Highlights
-
•
Pneumonia remains one of the major killers of children in a middle-income country such as Morocco.
-
•
A history of prematurity, a history of fever, leaving in a house with smokers, impaired consciousness, cyanosis, pallor, having ronchi on auscultation and human metapneumovirus infection are all independent risk factors for an adverse outcome.
-
•
A history of asthma is independently associated with a positive outcome.
-
•
Early identification of risk factors for an adverse outcome could improve overall prognosis.
Keywords: Acute respiratory infections, Pneumonia, Paediatrics, Risk factors, Prognosis, Morocco
Summary
Objectives
Data on prognostic factors among children with severe pneumonia are scarce in middle-income countries. We investigated prognostic factors for an adverse outcome among children admitted to the Hôpital d’Enfants de Rabat, Morocco with World Health Organization-defined clinically severe pneumonia (CSP).
Methods
Children aged 2–59 months admitted to the hospital and fulfilling the CSP definition were recruited into this 13-month prospective study. A poor prognosis was defined as death, a need for intensive care, or a Respiratory Index of Severity in Children (RISC) score ≥3. Multivariate logistic regression was performed to ascertain independent predictive factors for a poor prognosis.
Results
Of the 689 children included in this analysis, 55 (8.0%) required intensive care and 28 died (4.0%). Five hundred and two (72.8%) children were classified as having a good prognosis and 187 (27.2%) as having a poor prognosis. A history of prematurity (odds ratio (OR) 2.50, 95% confidence interval (CI) 1.24–5.04), of fever (OR 2.25, 95% CI 1.32–3.83), living in a house with smokers (OR 1.79, 95% CI 1.18–2.72), impaired consciousness (OR 10.96, 95% CI 2.88–41.73), cyanosis (OR 2.09, 95% CI 1.05–4.15), pallor (OR 2.27, 95% CI 1.34–3.84), having rhonchi on auscultation (OR 2.45, 95% CI 1.58–3.79), and human metapneumovirus infection (OR 2.13, 95% CI 1.13–4.02) were all independent risk factors for an adverse outcome, whereas a history of asthma (OR 0.46, 95% CI 0.25–0.84) was the only independent risk factor for a positive outcome.
Conclusions
The early identification of factors associated with a poor prognosis could improve management strategies and the likelihood of survival of Moroccan children with severe pneumonia.
1. Introduction
Acute respiratory infections (ARI) remain the leading cause of death in young children in low- and middle-income countries, accounting for almost 1.4 million annual deaths, with around one sixth of the deaths occurring in children under the age of five.1 Indeed, the death toll imposed by respiratory infections represents a massive burden for the fragile health systems in the developing world, where over 90% of all global deaths occur,2 and this cannot be sufficiently emphasized. Respiratory infections cause a variety of clinical syndromes, of which pneumonia – perhaps the most paradigmatic and severe of all – is the most commonly associated with an adverse outcome, causing over 90% of all deaths.3
Pneumonia is typically caused by bacterial agents, with Streptococcus pneumoniae 4 and Haemophilus influenzae type b (Hib)5 being the two most frequent underlying aetiologies. Respiratory viruses are typically the causes of upper and lower respiratory infections with a more benign course, although their pathogenic potential can sometimes be comparable to that of bacteria or even worse; respiratory syncytial virus (RSV) is one of the most common viruses involved in severe childhood disease.6 Whatever the underlying cause, case-fatality rates among under-fives hospitalized with pneumonia are reported to be around 19%.7
In the developing world, currently existing interventions to reduce pneumonia-related morbidity and mortality include the implementation of anti-pneumonia vaccines through the Expanded Programme on Immunization during infancy,8 and the adequate diagnosis and prompt treatment of clinical cases with antibiotics, in accordance with the Integrated Management of Childhood Illnesses (IMCI) algorithms,9 under the assumption that in such settings the majority of severe pneumonia cases are of bacterial origin.
Despite the increasing availability of preventive vaccines, morbidity amongst those children who do become infected remains high, possibly in relation to the many challenges faced in the diagnosis and management in resource-constrained settings. The early identification of risk factors for a poor outcome among pneumonia patients could help prioritize the management of those patients with an uncertain prognosis and perhaps increase their likelihood of surviving. Previous studies have described a series of factors including young age of the patient (or the patient's mother), malnutrition, anaemia, or the confirmation of grunting, cyanosis, or chest indrawing, among others, as harbingers of a negative outcome.10, 11, 12, 13
Scarce and limited epidemiological, clinical, and microbiological data are available regarding paediatric respiratory tract infections in the Kingdom of Morocco, a middle-income country in north-western Africa, although the World Health Organization (WHO) estimates that up to 26.5% of all deaths in children aged 1–59 months are secondary to pneumonia.14 In this analysis, part of a wider surveillance project on the aetiology of paediatric respiratory infections, we aimed to describe factors associated with a poor outcome among hospitalized children under the age of five admitted to a Moroccan reference hospital with a diagnosis of WHO-defined severe pneumonia.
2. Materials and methods
2.1. Study setting and procedures for recruited children
This prospective study was conducted from November 2010 to December 2011 at the Hôpital d’Enfants de Rabat (HER), in Morocco's capital. Children aged 2–59 months admitted to HER with respiratory symptoms were identified and approached for recruitment if they fulfilled the WHO definition of clinically severe pneumonia (CSP),15, 16 namely a history of cough or reported breathing difficulty and increased respiratory rate (RR) according to age,16 together with chest indrawing. After parents had signed an informed consent form, recruited children underwent standardized procedures upon admission, including pulse oximetry (Bionics PalmCare), an anteroposterior chest X-ray, nasal and pharyngeal swabs for the diagnosis of bacterial infection/carriage, and nasopharyngeal aspirates (NPA) for the diagnosis of respiratory viruses. A minimum of 2 ml venous blood was also collected for blood culture, full blood cell count, and biochemical determinations, including C-reactive protein (CRP) and procalcitonin (PCT).
