Abstract
Objectives
We studied paediatric patients with human adenovirus (HAdV) infection during the 2011 outbreak in northern Taiwan to define the clinical features of different HAdV genotypes in children.
Methods
Between January and December 2011, 637 patients <19 years of age exhibited culture-confirmed adenoviral infection in Chang Gung Memorial Hospital, and provided specimens available for genotyping by multiplex real-time PCR. Clinical data were collected retrospectively.
Results
Excluding five cases with multiple genotypes, 632 cases were included for analysis. Three genotypes were identified, including HAdV-3 (429/632; 67.6%), HAdV-7 (144/632; 22.6%) and HAdV-2 (59/632; 9.8%). Median age was 4.58 years (range 2 months to 18 years), with children infected with HAdV-3 significantly older (82.9% >3 years; p <0.001). Of the 621 inpatients, 98.2% had fevers and all exhibited respiratory symptoms, 75 patients (12.1%) had lower respiratory tract infections, 20 (3.2%) required intensive care (HAdV-2: 1; HAdV-3: 8; and HAdV-7: 11), and three died (all HAdV-7-infected). HAdV-3-infected patients were significantly more likely to have upper respiratory symptoms and a high serum C-reactive protein level >100 mg/L, whereas leucocytosis (white blood cell count >15 000/mm3) was more common in HAdV-2-infected patients (p 0.007). HAdV-7 infections were significantly associated with a longer duration of fever, leucopenia (white blood cell count <5000/mm3), thrombocytopenia (platelet count <150 000/mm3), lower respiratory tract infections, a longer length of hospital stay, and requiring intensive care (all p <0.001).
Conclusion
Childhood HAdV-2, HAdV-3 and HAdV-7 infections may exhibit different clinical manifestations. Although HAdV-3 was the most prevalent genotype observed during the 2011 Taiwan outbreak, HAdV-7 caused more severe disease characteristics and outcomes.
Keywords: Adenovirus, Children, Genotype 7, Outbreak, Taiwan
Introduction
Human adenovirus (HAdV) is a common pathogen in children that causes a variety of diseases, including respiratory disease, gastroenteritis, conjunctivitis and haemorrhagic cystitis [1], [2], [3], [4], [5], [6]. HAdV is a double-stranded DNA virus that can be subdivided into >60 genotypes in seven species classified from A to G based on genomic homology [7]. Each genotype can cause different clinical manifestations, with genotypes 2, 3, 4, 7, 14, 21 and 55 resulting in more severe and disseminated diseases, including pneumonia and encephalitis [8], [9], [10], [11], [12], [13], [14].
According to previous studies of adenoviral epidemiology in Taiwan, HAdV-3 has circulated annually since 1999 and remains the dominant circulating genotype [15] as compared with the low prevalence of HAdV-7. A large community outbreak of adenoviral infections was detected by the nationwide surveillance system of the Centre for Disease Control-Taiwan (CDC-Taiwan) between weeks 11 and 41 in 2011 [16], with the average HAdV-positivity rate increasing from 5.75% between 2008 and 2010 to 25.9% in 2011, along with an increase in the number of children admitted to the intensive care units of two medical centres in northern Taiwan owing to HAdV infection [17]. During this period, HAdV-7 re-emergence also occurred, with HAdV-7 proportions in Taiwan increasing significantly from 0.3% between 2008 and 2010 to 10% in 2011 [16].
Studies showed that HAdV-7 is associated with more severe clinical courses and complications [18], [19], [20], [21]; however, there are no reports concerning the clinical features associated with large outbreaks of HAdV-7 infections in children. To better understand the clinical features and complications of different HAdV infections in children, we conducted a retrospective study to investigate the clinical features of children with HAdV infections during the 2011 outbreak in Taiwan.
Materials and methods
Study design and population
We conducted a retrospective study of children with adenoviral infections in Chang Gung Memorial Hospital during 2011. Children <19 years old with culture-confirmed adenoviral infections were enrolled, excluding those infected with more than two types of virus or with insufficient samples for further genotype stratification. Demographic data were collected and analysed from all patients, whereas clinical symptoms, laboratory results, images, complications and clinical outcomes were collected and analysed from inpatients only.
