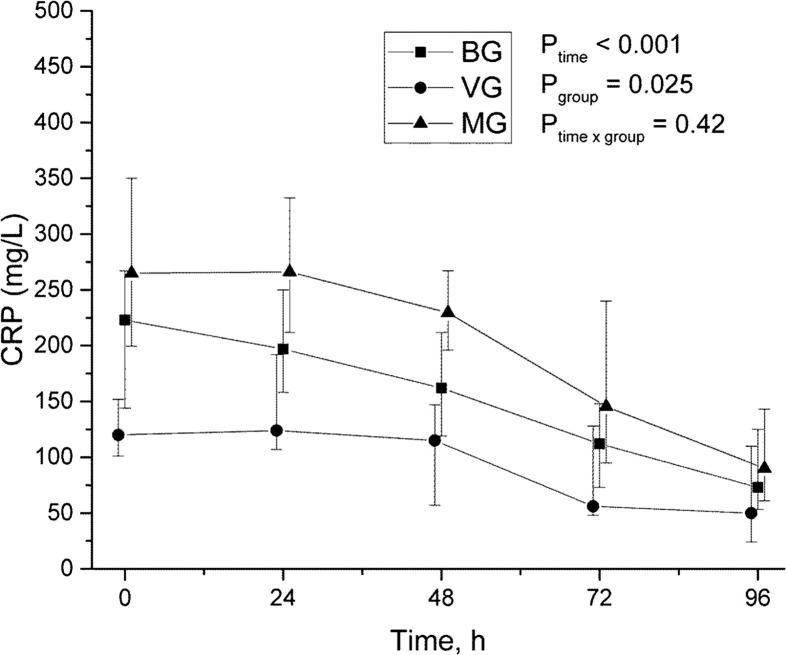
There were 21 (43%) pure bacterial infections (bacterial group, BG), 5 (10%) pure viral infections (viral group, VG), and 19 (39%) mixed bacterial-viral infections (mixed group, MG) among 45 mechanically ventilated SCAP patients. CRP and PCT levels were significantly higher in the MG and values decreased with time in all groups. PCT differed also in time and group analysis (P = 0.001), the highest being in the MG. IL-5 levels were significantly higher in the VG compared to others (Ptime = 0.001, Pgroup = 0.051 and Ptimexgroup = 0.016). IL-6 and IP-10 levels decreased over time (Ptime = 0.003 and Ptime = 0.021), but there were no differences between groups.
Conclusion
SCAP patients with viral etiology have higher IL-5 levels. Patients with mixed viral and bacterial group have higher PCT compared to other etiologies.
1. Introduction
Streptococcus pneumoniae is the main causative pathogen of community-acquired pneumonia (CAP) and intensive care unit (ICU)-treated severe community-acquired pneumonia (SCAP), however, concomitant viral infections occur up to 50% of all SCAP cases [1], [2], [3]. Mixed infections are related to higher inflammatory disease and represent relevant risk for hospitalization and ICU treatment [4], [5].
Elevated serum pro-inflammatory cytokines, such as interleukin-6 (IL-6), have been used in prognostic assessment and as a marker for the severity of the infection [6], [7]. In pneumonia, it has been suggested that the magnitude of cytokine secretion varies depending on the causative microorganism involved [8]. In pneumococcal pneumonia, higher interleukin-1 receptor antagonist (IL-1RA), IL-6, IL-8, IL-10, and TNF-α concentrations have been found compared to patients with non-pneumococcal etiology [8], [9], [10]. CAP patients with gram-negative etiology, Enterobacteriaceae have been shown in higher IL-8 and Klebsiella pneumoniae in higher IL-10 levels compared to other etiologies [11], [12]. In influenza pneumonia, IL-6, IL-8, IL-17, and transforming growth factor (TGF)-β1 have been shown to be elevated in an acute phase of the disease [13], [14]. Children with respiratory syncytial virus (RSV) pneumonia have presented high serum levels of IL-4, IL-10, and interferon gamma (IFN-γ) [14], [15]. However, the precise role of cytokines and the relationship between serum cytokine levels and microbiological etiology among mechanically ventilated SCAP patients is unclear [10], [11].