2.2. Definitions and clinical groups established
Admission and discharge diagnoses were coded using the International Classification of Diseases, 10th Revision (ICD-10).17 Hypoxemia implied an oxygen saturation (SaO2) <90%. Fever was defined as an axillary temperature of ≥37.5 °C. Nutritional status was based on weight-for-age Z scores (WAZ), calculated using the least mean square method and the 2000 Centers for Disease Control and Prevention (CDC) growth reference.18 Invasive bacterial disease implied the isolation of one or more non-contaminant bacteria in blood or pleural fluid.
For the purpose of this specific analysis, we defined two prognostic groups utilizing a composite definition based on outcome (discharged alive vs. in-hospital death), requirement for admission to the intensive care unit (ICU), and the Respiratory Index of Severity in Children (RISC) score, a simple and validated clinical score predicting the probability of death in children with lower respiratory tract infections (LRTI), based on parameters such as oxygen saturation, respiratory signs including indrawing or wheezing, and nutritional status.19 Thus, we defined a good prognosis as a RISC score <3, no need for admission to the ICU, and a discharge from hospital on the grounds of clinical improvement. In contrast, a poor prognosis was defined as the occurrence of a death outcome, or an ICU admission, or a RISC score of ≥3.
2.3. Laboratory methods
The full laboratory methods of the general study from which this analysis has been performed are detailed elsewhere.20 Blood samples were cultured using an automated blood culture system (BD Bactec; BD, New Jersey, USA), and bacterial isolates identified by Phoenix Automated Microbiology System (PHX system; BD) or colony morphology and biochemical tests. The detection of S. pneumoniae and H. influenzae type B was also studied by real-time PCR using a published in-house assay.21
The presence of DNA/RNA of influenza viruses A and B, RSV A and B, parainfluenza viruses (PIV) 1, 2, 3, and 4, rhinovirus (RV), adenovirus (ADV), enterovirus (EV), coronaviruses (CoV) 229E, NL63, and OC43, human metapneumovirus (hMPV), Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Bordetella pertussis in nasopharyngeal aspirates was also systematically investigated by means of the TrueScience RespiFinder Pathogen Identification Panel (Applied Biosystems, New York, USA).
2.4. X-ray interpretation
Chest X-rays were interpreted independently by two paediatricians following a WHO-designed X-ray interpretation protocol.22 Discordant results were resolved through a third reading. Evidence of consolidation and/or pleural effusion was defined as ‘endpoint pneumonia’. Other radiological endpoints included interstitial infiltrates or normal radiographs.
2.5. Data management and statistical analysis
All study questionnaires were double-entered into a study database using a program written in Filemaker Pro 12 (Filemaker Inc., Santa Clara, CA, USA). Statistical analyses were done using Stata 11 (StataCorp., College Station, TX, USA). Study variables were counted and summarized in frequency tables. Means with corresponding standard deviations (SD) or medians and interquartile ranges are presented for normally and non-normally distributed variables, respectively. Multivariate logistic regression was performed with good prognosis/poor prognosis as the outcome, using an automated backward and forward stepwise estimation. Variables previously shown in the literature to be related to a poor prognosis and other available data were investigated. All variables that were associated with an adverse outcome at a significance level of p < 0.10 in the univariate analysis were included in the initial model. The significance level for removal from the model was set at p = 0.06 and that for addition to the model at p = 0.05. The strength of association was determined by estimating the odds ratio (OR) and the 95% confidence intervals (CI).
2.6. Ethics
The protocol and informed consent documents were approved by the Ethics Committee of the Hospital Clinic (Barcelona, Spain) and by the Comité d’Éthique de la Recherché Biomédicale (Départ No. 1252–16Déc2009) of the Faculty of Medicine in Rabat.
3. Results
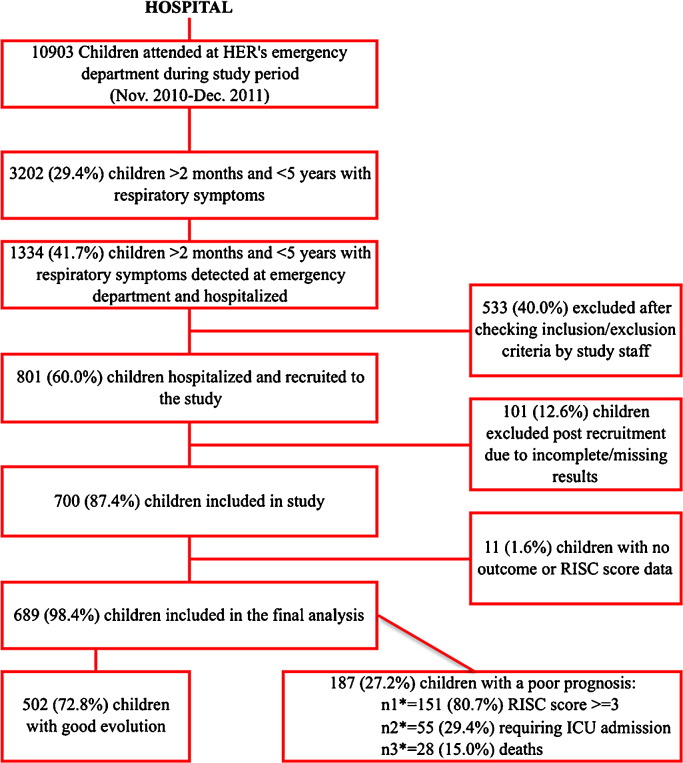
Out of almost 11 000 children seen in the emergency department of HER during the 13-month study period, 700 under the age of five were recruited into the study. Six hundred and eighty-nine (98.4%) of these children had all the necessary outcome data for inclusion in this specific risk factor analysis (Figure 1 ). There were 28 deaths among these 689 patients (4.0%), and 55 children (8.0%) required transfer to the ICU. The mean RISC score for the overall study population was 1.36 (SD 1.34). With regard to the two established risk groups, 502 (72.8%) children were assigned to the good prognosis group and 187 (27.2%) to the poor prognosis group (Figure 1).