Ethics statement
This retrospective study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (CGMH; No. 100-2518B). Clinical information was analysed anonymously so informed consent was waived.
Viral isolation, typing and sequencing
Procedures used for viral isolation and identification and designs of multiplex real-time PCR for HAdV genotyping are described in the Supplementary material (Data S1). HAdV sequencing was performed on full-length hexon genes as previously described [22]. PCR amplification was performed using specific primers (see Supplementary material, Table S1).
Definitions
We defined cases with lower respiratory tract infection (LRTI) as those having at least a pneumonia patch according to chest plain films or those without a pneumonia patch but with severe respiratory distress requiring oxygen supplementation and intensive care. In Taiwan, Mycoplasma pneumoniae caused frequent LRTIs in children >3 years old, and is usually screened for in this age group when pneumonia is suspected. Despite possible positivity for urinary pneumococcal antigen owing to asymptomatic carriage, some paediatricians still preferred using it as a screening tool, because Streptococcus pneumoniae is a common pneumonia pathogen in children <5 years old. For possible M. pneumoniae co-infection, we considered three situations as positive results (summarized in the Supplementary material, Data S1). Clinical laboratory data on admission and peak or nadir values during hospitalization were collected. White blood cell count >15 000/μL was defined as leucocytosis but a count of <5000/μL was defined as leucopenia. Platelet count >450 000/μL was defined as thrombocytosis and <150 000/μL as thrombocytopenia. Serum creatinine levels >1.0 mg/dL were considered evidence of impaired renal function, whereas a three-fold elevation in normal aspartate aminotransferase and alanine aminotransferase levels was considered evidence of impaired liver function.
Molecular phylogenetic analysis using the maximum likelihood method
All 107 complete hexon sequences were downloaded from GenBank and aligned using ClustalO [23] against 25 sequences provided in this study. The evolutionary history of these sequences was inferred using the maximum likelihood method based on the Hasegawa–Kishino–Yano model [24]. Evolutionary analyses were conducted using MEGA7 [25] and evaluated using 1000 bootstrap replicates.
Statistical analysis
We used the chi-square test or Fisher’s exact test to compare categorical variables. Non-categorical variables were compared by one-way independent analysis of variance with post-hoc analysis. We looked for factors associated with LRTI and intensive care admission, using a multivariate logistic regression analysis (variables with p values <0.20 in univariate analysis were entered in the model). Statistical significance was set at p <0.05.
Results
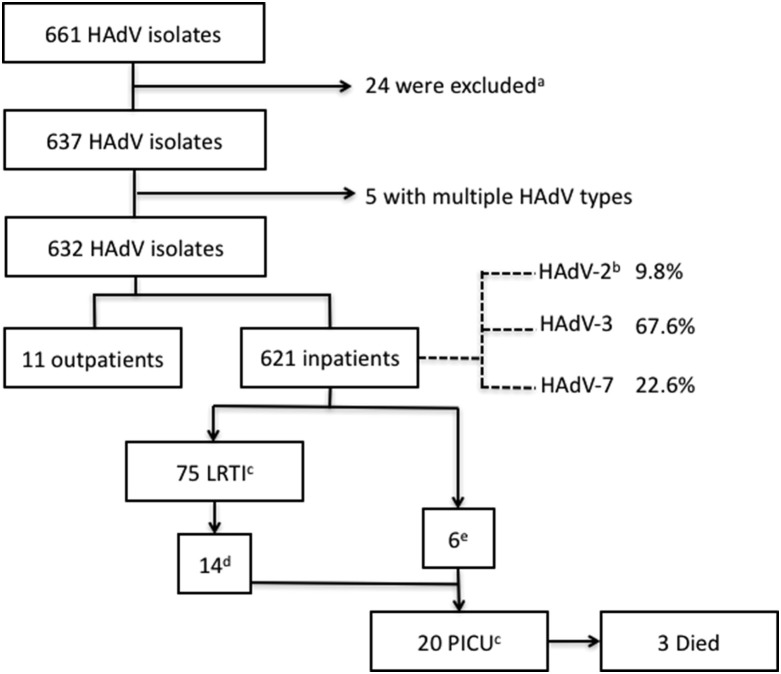
Of 1598 children with positive virus cultures, adenovirus alone was identified in 661 specimens (41.4%). Other viruses identified are listed in the Supplementary material (Table S2). Further genotyping by PCR was possible in 637 patients who had adequate specimens, most of which were obtained by throat swab (616/632; 97.5%; see Supplementary material, Table S3). After excluding five cases with multiple genotypes, a total of 632 cases were included for further analysis. Only three genotypes were identified, with HAdV-3 (429/632; 67.6%) being the most common, followed by HAdV-7 (144/632; 22.6%) and HAdV-2 (59/632; 9.8%). Of the 621 patients (98.3%) hospitalized, 75 (12%) had defined LRTIs, 20 (3.2%) required intensive care, and three (0.5%) eventually died (Fig. 1 ).