The purpose of this prospective study was to focus on analyzing cytokine kinetics during the first 96 h after ICU admission among SCAP patients with different etiologies of pneumonia, using CRP and PCT measurements as references.
2. Materials and methods
2.1. Patient population
The study population consisted of a cohort of SCAP patients admitted in a mixed ICU in a tertiary referral university hospital between June 2008 and May 2012. The first part of the study reporting SCAP etiology was been published on an earlier date [3]. Severe CAP was defined as an acute lower respiratory tract infection with fever or hypothermia, cough, and dyspnea acquired outside the hospital. The presence of pneumonia was confirmed by a chest radiograph that demonstrated a new pulmonary infiltrate. All patients fulfilled the criteria for sepsis, as well as, the Infectious Diseases Society of America’s and the American Thoracic Society’s (IDAS/ATS) major criteria for SCAP [1], [16]. Adult patients (older than 18 years) with SCAP who were expected to require intensive care treatment for more than 48 h and who were started on mechanical ventilation during the first 48 h following ICU admission were included in this study. The Hospital Ethics Committee approved the study protocol and written informed consent was requested from each patient or their next of kin.
2.2. Clinical data and laboratory measurements
A more detailed description of the methods, as well as, the microbiological analysis of study samples to define pneumonia etiology and classification of SCAP patients have been published earlier [3]. For this study the following data was analyzed: age, sex, presence of preexisting comorbidities, chronic pulmonary disease, and smoking. Serum C-reactive protein (CRP) and proclacitonin (PCT) (Siemens Centaur XP, Advia Centaur BRAHMS PCT) levels were recorded daily. The sum of Pulmonary Severity Index (PSI) [17] and CURB-65 (Confusion-Urea-Respiratory rate-Blood pressure) score [18] were also reported. SCAP patients were classified in three different groups according to microbial etiology: bacterial group (BG), if etiological examinations revealed only bacteria; viral group (VG), if only viral findings were recovered; and mixed group (MG), if etiological examinations yielded both bacterial and viral findings.
2.3. Serum cytokine detection
For serum cytokine detection, blood samples were collected together with CRP and PCT samples at time points 0, 12, 24, 48, 72, and 96 h after study inclusion. The serum samples were kept at −75 °C until they were analyzed at the Virus Diagnostics Laboratory, University of Turku. Serum cytokine levels were determined by 27-plex immunoassay (IL-1β, IL-1rα, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, eotaxin, FGF basic, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, platelet-derived growth factor (PDGF-ββ), MIP-1, RANTES, TNF-α, vascular endothelial growth factor (VEGF)) from Bio-Rad Laboratories, Inc. (California, United States)). According to the manufacturer’s instructions, except that the amount of beads, detection antibodies, and streptavidin-phycoerythrin conjugate were used at 50% of their recommended concentration, which was tested previously as appropriate for the analysis system. The results were analyzed with Bio-Plex Manager 6.0 software. For statistical analyses and calculating the geometric mean cytokine levels, samples under the detection limit were given a value that was the detection limit divided by two. This was done in order to enable the inclusion of negative values (0 values) for geometric mean calculations.
2.4. Statistical analysis
The summary measurements are presented as median with 25th-75th percentiles, unless stated otherwise. The baseline comparisons between the three study groups for continuous variables were performed by analysis of variance or by the Brown–Forsythe test if the homogeneity of variance test rejected the homogeneity assumption. Categorical data was analyzed using Fisher’s exact test. A linear mixed model (LMM) was used for repeatedly measured data. The p-values reported for LMM are Ptime for change over time, Pgroup for average between group difference, and Ptime*group for interaction between time and group. Two-tailed p-values < 0.05 were considered statistically significant. A non-parametric Spearman's correlation coefficient (rho) was calculated. All analyses were performed using SPSS for Windows (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) and SAS (version 9.4, SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient characteristics
The microbial etiology of 45 ventilated SCAP patients was identified as follows: a bacterial etiology (bacterial group, BG) was diagnosed for 21 (47%) patients, combined bacterial-viral etiology (mixed group, MG) for 19 (42%), and viral etiology (viral group, VG) for 5 (10%) patients. The causative microbes are presented in Table 1 .
Table 1.