Figure 1.
Study flowchart.
The socio-demographic characteristics and health history factors of the patients according to the prognosis group are shown in Table 1 . Patients with a worse outcome were significantly younger; no difference was found regarding gender. No significant differences were seen in terms of any seasonality of admission in relation to outcome (data not shown). A history of having been born prematurely, having received antibiotics preceding admission, or having an already known co-morbidity had a significant association with a poorer outcome, while a history of previous admission for LRTI or wheezing was strongly associated with a good prognosis. Finally, being exposed to tobacco smoking at home was also significantly associated with a worse outcome.
Table 1.
Baseline socio-demographic characteristics of recruited patients, according to the prognosis groupa
| Good prognosis (n = 502) | Poor prognosis (n = 189) | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, months, mean ± SD | 23.40 ± 14.80 | 16.00 ± 12.10 | 0.001 | |
| Age <12 months | 123 (24.50) | 89 (47.59) | 2.80 (1.97–2.98) | 0.001 |
| Female sex | 187 (37.25) | 62 (33.16) | 0.84 (0.59–1.19) | 0.32 |
| Patient history | ||||
| Prematurity | 28 (5.59) | 23 (12.30) | 2.37 (1.33–4.33) | 0.003 |
| Breastfeeding ≥6 months | 308 (61.72) | 109 (58.30) | 0.87 (0.62–1.22) | 0.41 |
| Vitamin A supplementation | 461 (91.83) | 168 (89.84) | 0.79 (0.45–1.40) | 0.67 |
| Past morbidity and co-morbidity | ||||
| Previous admission for ARI | 182 (36.25) | 39 (20.86) | 0.46 (0.31–0.68) | 0.001 |
| History of wheezing or asthma | 164 (32.67) | 22 (11.76) | 0.27 (0.17–0.44) | 0.001 |
| Antibiotic preceding admission | 136 (27.09) | 68 (36.36) | 1.54 (1.08–2.20) | 0.018 |
| Existing co-morbidity | 13 (2.60) | 12 (6.50) | 3.06 (1.37–6.83) | 0.016 |
| Vaccination history | ||||
| Adequate vaccination status according to age | 438 (87.43) | 158 (84.95) | 0.81 (0.50–1.31) | 0.39 |
| At least one dose of Hib vaccine | 492 (98.01) | 181 (96.80) | 0.61 (0.22–1.70) | 0.34 |
| Proxy of socioeconomic level | ||||
| Parents with high level of education | 68 (13.55) | 24 (12.83) | 0.94 (0.57–1.55) | 0.93 |
| Medical insurance | 137 (27.30) | 40 (21.40) | 0.72 (0.48–1.08) | 0.28 |
| Father's occupation status | 396 (79.68) | 145 (79.23) | 0.97 (0.64–1.47) | 0.89 |
| Mother's occupation status | 60 (11.98) | 20 (10.70) | 0.88 (0.51–1.50) | 0.64 |
| Family environment | ||||
| Smoking exposure at home | 192 (38.32) | 87 (46.52) | 1.04 (1–1.97) | 0.05 |
| Number of persons in the house >6 | 189 (37.65) | 75 (40.11) | 1.11 (0.80–1.57) | 0.55 |
| Rooms in the house >3 | 164 (32.67) | 72 (38.50) | 1.30 (0.91–1.83) | 0.15 |
OR, odds ratio; CI, confidence interval; SD, standard deviation; ARI, acute respiratory infection; Hib, Haemophilus influenzae type b.
Results are n (%), unless stated otherwise.
In terms of the current admission history and other clinical indicators, patients with a worse outcome were clearly more severely ill on admission (including a significantly higher mean temperature, heart rate, and respiratory rate, and a lower oxygen saturation) and had a significantly longer history of symptoms (Table 2 ). They were also significantly more likely to have required antibiotics during hospitalization (OR 8.75, p < 0.001). Their nutritional status was also significantly poorer, with up to 16.5% (30/182) being severely malnourished. In terms of respiratory signs, only wheezing was significantly more common among children with a good prognosis. Radiologically confirmed pneumonia was again much more frequent (32.9% vs. 14.5%, p = 0.001) amongst children with a poorer outcome, and hospitalizations were significantly more prolonged in this group (9.96 days vs. 4.31 days, p = 0.001).
Table 2.