Fig. 1.
Flow chart describing case selection. a Patients excluded due to insufficient samples. b HAdV-2, -3 and -7, human adenovirus type 2, 3, and 7. c LRTI, lower respiratory tract infection; PICU, paediatric intensive care unit. d LRTI patients (14/75) exhibiting severe respiratory distress. e These patients included three with neurological dysfunction, two suffering from shock, and one with massive bleeding in the gastrointestinal tract.
Demographic and clinical characteristics
The median age was 4.58 years (range: 2 months to 18 years), with HAdV-3-infected children being significantly older than children harbouring other infections (Table 1 ). Detailed clinical characteristics of hospitalized children are shown in Table 1. HAdV-3-infected children were more likely to experience symptoms of upper respiratory tract infection, including cough, rhinorrhoea, nasal congestion and exudative tonsillitis. HAdV-7-infected children experienced longer duration of fever and were more likely to be less active. Skin rash accompanied with oral ulcers (6.9%) mimicking clinical symptoms of enterovirus infection was also observed, particularly in HAdV-7-infected patients. All clinical diagnoses are listed in the Supplementary material (Table S4).
Table 1.
Demographic data and clinical characteristics of 621 hospitalized children infected with different HAdV genotypes
| Characteristics | HAdV-2, n (%) |
HAdV-3, n (%) |
HAdV-7, n (%) |
pa |
|---|---|---|---|---|
| Case number | 58 | 422 | 141 | |
| Male | 33 (56.9) | 237 (56.1) | 89 (63.1) | 0.346 |
| Age, mean ± SD (months) | 38.90±23.01 | 61.59±30.54 | 57.98±46.52 | <0.001 |
| >3 years | 31 (53.4) | 350 (82.9) | 84 (59.6) | <0.001 |
| General symptoms | ||||
| Fever | 54 (93.1) | 417 (98.8) | 138 (97.9) | 0.012 |
| Fever >39°C | 51 (87.9) | 396 (93.8) | 133 (94.3) | 0.209 |
| Duration, mean ± SD (days) | 5.33±3.03 | 5.39±2.13 | 6.58±2.90 | <0.001 |
| >7 days | 17 (29.3) | 36 (8.5) | 40 (28.4) | <0.001 |
| Decreased appetite | 49 (84.5) | 352 (83.4) | 128 (90.8) | 0.102 |
| Decreased activity | 33 (56.9) | 282 (66.8) | 116 (82.3) | <0.001 |
| Respiratory symptoms | ||||
| Cough | 47 (81.0) | 362 (85.8) | 106 (75.2) | 0.014 |
| Rhinorrhoea | 40 (69.0) | 306 (72.5) | 79 (56.0) | 0.007 |
| Nasal congestion | 29 (50.0) | 234 (55.5) | 53 (37.6) | 0.001 |
| Sore throat | 17 (29.3) | 189 (44.8) | 58 (41.1) | 0.077 |