Admission values of four evaluated cytokines in three pneumonia etiology groups.
| Cytokinea | Bacterial groupb | Mixed groupc | Viral groupd | p-value |
|---|---|---|---|---|
| (BG) | (MG) | (VG) | ||
| n = 21 | n = 19 | n = 5 | ||
| IL-5 | 10.1 (7.1–18.9) | 7.6 (5.8–11.7) | 18.7 (13.8–45.1) | 0.188 |
| IL-6 | 187.1 (48.9–1317.5) | 353.9 (68.3–2774.3) | 174.1 (52.5–595.1) | 0.524 |
| TNF-α | 96.1 (56.1–147.8) | 71.0 (57.4–154.4) | 94.2 (80.7–193.7) | 0.546 |
| IP-10 | 2226.5 (562.3–15959.9) | 6767.3 (1707.1–22754.9) | 363.2 (353.9–193.7) | 0.509 |
All values are presented pg/ml, IL = interleukin, TNF-α = tumor necrosis factor alfa, IP-10 = interferon gamma induced protein 10.
Bacterial group (BG) consisted of following bacteriae: Streptococcus pneumoniae; n = 15, Mycoplasma pneumoniae; n = 3, Staphylococcus aureus; n = 1, Escherichia coli; n = 1, Klebsiella pneumoniae and Mycoplasma pneumoniae; n = 1.
Mixed group (MG): S.pneumoniae + rhinovirus; n = 7, S.pneumoniae + parainfluenzavirus 3; n = 1, S.pneumoniae + coronavirus; n = 1, S.pneumoniae + adenovirus; n = 1, S.pneumoniae + respiratory syncytial virus; n = 1, S.pneumoniae + enterovirus; n = 1, S.pneumoniae + enterovirus + adenovirus; n = 1, Pseudomonas aeruginosa + rhinovirus; n = 1, Haemophilus influenzae + Moraxella catharralis + rhinovirus; n = 1, S.aureus + rhinovirus; n = 1, H.influenzae + rhinovirus; n = 1, M.pneumoniae + rhinovirus; n = 1, M.pneumoniae + rhinovirus + adenovirus; n = 1.
Viral group (VG): rhinovirus; n = 2, coronavirus; n = 1, adenovirus; n = 1, influenza A virus; n = 1 [3].
The median ages of these three groups were as follows: BG 53 (25th and 75th percentiles 49–58), MG 55 (44–65), and VG 48 (44–57) (P > 0.9) years, and there were 10 (48%) males in BG, 8 (42%), and 2 (40%) in MG and VG, respectively. Fifteen (71%) patients in BG, 13 (68%) in MG, and 4 (80%) in VG had chronic co-morbidities. One patient (20%) in VG had chronic obstructive pulmonary disease while the corresponding figures in BG and MG were 1 (5%) and 2 (11%), respectively. Smoking was the most common in VG where 60% (3/5) of the patients were smokers, in BG and MG there were 9 smokers (45% and 47%) in each group. 11 patients (52%) in BG, 11 (58%) in MG, and 2 (40%) in VG were treated with corticosteroid due to septic shock during their ICU stay.
Patients in BG had a somewhat higher median PSI score; (123 [25th and 75th percentiles 85–138], P = 0.66), while the corresponding figures in MG and VG were 103 (87–143) and 107 (78–134), respectively. The CURB-65 score was 3 (2–3) in BG, 3 (2–4) in MG, and 2 (2–3) in VG (P = 0.73).
3.2. Cytokine kinetics
According to the 27-plex immunoassay results only IL-5, IL-6, and IP-10 showed significant changes over time or between groups. These cytokines showed the highest values during the first 24 h after enrollment of the study. Serum cytokine levels on admission are shown in Table 1.
Serum IL-5 levels were highest in the viral group (Ptime = 0.001, Pgroup = 0.051, and Ptimexgroup = 0.016, Fig. 1 ). Both IL-6 and IP-10 levels decreased during the study period (Ptime = 0.003 and Ptime = 0.021), but there were no differences between groups (Figs. 2 and 3 ). There were no significant differences in TNF-α between groups (Fig. 4 ).
Fig. 1.
Time-group-analysis curve of interleukin (IL)-5.
Fig. 2.