Clinical history, physical examination findings on admission, and outcome of recruited patients, according to the prognosis groupa
| Good prognosis (n = 502) | Poor prognosis (n = 189) | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| History of the current disease | ||||
| Time interval between onset and admission (Days): mean ± SD | 3.40 ± 4.29 | 5.56 ± 7.85 | NA | 0.001 |
| History of fever | 312 (62.28) | 157 (83.96) | 3.17 (2.06–4.87) | 0.001 |
| History of cough | 495 (98.80) | 182 (97.85) | 1.08 (1.04–1.13) | 0.35 |
| History of runny nose | 390 (77.84) | 139 (74.33) | 0.82 (0.56–1.21) | 0.33 |
| History of vomiting | 249 (49.70) | 101 (54.01) | 1.19 (0.85–1.67) | 0.31 |
| History of diarrhoea | 80 (15.97) | 57 (30.48) | 2.31 (1.56–3.42) | 0.001 |
| Difficulties breastfeeding | 141 (30.79) | 121 (67.22) | 4.61 (3.19–6.67) | 0.001 |
| Impaired consciousness | 3 (0.60) | 17 (9.09) | 16.63 (4.81–57.45) | 0.001 |
| Symptoms and signs on admission | ||||
| Malnourished (WAZ < −1 SD) | 118 (23.89) | 86 (47.25) | 2.85 (1.99–4.07) | 0.001 |
| WAZ > −1 SD | 376 (76.11) | 96 (52.75) | 0.35 (0.24–0.5) | 0.001 |
| WAZ < −1 to > −3 SD | 111 (22.47) | 56 (30.77) | 1.53 (1.05–2.24) | 0.02 |
| WAZ < −3 SD | 7 (1.42) | 30 (16.48) | 13.73 (5.91–31.89) | 0.001 |
| Axillary temperature, °C, mean ± SD | 37.65 ± 0.83 | 38.07 ± 0.88 | NA | 0.001 |
| Hyperpyrexia (axillary temperature >39 °C) | 50 (9.98) | 45 (24.32) | 2.90 (1.84–4.56) | 0.001 |
| Respiratory rate, per min, mean ± SD | 58.68 ± 12.54 | 61.14 ± 17.98 | 1.01 (1–1.02) | 0.04 |
| Heart rate, per min, mean ± SD | 125.86 ± 22.73 | 131.12 ± 24.61 | 1.01 (1–1.02) | 0.008 |
| Oxygen saturation, %, mean ± SD | 95.37 ± 3.12 | 93.31 ± 6.35 | NA | 0.001 |
| WHO oxygen desaturation <90% | 17 (3.53) | 35 (19.44) | 6.58 (3.50–12.38) | 0.001 |
| Cyanosis | 22 (4.38) | 40 (21.40) | 5.93 (3.34–10.52) | 0.001 |
| Pallor | 55 (11) | 61 (32.62) | 3.93 (2.56–6.04) | 0.001 |
| Nasal flaring | 367 (73.11) | 139 (74.33) | 1.07 (0.73–1.57) | 0.74 |
| Wheezing | 416 (82.87) | 36 (19.22) | 0.05 (0.02–0.08) | 0.001 |
| Crackles | 40 (7.97) | 34 (18.18) | 2.56 (1.56–4.22) | 0.001 |
| Rhonchi | 215 (42.83) | 138 (73.80) | 3.76 (2.55–5.53) | 0.001 |
| Radiologically confirmed pneumonia | 64 (14.48) | 47 (32.87) | 2.89 (1.84–4.52) | 0.001 |
| Outcome | ||||
| Received antibiotics during admission | 139 (27.7) | 144 (77.0) | 8.75 (5.64–13.57) | 0.001 |
| Length of admission (Days): mean ± SD | 5.24 ± 4.31 | 7.09 ± 9.96 | NA | 0.001 |
| RISC score, mean ± SD | 0.76 ± 0.80 | 2.98 ± 1.14 | NA | 0.001 |
| Transferred to ICU | 0 (0) | 55 (29.41) | NA | 0.001 |
| Died | 0 (0) | 28 (14.97) | NA | 0.001 |
OR, odds ratio; CI, confidence interval; SD, standard deviation; NA, Not applicable; WAZ, weight-for-age Z score; WHO, World Health Organization; RISC, Respiratory Index of Severity in Children; ICU, intensive care unit.
Results are n (%), unless stated otherwise.
Laboratory and microbiology findings also showed important differences between the two groups, with children with a worse outcome being significantly more anaemic and more frequently demonstrating elevated biomarkers of infection (PCT and CRP). Importantly, no differences were seen according to the outcome group in the mean white blood cell count or in the prevalence of leukopenia or leukocytosis (Table 3 ). As expected, cases of bacteraemia were significantly more frequent among the group with a poor outcome, and so were infections with hMPV. In contrast, infections with RV appeared to be associated with a good outcome (p = 0.001).
Table 3.