| Exudate coating | 21 (36.2) | 202 (47.9) | 47 (33.9) | 0.005 |
| Oral ulcers | 1 (1.7) | 24 (5.7) | 18 (12.8) | 0.004 |
| Conjunctivitis | 9 (15.5) | 79 (18.7) | 24 (17.0) | 0.786 |
| Extrapulmonary symptoms | ||||
| Skin rash | 4 (6.9) | 22 (5.2) | 17 (12.1) | 0.021 |
| Headache | 4 (6.9) | 51 (12.1) | 11 (7.9) | 0.232 |
| Abdominal pain | 12 (20.7) | 124 (29.4) | 38 (27.0) | 0.365 |
| Vomiting | 13 (22.4) | 119 (28.2) | 41 (29.1) | 0.611 |
| Diarrhoea | 10 (17.2) | 117 (27.7) | 57 (27.0) | 0.365 |
| Underlying diseases | 1 (1.7) | 10 (2.4) | 3 (2.1) | 0.947 |
| Neurology | 0 | 2 | 1 | |
| Pulmonary | 0 | 1 | 0 | |
| Gastrointestinal | 0 | 1 | 2 | |
| Cardiovascular | 0 | 0 | 0 | |
| Others | 1 | 6 | 0 | |
| Complication | ||||
| LRTI | 3 (3.4) | 40 (9.0) | 32 (20.6) | <0.001 |
| Respiratory distress | 1 (1.7) | 13 (3.1) | 8 (5.7) | 0.259 |
| Intubation | 1 (1.7) | 3 (0.7) | 3 (2.1) | 0.333 |
| Duration (days) | 2 | 6.67±0.58 | 25.33±32.62 | 0.017 |
| Pneumonia patch | 3 (6.9) | 32 (8.5) | 28 (19.9) | <0.001 |
| Single lobe | 100% | 66% | 61% | |
| Multiple lobes | 0% | 34% | 39% | |
| Pleural effusion | 0 | 3 (0.7) | 8 (5.7) | <0.001 |
| PICU | 1 (1.7) | 8 (1.9) | 11 (7.8) | <0.001 |
| Duration (days) | 4 | 6.38±2.72 | 11.45±18.93 | 0.114 |
| Mortality | 0 | 0 | 3 | |
| ECMO use | 0 | 0 | 2 | |
| Antibiotics treatment | 30 (51.7) | 275 (65.2) | 76 (53.9) | <0.001 |
| Co-infection with Mycoplasma pneumoniae | 0 | 5 (1.2) | 4 (2.8) | 0.305 |
| Co-infection with pneumococcus | 0 | 0 | 4 (2.8) | 0.15 |
| LOS (days) | 3.84±1.25 | 4.40±3.69 | 6.18±7.32 | <0.001 |
Abbreviations: ECMO, extracorporeal membrane oxygenation; HAdV, human adenovirus; LOS, length of hospital stay; LRTI, lower respiratory tract infection; PICU, paediatric intensive care unit.
Statistical significance: p <0.05 via post hoc analysis.
Laboratory parameters
Detailed laboratory data are shown in Table 2 . Leucocytosis was noted in 163 (26.2%) children, and a serum C-reactive protein (CRP) level ≥40 mg/L was identified in 423 (68.1%) children. HAdV-2-infected children showed leucocytosis and thrombocytosis, whereas HAdV-3-infected children were characterized by high serum CRP levels. By contrast, HAdV-7-infected children were significantly more likely to show leucopenia, thrombocytopenia, and impaired liver and renal function.
Table 2.