Time-group-analysis curve of IL-6.
Fig. 3.
Time-group-analysis curve of interferon gamma induced protein (IP)-10.
Fig. 4.
Time-group-analysis curve of tumour necrosis factor (TNF)-alfa.
3.3. Correlation of cytokine levels to start of mechanical ventilation and onset of symptoms
The median time from the start of mechanical ventilation to the first study sample collection was 7.0 h and no statistically significant correlation was found between cytokine levels and the time gap. Spearman’s correlation coefficient calculations were as follows; IL-5 (rho = 0.161, P = 0.308), IL-6 (rho= 0.132, P = 0.394), IP-10 (rho = 0.06, P = 0.971) and TNF-α (rho = −0.235, P = 0.125), respectively. The median time between onset of symptoms and the first study sample was 4 days. Nor statistically significant correlations were found between cytokine levels and the time gap from onset symptoms; IL-5 (rho = −0.135, P = 0.383), IL-6 (rho = 0.0, P > 0.9), IP-10 (rho = 0.27, P = 0.88) and TNF-α (rho = −0.144, P = 0.36).
3.4. CRP and procalcitonin kinetics
CRP and PCT levels were significantly higher in MG and values decreased according to time in all groups (Fig. 5, Fig. 6 ). PCT differed also in time and group analysis (P = 0.001) being highest in MG (Fig. 6).
Fig. 5.
Time-group-analysis curve of C-reactive protein.
Fig. 6.
Time-group-analysis curve of procalcitonin.
4. Discussion
In this prospective study consisting of 45 severely ill patients requiring mechanical ventilation and fulfilling the major criteria of severe community-acquired pneumonia, we found that patients in the viral group showed highest serum IL-5 levels during the first 96 h in the ICU as compared to patients with bacterial or mixed viral. The mixed group showed the highest PCT values compared to patients with bacterial or viral etiologies.
Our results showed that SCAP patients with a viral etiology had the highest serum IL-5 concentrations. It has previously been shown that children with a laboratory confirmed influenza A (2009 H1N1) virus pneumonia had higher IL-5 concentrations compared to children without a lower respiratory tract infection [19], [20], [21]. In influenza A H1N1 virus pneumonia serum IL-5 levels were even higher among those patients requiring mechanical ventilation [19] or in influenza patients without viral pneumonia [21]. In our adult study population, we had only one pneumonic patient with a H1N1 influenza viral infection, while the other pneumonias were due to adeno-, corona-, or rhinovirus infections. Our study suggests that high IL-5 concentrations may also be related to viral pneumonias. This is supported by the fact that in children elevated serum IL-5 levels have also been found in wheezing caused by a rhinovirus or respiratory syncytial virus infection [22].
IL-6 and TNF-α, together act as a general marker of inflammation in pneumonia, regulating the expression of acute phase proteins [8]. In our study serum IL-6 levels decreased over time in all groups. In general, high IL-6 concentrations in serum and bronchoalveolar lavage fluid have been reported in all pneumonia patients. In addition, high serum IL-6 levels correlate to the presence of pneumonia, disease severity, and poor outcome [10], [12], [23], [24], [25]. In an earlier CAP study with 685 patients of whom 3% required mechanical ventilation and 2.7% with septic shock showed that bacteremic patients had higher serum IL-6 levels compared to CAP patients with an unknown microbial etiology [26]. However, in our study there were no differences between etiologies. Our patients were all severely acutely ill, requiring mechanical ventilation, and 43% had septic shock. Although high serum TNF-α levels have been reported in patients with CAP or SCAP [28], we did not find any significant differences in TNF-α concentrations between the three etiological groups. This apparent discrepancy between our study and the one of Calbo and colleagues, may be due to different serum sampling times and half-lives of cytokines; the half-life of TNF-α after initial cytokine response is relatively short, only 15 min, compared to for instance, IL-6, which according to literature may vary from 2 to 15 h [29]. Thus, in our study serum TNF-α levels may have returned to basal levels at the time of hospital admission.