Laboratory and microbiology findings for the patients recruited, according to the prognosis groupa
| Good prognosis (n = 502) | Poor prognosis (n = 189) | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Biomarkers | ||||
| High PCT levels (>5 ng/ml) | 98 (20.04) | 92 (49.73) | 3.94 (2.69–5.77) | 0.001 |
| High CRP levels (>5 mg/dl) | 100 (20.16) | 60 (32.61) | 1.91 (1.30–2.80) | 0.004 |
| Haemogram | ||||
| WBC count, mean ± SD | 15.82 ± 7.70 | 16.43 ± 8.01 | NA | 0.42 |
| Abnormal WBC (<5 or >20, × 109/l) | 84 (23.33) | 38 (25.85) | 1.15 (0.74–1.79) | 0.54 |
| Haemoglobin, g/l, mean ± SD | 11.62 ± 2.82 | 10.56 ± 1.87 | NA | 0.001 |
| Anaemia <9 g/l | 27 (7.44) | 24 (16.11) | 2.39 (1.32–4.32) | 0.003 |
| Biochemistry | ||||
| Creatinine, Micromol/L, mean ± SD | 4.05 ± 0.53 | 4.46 ± 2.6 | NA | 0.34 |
| Sodium, Micromol/L, mean ± SD | 135.73 ± 3.9 | 136.72 ± 6.47 | NA | 0.42 |
| Potassium, Micromol/L, mean ± SD | 4.62 ± 0.55 | 4.64 ± 0.72 | NA | 0.88 |
| Glycaemia, Micromol/L, mean ± SD | 95.97 ± 40.61 | 114.5 ± 63.61 | NA | 0.12 |
| Bacteraemia | 10 (2.03) | 14 (7.50) | 3.9 (1.68–9.01) | 0.006 |
| PCR results, microorganism isolated | ||||
| Human metapneumovirus | 33 (6.76) | 27 (15.08) | 2.48 (1.44–4.29) | 0.007 |
| Rhinovirus | 282 (56.97) | 72 (40.22) | 0.50 (0.35–0.72) | 0.001 |
| Coronavirus | 41 (8.28) | 13 (7.26) | 0.87 (0.45–1.66) | 0.66 |
| Bordetella pertussis | 3 (0.61) | 2 (1.12) | 1.85 (0.31–11.16) | 0.49 |
| Mycoplasma | 7 (1.41) | 3 (1.68) | 1.18 (0.30–4.61) | 0.80 |
| Influenza virus | 14 (2.83) | 10 (5.60) | 2.03 (0.88–4.66) | 0.08 |
| Parainfluenza virus | 92 (24.58) | 44 (18.60) | 1.43 (0.95–2.15) | 0.08 |
| Respiratory syncytial virus | 93 (18.80) | 32 (17.88) | 0.94 (0.60–1.47) | 0.78 |
| Adenovirus | 86 (17.37) | 30 (16.76) | 0.96 (0.61–1.51) | 0.85 |
OR, odds ratio; CI, confidence interval; SD, standard deviation; NA, Not applicable; PCT, procalcitonin; CRP, C-reactive protein; WBC, white blood cell count.
Results are n (%), unless stated otherwise.
The multivariate analysis of factors associated with the prognosis (Table 4 ) showed that a history of prematurity (OR 2.50, 95% CI 1.24–5.04), of fever (OR 2.25, 95% CI 1.32–3.83), living in a house with smokers (OR 1.79, 95% CI 1.18–2.72), impaired consciousness (OR 10.96, 95% CI 2.88–41.73), cyanosis (OR 2.09, 95% CI 1.05–4.15), pallor (OR 2.27, 95% CI 1.34–3.84), having rhonchi on auscultation (OR 2.45, 95% CI 1.58–3.79), and hMPV infection (OR 2.13, 95% CI 1.13–4.02) were all independent risk factors for an adverse outcome, whereas a history of asthma (OR 0.46, 95% CI 0.25–0.84) was the only independent risk factor for a positive outcome.
Table 4.
Independent risk factors for a poor prognosis among admitted Moroccan children with WHO-defined severe pneumonia, according to multivariate analysisa
| Risk factors for a poor prognosis | Adjusted OR | 95% CI |
p-Valueb | |
|---|---|---|---|---|
| Lower | Upper | |||
| History of prematurity | 2.50 | 1.24 | 5.04 | 0.010 |
| History of asthma | 0.46 | 0.25 | 0.84 | 0.012 |
| History of fever | 2.25 | 1.32 | 3.83 | 0.003 |
| Smoker at home | 1.79 | 1.18 | 2.72 | 0.006 |
| Pallor | 2.27 | 1.34 | 3.84 | 0.002 |
| Cyanosis | 2.09 | 1.05 | 4.15 | 0.035 |
| Rhonchi | 2.45 | 1.58 | 3.79 | <0.001 |
| Impaired consciousness | 10.96 | 2.88 | 41.73 | <0.001 |
| Human metapneumovirus infection | 2.13 | 1.13 | 4.02 | 0.019 |
WHO, World Health Organization; OR, odds ratio; CI, confidence interval.
Other variables assessed in the model included having a chronic underlying disease, fever on admission, hyperpyrexia, crackles, diarrhoea, high C-reactive protein, high procalcitonin, bacteraemia, respiratory syncytial virus infection, and rhinovirus infection.
Likelihood ratio Chi-square test, 1 degree of freedom.
4. Discussion
The burden and impact of acute LRTIs in Morocco has not been sufficiently addressed. The few available publications23, 24, 25, 26 have patchily presented data on specific microorganisms or generic laboratory findings, but to our knowledge, this project has been the first to comprehensively describe the burden, aetiology, and clinical characterization of severe paediatric pneumonia in a tertiary referral Moroccan hospital.20 In this setting, paediatric respiratory disease results in unacceptably high associated case-fatality rates (28 deaths, giving a case-fatality rate (CFR) of 4.0%, which is towards the upper bound of what has previously been described for similar or even poorer settings, ranging from 0.8% to 4.8%7, 27) and affects mostly infants.7 About a quarter of the patients recruited into this study could be classified as having a poor prognosis, and 8% of these patients required intensive care. Importantly, the management of these patients seems to have been adequately matched to the severity of their symptoms, with a good availability of intensive care and a very high coverage of oxygen and antibiotics, including broad-spectrum ones.20, 28 Nosocomial infections, which in other settings may be an important cause of in-hospital mortality, appear to have played a moderate role in this series, as only about 14.2% of the patients (4/28) had an admission duration longer than 10 days.