Laboratory findings from 621 hospitalized children infected with different HAdV genotypes
| Laboratory data | HAdV-2, n (%) |
HAdV-3, n (%) |
HAdV-7, n (%) |
p |
|---|---|---|---|---|
| Case number | 58 | 422 | 141 | |
| Haemogram | ||||
| WBC count (1000/μL)a | 15.36±5.85 | 12.93±5.48 | 9.39±4.69 | <0.001 |
| Peak or nadirb WBC count | 15.80±5.74 | 13.19±5.55 | 9.92±5.00 | <0.001 |
| <5000/μL | 0 | 7 (1.7) | 17 (12.1) | <0.001 |
| >15 000/μL | 28 (48.3) | 117 (27.7) | 18 (12.7) | <0.001 |
| Hb, mean±SD (g/dl) | 13.51±5.63 | 9.92±5.00 | 12.69±5.69 | 0.126 |
| Platelet, mean±SD (1000/μL) | 297.74±101.05 | 249.58±72.74 | 213.74±76.21 | <0.001 |
| <150 000/μL | 0 | 12 (2.8) | 22 (15.6) | <0.001 |
| >450 000/μL | 4 (6.9) | 8 (1.9) | 1 (0.7) | <0.001 |
| Biochemistry | ||||
| CRP (mg/L)a | 42.87±37.07 | 77.81±56.39 | 38.46±37.86 | <0.001 |
| Peak CRP (mg/L)b | 46.75±38.97 | 84.47±55.15 | 44.83±39.76 | <0.001 |
| <40 mg/L | 30 (51.7) | 93 (22.0) | 75 (53.2) | <0.001 |
| >100 mg/L | 7 (12.1) | 153 (36.3) | 14 (9.9) | <0.001 |
| BUN, mean±SD (mg/dL) | 8.99±3.54 | 7.84±3.55 | 8.52±5.78 | 0.463 |
| Cr, mean±SD (mg/dL) | 0.33±0.10 | 0.37±0.11 | 0.62±1.79 | 0.146 |
| >1.0 mg/dL | 2/24 (8.3) | 5/176 (2.8) | 7/66 (10.6) | 0.043 |
| AST (U/L) | 50.91±79.06 | 41.02±66.22 | 57.97±57.66 | 0.104 |
| >3-fold of normal value | 0/33 | 0/250 | 3/88 (3.4) | 0.008 |
Abbreviations: AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; CRP, C-reactive protein; HAdV, human adenovirus; Hb, haemoglobin; WBC, white blood cell;.
Note: Haemogram and CRP were checked in all 621 cases. Cr was checked in 266 cases (HAdV-2: 24 cases; HAdDV-3: 176 cases; and HAdV-7: 66 cases) and AST was checked in 371 cases (HAdV-2: 24 cases; HAdDV-3: 176 cases; and HAdV-7: 66 cases).
First laboratory examination on admission.
The highest or lowest laboratory value during hospitalization.
LRT involvement and radiological findings
LRTI was diagnosed in 75 cases, including 63 with a pneumonia patch observed on chest plain films and 12 with respiratory distress requiring both oxygen supplementation and intensive care, but without observation of a pneumonia patch. Most of these patients (91%) were previously healthy. Among the 63 pneumonia cases, most (65%) involved a single lobe and 11 (17%) exhibited pleural effusion. HAdV-7-infected patients were more likely to develop LRTI. Seven (9.3%) of the 75 LRTI cases eventually developed respiratory failure and required intubation, with three cases (43%) having underlying diseases. HAdV-7-infected patients also exhibited longer durations of intubation (Table 1). Compared with patients without LRTIs, those with LRTIs were more likely to show longer fever duration, leucopenia, and longer hospital stays and antibiotics treatment (Table 3 ). In multivariate analysis, underlying diseases, fever >7 days, leucopenia, antibiotic prescription and HAdV-7 infection were all independently associated with the risk of LRTI (see Supplementary material, Table S5). All 75 patients with LRTI had blood samples sent for bacterial culture and three samples yielded positive results (all coagulase-negative staphylococcus). Urine samples were collected for 38 cases. Bacterial cultures showed only one positive result, which was for Escherichia coli in the HAdV-7 group. Thirty-six cases were tested for M. pneumoniae co-infection, with most (63.9%) aged >3 years. Positive tests were obtained for 9 of 36 (25%) cases, including a positive PCR result in one patient. Among the 46 cases screened for pneumococcus, 63% were <5 years old. Four (8.7%) of 46 cases exhibited a positive result for urinary pneumococcal antigen. Sputum specimens from 18 cases underwent bacterial culture, with two yielding results indicating methicillin-resistant Staphylococcus aureus (on days 18 and 33 following admission, respectively). Pleural fluid specimens from two cases underwent microbiological testing, with both specimens subsequently positive for HAdV-7 according to virus culture, but negative for bacteria. Co-infections with M. pneumoniae or S. pneumoniae were not statistically different among patients infected with HAdV-2, -3, or -7.