In our series, IP-10 decreased over time in all groups. In a study with 74 children (≤5 years old) hospitalized with viral and/or bacterial community-acquired pneumonia, patients with identified mixed-detection had significantly higher serum IP-10 levels than those with a single detection [27]. An in vitro study has shown that compared to a single pathogen infection, a mixed bacterial-viral co-infection synergistically increased IP-10 expression by modulating the JAK-STAT signaling pathway [27]. Also in our series the mixed group had the highest but not statistically significant IP-10 values.
It is worth noticing that confounding factors may contribute to the observed differences in cytokine concentrations. Cytokine levels vary over time, and mechanical ventilation is known to induce inflammatory responses [30], [31]. In our series, the median time from the start of mechanical ventilation to first study sample collection was 7.0 h and there was no correlation between the time gap and cytokine levels. Additionally the median time of sampling relative to the onset of symptoms was 4 days and no correlation was found between the time gap and cytokine levels.
This study showed highest procalcitonin among the MG patients. This is in accordance with previous studies on CAP patients [5], [26], [32].
The present study has several limitations that should be mentioned. Our patient material consisted only of mechanically ventilated SCAP patients in a single center university hospital which limits the generalization of the results to other pneumonia cases or other centers. However, there are thus far only a few studies concerning the patterns of cytokines in SCAP patients. Our study group was relatively small especially in respect to viral pneumonias. Thus, to confirm the elevated serum IL-5 as an informative biomarker for viral infections further studies with a larger patient population are needed. If the ICU admission occurred at nighttime there was at least a few hours delay in obtaining the first study samples, due to the lack of informed consent or laboratory personnel. Therefore, it is possible that we may have missed some very early cytokine findings, specifically, those concerning TNF-α. Moreover, our results may have been hampered by the fact that 28% of our patients had already received antibiotics before the hospital admission. Our finding should be regarded as preliminary until confirmed in a larger cohort of patients.
5. Conclusion
This is the first study concerning serum biomarker profiles in adult mechanically ventilated SCAP patients with bacterial, mixed bacterial-viral and viral etiologies. SCAP patients with viral etiology have higher IL-5 levels, and patients in the mixed viral and bacterial group have higher PCT compared to other etiologies. The importance of IL-5 as a diagnostic biomarker in viral SCAP should be studied among larger patient cohorts.
Acknowledgments
Acknowledgements
The authors wish warmly thank RN Sinikka Sälkiö for her assistance with patient screening and the study samples.
Funding sources
This work was supported by the Finnish State Funding for Health Research.
Conflict of interests
The authors have no conflicts of interests.
References
- 1.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C., Dowell S.F., File T.M., Jr, Musher D.M., Niederman M.S., Torres A., Whitney C.G. Infectious diseases society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi S.H., Hong S.B., Ko G.B., Lee Y., Park H.J., Park S.Y., Moon S.M., Cho O.H., Park K.H., Chong Y.P., Kim S.H., Huh J.W., Sung H., Do K.H., Lee S.O., Kim M.N., Jeong J.Y., Lim C.M., Kim Y.S., Woo J.H., Koh Y. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am. J. Respir. Crit. Care Med. 2012;186:325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 3.Karhu J., Ala-Kokko T.I., Vuorinen T., Ohtonen P., Syrjälä H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin. Infect. Dis. 2014;59:62–70. doi: 10.1093/cid/ciu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cillóniz C., Ewig S., Ferrer M., Polverino E., Gabarrus A., Puig de la Bellacasa J., Mensa J., Torres A. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit. Care. 2011;15:R209. doi: 10.1186/cc10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bello S., Minochle E., Fandos S., Lasierra A.B., Ruiz M.A., Simon A.L., Panadero C., Lapresta C., Menendez R., Torres A. Inflammatory response in mixed viral-bacterial community-acquired pneumonia. BMC Pulm. Med. 2014;14:123. doi: 10.1186/1471-2466-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antunes G., Evans S.A., Lordan J.L., Frew A.J. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur. Respir. J. 2002;20:990–995. doi: 10.1183/09031936.02.00295102. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez P., Ferrer M., Marti V., Reyes S., Martinez R., Menendez R., Ewig S., Torres A. Inflammatory biomarkers and prediction for intensive care admission in severe community-acquired pneumonia. Crit. Care Med. 2011;39:2211–2217. doi: 10.1097/CCM.0b013e3182257445. [DOI] [PubMed] [Google Scholar]
- 8.Bordon J., Aliberti S., Fernandez-Botran R., Uriarte S.M., Rane M.J., Duvvuri P., Peyrani P., Morlacchi L.C., Blasi F., Ramirez J.A. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Intern. J. Infect. Dis. 2013;17:e75–e83. doi: 10.1016/j.ijid.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Endeman H., Meijvis S.C.A., Rijkers G.T., van Velzen-Blad H., van Moorsel C.H.M., Grutters J.C., Biesma D.H. Systemic cytokine response in patients with community acquired pneumonia. Eur. Respir. J. 2011;37:1431–1438. doi: 10.1183/09031936.00074410. [DOI] [PubMed] [Google Scholar]
- 10.Paats M.S., Bergen I.M., Hanselaar W.E.J.J., Groenix van Zoelen E.C., Hoogsteden H.C., Hendriks K.W., van den Eerden M.M. Local and systemic cytokine profiles in nonsevere and severe community-acquired pneumonia. Eur. Respir. J. 2013;41:1378–1385. doi: 10.1183/09031936.00060112. [DOI] [PubMed] [Google Scholar]
- 11.Rendon A., Rendon-Ramirez E.J., Rosas-Taraco A.G. Relevant cytokines in the management of community-acquired pneumonia. Curr. Infect. Dis. Rep. 2016;18:10. doi: 10.1007/s11908-016-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C.L., Chan M.G., Chang G.C., Lee Y.L., Chin C.S., Chang K.M., Hsu J.Y. Etiology and cytokine expression in patients requiring mechanical ventilation due to severe community-acquired pneumonia. J. Formos. Med. Assoc. 2006;105:49–55. doi: 10.1016/s0929-6646(09)60108-x. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Ramirez H.G., Salinas-Carmona M.C., Barboza-Quintana O., Melo-de la Ganza A., Cecenas-Falcon L.A., Rangel-Martinez L.M., Rosas-Taraco A.G. CD206+ cell member differentiates Influenza A(H1N1)pdm09 from seasonal Influenza A in fatal cases. Mediat. Inflamm. 2014:1–8. doi: 10.1155/2014/921054. (article ID 921054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davey R.T., Jr, Lynfield R., Dwyer D.E., Losso M.H., Cozzi-Lepri A., Wentworth D., Lane H.C., Dewar R., Rupert A., Metcalf J.A., Pett S.L., Uyeki T.M., Bruguera J.M., Angus B., Cummins N., Lundgren J., Neaton J.D., INSIGHT FLU 002 & 003 Study Groups The association between serum biomarkers and disease outcome in influenza A (H1N1)pdm09 virus infection. Results of two international observational cohort studies. PlosOne. 2013;(8):e57121. doi: 10.1371/journal.pone.0057121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Q., Shao W.X., Shang S.Q., Pan Y.X., Shen H.Q., Chen X.J. Epidemiological characteristics and immune status of children with Respiratory Syncytial Virus. J. Med. Virol. 2015;87:323–329. doi: 10.1002/jmv.24047. [DOI] [PubMed] [Google Scholar]
- 16.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine M.J., Auble T.E., Yealy D.M., Hanusa B.H., Weissfeld L.A., Singer D.E., Coley C.M., Marrie T.J., Kapoor W.N. A prediction rule to identify low-risk patients with community-acquired pneumonia. New Engl. J. Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 18.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I., Lewis S.A., Macfarlane J.T. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terai M., Honda T., Yamamoto S., Yoshida M., Tsuchiya N., Moriyama Y., Matsui T., Tokutake S., Suzuki E., Shirato Y., Muto A., Hayashi K., Hamada H. Early induction of nterleukin-5 and peripheral eosinophilia in acute pneumonia in Japanese children infected by pandemic 2009 influenza A in the Tokyo area. Microbiol. Immunol. 2011;55:341–346. doi: 10.1111/j.1348-0421.2011.00320.x. [DOI] [PubMed] [Google Scholar]