In this analysis, we aimed to identify patient risk factors (socio-economic, clinical, and laboratory) upon arrival that could guide the clinician's response and help tailor the scarce resources for improved patient management. Such factors, extensively described in the pneumonia literature10, 11, 12, 13, 19, 29, 30, 31, 32, 33, 34 and currently being specifically investigated as part of the PERCH (aetiology of pneumonia) multisite case–control study,35 may change according to the local or regional epidemiology of LRTI, warranting locally specific analyses. In this series, and similar to what other authors have already described, impaired consciousness,10, 13, 33 cyanosis as a proxy of hypoxemia,11, 12, 13, 19, 36 and pallor13, 34, 37 were three clinical signs strongly and independently associated with a poor prognosis, together with the presence of rhonchi. Wheezing, normally associated with relatively benign LRTI (bronchitis and/or bronchiolitis), and usually considered less frequent in developing settings in comparison to rich countries,38 was very common in our series (present in up to two-thirds of the patients) and highly associated with viral infections,20 but carried a low associated CFR and risk of a poor outcome. Although wheezing was not included in the final multivariate model because of being part of the composite definition of the outcome (being part of the RISC score), we found that a history of asthma was the only protective factor against a negative outcome in our series.
Young age (infants <12 months vs. older children) was also confirmed as a risk factor for a poor prognosis, as has been shown in other studies.11, 19, 32, 33 This sets the rationale for extra care when facing young infants with a respiratory symptomatology, likely warranting a more conservative approach in terms of management.
Deficient nutritional status or low birth weight has clearly been established as a risk factor both for morbidity and mortality among LRTI patients.10, 19, 30, 31, 32, 33 In our series, a history of prematurity (intrinsically linked with a low birth weight) was independently associated with a high risk of dying. Similarly, under-nutrition, although not included in the final model because of being part of the outcome definition of the RISC score, was also found to be strongly associated with a higher likelihood of death, and was highly prevalent, affecting about a quarter of the recruited patients with a good outcome and almost half of those with a bad outcome. In recent years, Morocco has made important efforts to introduce programs for the prevention and management of childhood diseases, including the promotion of exclusive breastfeeding, supplementation of iron and vitamin A, and the introduction of anti-pneumonia vaccines, all of which may have an impact on the incidence of new malnutrition cases. However, malnutrition prevalence rates remain high in Morocco,25, 39 and unless adequately addressed, will continue to negatively affect the survival of patients with pneumonia.
Perhaps the most interesting finding in this analysis was the adverse outcome associated with the specific pneumonia cases secondary to hMPV. Among the 62 patients (9.1%) with this infection, seven (11.3%) required ICU admission, three died, and the mean RISC score was 1.84. The impact and real burden of this relatively newly described human virus needs to be investigated further,40, 41, 42 but our data clearly show its pathogenicity potential and suggest that active surveillance for this virus should be maintained in Morocco.
Our study also confirmed the previously well-documented association of smoking in the household and an adverse outcome.43, 44 However, we were not able to confirm the association of certain well-known risk factors with a higher risk of death.10, 11, 12, 13 Indeed, parental education, lack of breastfeeding, vaccination status, unavailability of adequate medical care, co-morbidities and co-infections, metabolic acidosis, and other laboratory findings (leukocytosis, abnormal biomarkers, etc.), although assessed for some or the majority of outpatients, could not be independently related to a poorer outcome, even though the study sample size was relatively large and the univariate association was evident. This does not limit the strength of our results, but, in our opinion, may express that in this setting their validity as prognostic markers appears less critical than in other settings. Our study suffers from further limitations, including the fact that it was conducted in an urban referral tertiary hospital with good accessibility to intensive care, a factor that may have influenced the outcome for some of these patients, thus underestimating the real impact that pneumonia may have in other less advantaged or more rural areas of the country.
In conclusion, the wide implementation of anti-pneumococcal and Hib vaccines and other known preventive measures will certainly result in a significant decrease in pneumonia-related morbidity and mortality globally. However, in regions where pneumonia remains a major cause of disease and death, the emphasis on early identification of risk factors known to be globally or locally associated with a poor outcome and the establishment of adequate case management strategies can make an enormous difference in the prognosis of these patients. In this respect, this study represents an important addition to the significant knowledge gaps related to paediatric pneumonia in Morocco, and more widely in the Maghreb area, and provides good evidence for new approaches to prevent and better manage this deadly disease.
Acknowledgements
We want to acknowledge the facilitating role played by Pascal Andignac, Eva López, Younes Ben Azzouz, Maria José López, Robert Álvarez, and the rest of the team at Fundació Clínic Maroc, together with the nursing and clinical personnel in HER, Rabat (Emergency ward, P1, and ICU), and the technical personnel at the research laboratory of CHIS. We also thank Dr Jordi Vila and Dr Míriam Álvarez, who contributed to the general set-up of laboratory methods at the research laboratory. Edward B. Hayes played a critical role in the design of the study protocol. Finally, we are also thankful to the mothers, guardians, and study participants.
Funding sources: Spanish Agency of International Cooperation for Development (AECID) through grant 07-CO1-021 awarded to Fundació Clínic per a la Recerca Biomèdica (Convenio de Fortalecimiento del sistema nacional de salud, con especial atención a la salud materno-infantil, Marruecos, 2008–2012). JR has a fellowship from the program I3, of the ISCIII (grant number CES11/012). QB has a fellowship from the program Miguel Servet of the ISCIII (grant number CP11/00269).