Table 3.
Comparison of clinical features between 621 hospitalized children with or without LRTIs or PICU admission
| Characteristics | LRTI, n (%) |
Non-LRTI, n (%) |
p | PICU, n (%) |
Non-PICU, n (%) |
p |
|---|---|---|---|---|---|---|
| Case number | 75 | 546 | 20 | 601 | ||
| Genotypes | ||||||
| HAdV-2 | 3 (4.0) | 55 (10.1) | 0.09 | 2 (10.0) | 56 (9.3) | 0.918 |
| HAdV-3 | 40 (53.3) | 382 (69.9) | 0.004 | 8 (40.0) | 414 (68.9) | 0.006 |
| HAdV-7 | 32 (42.7) | 109 (19.9) | <0.001 | 10 (50.0) | 131 (21.8) | 0.003 |
| Fever | 74 (98.6) | 535 (98.0) | 0.757 | 19 (95.0) | 590 (98.2) | 0.311 |
| Fever>39°C | 72 (96.0) | 508 (93.0) | 0.331 | 18 (90.0) | 561 (93.5) | 0.536 |
| Duration (days) | 7.96±7.46 | 4.20±3.37 | <0.001 | 8.7±5.31 | 5.55±2.26 | <0.001 |
| >7 days | 36 (48.0) | 57 (10.4) | <0.001 | 11 (55) | 82 (13.6) | <0.001 |
| Peak or nadir leucocyte count, mean±SD | 12.99±7.46 | 12.68±5.47 | 0.673 | 15.72±8.13 | 12.59±5.58 | 0.004 |
| <5000/μL | 8 (10.6) | 16 (2.9) | <0.001 | 2 (10.0) | 22 (3.7) | 0.148 |
| >15 000/μL | 22 (29.3) | 141 (25.8) | 0.227 | 9 (45.0) | 154 (25.6) | 0.053 |
| Peak CRP, mean±SD (mg/L) | 85.05±38.97 | 70.37±52.8 | 0.033 | 109.57±86.44 | 70.76±52.03 | 0.021 |
| >100 mg/L | 28 (37.7) | 146 (26.8) | 0.055 | 8 (40.0) | 166 (27.6) | 0.225 |
| Underlying diseases | 6 (8) | 8 (1.5) | 0.003 | 6 (30) | 8 (1.3) | <0.001 |
| Neurology | 3 | 0 | 3 | 0 | ||
| Pulmonary | 0 | 1 | 0 | 1 | ||
| Gastrointestinal | 3 | 0 | 3 | 0 | ||
| Cardiovascular | 0 | 7 | 0 | 7 | ||
| Others | 1 | 6 | 1 | 6 | ||
| Antibiotic treatment | 72 (96.0) | 309 (56.6) | <0.001 | 20 (100) | 361 (60.1) | <0.001 |
| LOS (days) | 9.13±9.42 | 4.20±3.37 | <0.001 | 20.0±17.89 | 4.24±2.17 | <0.001 |
Abbreviations: CRP, C-reactive protein; HAdV-2, -3 and -7, human adenoviruses types 2, 3 and 7; LOS, length of hospital stay; LRTI, lower respiratory tract infection; PICU, paediatric intensive care unit.