- 20.Takano T., Tajiri H., Kashiwagi Y., Kimura S., Kawashima H. Cytokine and chemokine response in children with the 2009 pandemic influenza (H1N1) virus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:117–120. doi: 10.1007/s10096-010-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto Y., Kawamura Y., Nakai H., Sugata K., Yoshikawa A., Ihira M., Ohashi M., Kato T., Yoshikawa T. Cytokine and chemokine responses in pediatric patients with severe pneumonia associated with pandemic A/H1N1/2009 influenza virus. Microbiol. Immunol. 2012;56:651–655. doi: 10.1111/j.1348-0421.2012.00489.x. [DOI] [PubMed] [Google Scholar]
- 22.Kato M., Tsukagoshi H., Yoshizumi M., Saitoh M., Kozawa K., Yamada Y., Maruyama K., Hayashi Y., Kimura H. Different cytokine profile and eosinophil activation are involved in rhinovirus and RS virus-induced acute exacerbation of childhood wheezing. Pediatr. Aller. Immunol. 2011;22:e87–e94. doi: 10.1111/j.1399-3038.2010.01026.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y.L., Chen W., Chen L.Y., Chen C.H., Lin Y.C., Liang S.J., Shih C.M. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. J. Crit. Care. 2010;25:e7–e13. doi: 10.1016/j.jcrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Zopel K., Martus P., Pletz M.W., Ewig S., Prediger M., Welte T., Bühlig F., CAPNETZ study group Interleukin 6, lipopolysaccharide-binding protein and interleukin 10 in the prediction of risk and etiologic patterns in patients with community-acquired pneumonia: results from the German competence network CAPNETZ. BMC Pulm. Med. 2012;12:6. doi: 10.1186/1471-2466-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacci M.R., Leme R.C.P., Zing N.P.C., Murad N., Adami F., Hinnig P.F., Feder D., Chagas A.C.P., Fonseca F.L.A. IL-6 and TNF-α serum levels are associated with early death in community-acquired pneumonia patients. Bras. J. Med. Biol. Res. 2015;48:427–432. doi: 10.1590/1414-431X20144402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menéndez R., Sahuquillo-Arce J.M., Reyes S., Martínez R., Polverino E., Cillóniz C., Córdoba J.G., Montull B., Torres A. Cytokine activation patterns and biomarkers are influenced by microorganisms in community-acquired pneumonia. Chest. 2012;141:1537–1545. doi: 10.1378/chest.11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann J., Machado D., Terrier O., Pouzol S., Messaoudi M., Basualdo W., Espínola E.E., Guillen R.M., Rosa-Calatrava M., Picot V., Bénet T., Endtz H., Russomando G., Paranhos-Baccalà G. Viral and bacterial co-infection in severe pneumonia triggers innate immune responses and specifically enhances IP-10: translational study. Sci. Rep. 2016;6:38532. doi: 10.1038/srep38532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calbo E., Alsina M., Rodrìguez-Carballeira, Lite J., Garau J. The impact of time on the systemic inflammatory response in pneumococcal pneumonia. Eur. Resp. J. 2010;35:614–618. doi: 10.1183/09031936.00052709. [DOI] [PubMed] [Google Scholar]
- 29.Oliver J.C., Bland L.A., Oettinger C.W., Arduino M.J., McAllister S.K., Aguero M.S., Favero M.S. Cytokine kinetic in an in vitro whole blood model following an endotoxin challenge. Lymphok. Cytok. Res. 1993;12:115–120. [PubMed] [Google Scholar]
- 30.Wolthuis E., Vlaar A.P.J., Choi G., Roelofs J.J.T.H., Juffermans N.P., Schultz M. Mechanical ventilation using non-injurious ventilator settings causes lung injury in the absence of pre-existing lung injury in mice. Crit. Care. 2009;13:R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ying-Nan J., Kai-Jiang Y., Guo-Nian W. Budesonide ameliorates lung injury induced by large volume ventilation. BMC Pulm. Med. 2016;16:90. doi: 10.1186/s12890-016-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruger S., Ewig S., Papassotiriou J., Kunde J., Marre R., von Baum H., Sutter N., Welte T., CAPNETZ study group Inflammatory parameters predict etiologic pattern but do not allow for individual prediction of etiology in patients with CAP. Results from the German Competence network CAPNETZ. Respir. Res. 2009;10:65. doi: 10.1186/1465-9921-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]