Conflict of interest: The authors declare that they have no conflict of interest. No material submitted as part of the manuscript infringes existing copyrights, or the rights of a third party.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
References
- 1.Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.The United Nations Children's Fund (UNICEF)/World Health Organization (WHO) UNICEF/WHO; New York: 2006. Pneumonia: the forgotten killer of children. [Google Scholar]
- 3.Black R.E., Cousens S., Johnson H.L., Lawn J.E., Rudan I., Bassani D.G. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien K.L., Wolfson L.J., Watt J.P., Henkle E., Deloria-Knoll M., McCall N. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 5.Watt J.P., Wolfson L.J., O’Brien K.L., Henkle E., Deloria-Knoll M., McCall N. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–911. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 6.Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair H., Simoes E.A., Rudan I., Gessner B.D., Azziz-Baumgartner E., Zhang J.S. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obaro S.K., Madhi S.A. Bacterial pneumonia vaccines and childhood pneumonia: are we winning, refining, or redefining? Lancet Infect Dis. 2006;6:150–161. doi: 10.1016/S1473-3099(06)70411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull World Health Organ. 1997;75(Suppl 1):7–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Demers A.M., Morency P., Mberyo-Yaah F., Jaffar S., Blais C., Somse P. Risk factors for mortality among children hospitalized because of acute respiratory infections in Bangui, Central African Republic. Pediatr Infect Dis J. 2000;19:424–432. doi: 10.1097/00006454-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Djelantik I.G., Gessner B.D., Sutanto A., Steinhoff M., Linehan M., Moulton L.H. Case fatality proportions and predictive factors for mortality among children hospitalized with severe pneumonia in a rural developing country setting. J Trop Pediatr. 2003;49:327–332. doi: 10.1093/tropej/49.6.327. [DOI] [PubMed] [Google Scholar]
- 12.Suwanjutha S., Ruangkanchanasetr S., Chantarojanasiri T., Hotrakitya S. Risk factors associated with morbidity and mortality of pneumonia in Thai children under 5 years, Southeast Asian. J Trop Med Public Health. 1994;25:60–66. [PubMed] [Google Scholar]
- 13.Tiewsoh K., Lodha R., Pandey R.M., Broor S., Kalaivani M., Kabra S.K. Factors determining the outcome of children hospitalized with severe pneumonia. BMC Pediatr. 2009;9:15. doi: 10.1186/1471-2431-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . Country by country Morocco. WHO; Geneva: 2012. Global Health Observatory Data Repository. [Google Scholar]
- 15.Mulholland E.K., Simoes E.A., Costales M.O., McGrath E.J., Manalac E.M., Gove S. Standardized diagnosis of pneumonia in developing countries. Pediatr Infect Dis J. 1992;11:77–81. doi: 10.1097/00006454-199202000-00004. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . Second edition. WHO; Geneva: 2013. Pocket book for hospital care of children: guidelines for the management of common illness with limited resources. [Google Scholar]
- 17.International statistical classification of diseases and related health problems 10th revision (ICD-10). Geneva: WHO; 2010. Available at: http://apps.who.int/classifications/icd10/browse/2010/en (accessed May 2013).
- 18.Centers for Disease Control and Prevention. CDC growth charts. Atlanta, GA: CDC; Available at: http://www.cdc.gov/growthcharts/ (accessed May 2013).
- 19.Reed C., Madhi S.A., Klugman K.P., Kuwanda L., Ortiz J.R., Finelli L. Development of the Respiratory Index of Severity in Children (RISC) score among young children with respiratory infections in South Africa. PLoS One. 2012;7:e27793. doi: 10.1371/journal.pone.0027793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jroundi I., Mahraoui C., Benmessaoud R., Moraleda C., Tligui H., Seffar M. The epidemiology and aetiology of infections in children admitted with clinical severe pneumonia to a university hospital in Rabat, Morocco. J Trop Pediatr. 2014;60(4):270–278. doi: 10.1093/tropej/fmu010. [DOI] [PubMed] [Google Scholar]
- 21.Selva L., Benmessaoud R., Lanaspa M., Jroundi I., Moraleda C., Acacio S. Detection of Streptococcus pneumoniae and Haemophilus influenzae type B by real-time PCR from dried blood spot samples among children with pneumonia: a useful approach for developing countries. PLoS One. 2013;8:e76970. doi: 10.1371/journal.pone.0076970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherian T., Mulholland E.K., Carlin J.B., Ostensen H., Amin R., de Campo M. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–359. [PMC free article] [PubMed] [Google Scholar]
- 23.Benbachir M., Elmdaghri N., Belabbes H., Haddioui G., Benzaid H., Zaki B. Eleven-year surveillance of antibiotic resistance in Streptococcus pneumoniae in Casablanca (Morocco) Microb Drug Resist. 2012;18:157–160. doi: 10.1089/mdr.2011.0130. [DOI] [PubMed] [Google Scholar]
- 24.El Mdaghri N., Jilali N., Belabbes H., Jouhadi Z., Lahssoune M., Zaid S. Epidemiological profile of invasive bacterial diseases in children in Casablanca, Morocco: antimicrobial susceptibilities and serotype distribution. East Mediterr Health J. 2012;18:1097–1111. doi: 10.26719/2012.18.11.1097. [DOI] [PubMed] [Google Scholar]
- 25.Ministry of Health . Ministry of Health; Morocco: 2011. Enquête nationale sur la population et la santé familiale (ENPSF) [Google Scholar]
- 26.Warda K., Oufdou K., Zahlane K., Bouskraoui M. Antibiotic resistance and serotype distribution of nasopharyngeal isolates of Streptococcus pneumoniae from children in Marrakech region (Morocco) J Infect Public Health. 2013;6(6):473–481. doi: 10.1016/j.jiph.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Ayieko P., Okiro E.A., Edwards T., Nyamai R., English M. Variations in mortality in children admitted with pneumonia to Kenyan hospitals. PLoS One. 2012;7:e47622. doi: 10.1371/journal.pone.0047622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jroundi I., Benmessaoud R., Mahraoui C., Moraleda C., Tligui H., Seffar M. Antibiotic usage prior and during hospitalization for clinical severe pneumonia in children under five years of age in Rabat, Morocco. Antibiotics. 2013;2:450–464. doi: 10.3390/antibiotics2040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azab S.F., Sherief L.M., Saleh S.H., Elsaeed W.F., Elshafie M.A., Abdelsalam S.M. Impact of the socioeconomic status on the severity and outcome of community-acquired pneumonia among Egyptian children: a cohort study. Infect Dis Poverty. 2014;3:14. doi: 10.1186/2049-9957-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chisti M.J., Tebruegge M., La Vincente S., Graham S.M., Duke T. Pneumonia in severely malnourished children in developing countries—mortality risk, aetiology and validity of WHO clinical signs: a systematic review. Trop Med Int Health. 2009;14:1173–1189. doi: 10.1111/j.1365-3156.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson A.W., Osinusi K., Aderele W.I., Gbadero D.A., Olaleye O.D., Adeyemi-Doro F.A. Etiologic agents and outcome determinants of community-acquired pneumonia in urban children: a hospital-based study. J Natl Med Assoc. 2008;100:370–385. doi: 10.1016/s0027-9684(15)31269-4. [DOI] [PubMed] [Google Scholar]
- 32.Nacul L.C., Kirkwood B.R., Carneiro A.C., Pannuti C.S., Magalhaes M., Arthur P. Aetiology and clinical presentation of pneumonia in hospitalized and outpatient children in Northeast Brazil and risk factors for severity. J Health Popul Nutr. 2005;23:6–15. [PubMed] [Google Scholar]
- 33.Ramachandran P., Nedunchelian K., Vengatesan A., Suresh S. Risk factors for mortality in community acquired pneumonia among children aged 1–59 months admitted in a referral hospital. Indian Pediatr. 2012;49:889–895. doi: 10.1007/s13312-012-0221-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang L.J., Mu S.C., Lin C.H., Lin M.I., Sung T.C. Fatal community-acquired pneumonia: 18 years in a medical center. Pediatr Neonatol. 2013;54:22–27. doi: 10.1016/j.pedneo.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Wonodi C.B., Deloria-Knoll M., Feikin D.R., DeLuca A.N., Driscoll A.J., Moisi J.C. Evaluation of risk factors for severe pneumonia in children: the Pneumonia Etiology Research for Child Health study. Clin Infect Dis. 2012;54(Suppl 2):S124–S131. doi: 10.1093/cid/cir1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subhi R., Adamson M., Campbell H., Weber M., Smith K., Duke T. The prevalence of hypoxaemia among ill children in developing countries: a systematic review. Lancet Infect Dis. 2009;9:219–227. doi: 10.1016/S1473-3099(09)70071-4. [DOI] [PubMed] [Google Scholar]
- 37.Moschovis P.P., Banajeh S., MacLeod W.B., Saha S., Hayden D., Christiani D.C. Childhood anemia at high altitude: risk factors for poor outcomes in severe pneumonia. Pediatrics. 2013;132:e1156–e1162. doi: 10.1542/peds.2013-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel S.P., Jarvelin M.R., Little M.P. Systematic review of worldwide variations of the prevalence of wheezing symptoms in children. Environ Health. 2008;7:57. doi: 10.1186/1476-069X-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ministry of Health. (2011) Santé en chiffres. Morocco: Ministry of Health; Available at: http://srvweb.sante.gov.ma/Publications/Etudes_enquete/Documents/SC2011.pdf (accessed).
- 40.Hamelin M.E., Boivin G. Human metapneumovirus: a ubiquitous and long-standing respiratory pathogen. Pediatr Infect Dis J. 2005;24:S203–S207. doi: 10.1097/01.inf.0000188158.27840.7c. [DOI] [PubMed] [Google Scholar]
- 41.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caracciolo S., Minini C., Colombrita D., Rossi D., Miglietti N., Vettore E. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: virologic and clinical features. Pediatr Infect Dis J. 2008;27:406–412. doi: 10.1097/INF.0b013e318162a164. [DOI] [PubMed] [Google Scholar]
- 43.Chisti M.J., Duke T., Robertson C.F., Ahmed T., Faruque A.S., Bardhan P.K. Co-morbidity: exploring the clinical overlap between pneumonia and diarrhoea in a hospital in Dhaka, Bangladesh. Ann Trop Paediatr. 2011;31:311–319. doi: 10.1179/1465328111Y.0000000033. [DOI] [PubMed] [Google Scholar]
- 44.Ujunwa F., Ezeonu C. Risk factors for acute respiratory tract infections in under-five children in Enugu Southeast Nigeria. Ann Med Health Sci Res. 2014;4:95–99. doi: 10.4103/2141-9248.126610. [DOI] [PMC free article] [PubMed] [Google Scholar]