Complications and clinical outcomes
Twenty (3.2%) patients were admitted to the intensive care unit, including 14 with respiratory distress, three with shock, and one each with acute encephalitis and status epilepticus with hydrocephalus, respectively. Among these, six (33%) had underlying chronic systemic diseases, including neurological diseases (cerebral palsy) for three cases, tracheo-oesophageal fistula for two, and cystic fibrosis for one. These 20 patients experienced a longer duration of fever and length of hospital stay, and were more likely to have pneumonia involving multiple lobes (61.5% versus 25.5%; p 0.012) and receive antibiotics treatment (100% versus 60.1%; p <0.001) (Table 3). Compared with those infected with non-HAdV-7 HAdV, patients infected with HAdV-7 were more likely to require intensive care (OR 4.43, 95% CI 1.78–10.91) and have a longer hospital stay. Three HAdV-7-infected patients eventually died, and extracorporeal-membrane oxygenation therapy was performed on two of these three patients. These two cases exhibited an underlying disease of cerebral palsy. Multivariate regression analysis revealed that underlying diseases, fever duration >7 days and HAdV-7 infection were predictive risk factors for severe infection requiring intensive care (see Supplementary material, Table S5).
Phylogenetic tree of the hexon gene
Because HAdV-7 caused more severe clinical symptoms, two, one and 22 hexon sequences from HAdV-7 isolated in 2002, 2004 and 2011, respectively, were analysed in this study. The 22 sequences isolated in 2011 were identical, but contained four substitutions at nucleotide positions 771, 1286, 1319 and 1785 not present in the other three sequences from 2002 and 2004. These substitutions included two synonymous (F257 and I595) and two non-synonymous (S429Y and Q440L) amino acid mutations. To compare these 22 strains with 82 others available in GenBank, a phylogenetic tree (Fig. 2 ) was constructed, revealing that these strains were grouped with and identical to 22 other strains, including Taiwanese strains isolated in 2011, Chinese strains isolated in 2009 and 2015, a Russian strain isolated in 2014 (Accession no. KU361344), and a strain from the USA isolated in 2014 (Accession no. KT963081). The three sequences from 2002 and 2004 were identical to a strain isolated in the USA (Accession no. AY601634) in 1997. These findings indicated that the hexon gene sequences isolated from HAdV-7-infected Taiwanese children were highly conserved.
Fig. 2.
Phylogenetic tree for human adenovirus type 7 (HAdV-7) hexon genes. This phylogenetic tree was derived from the HAdV-7 hexon gene and evaluated using 1000 bootstrap replicates. The 25 sequences provided in this study are labelled by their strain names. Among these, 11 severe and 11 non-severe cases are marked with black and white circles, respectively, and three cases isolated in 2002 and 2004 are marked with triangles. Other sequences downloaded from GenBank are labelled by either accession number, location and year or by strain name.
Discussion
HAdV-2, -3 and -7 were the main causative agents of HAdV infections in the 2011 outbreak in Taiwan. Although HAdV-3 was the dominant circulating genotype, HAdV-7 caused more severe clinical symptoms, and HAdV-7-infected children were more likely to exhibit lower respiratory complications and higher mortality rates, especially in those with underlying neurological diseases.
HAdV is a common pathogen associated with acute respiratory infection in children. Previous epidemiological and molecular studies [26], [27] indicated that respiratory syncytial virus was the most common pathogen in paediatric inpatients with acute respiratory infection and, compared with respiratory syncytial virus, human rhinovirus and bocavirus, they also found that HAdV tended to infect older children and was more likely to cause leucocytosis, high CRP levels, longer hospital stays, pneumonia and more frequent antibiotics prescriptions [26]. Among our studied cohort, 41.4% of paediatric patients with acute respiratory infections exhibited HAdV infection, with ∼3.4% exhibiting co-infection identified by viral culture instead of real-time PCR. This might explain the underestimated rate of co-infection; however, our findings might reflect a community outbreak of HAdV, whereas most cases admitted to the hospital were due to more severe clinical presentations. Notably, none of our cases involved a diagnosis of bronchiolitis. This finding might be explained by 75% of the patients being >3 years old, as well as diagnoses by clinical physicians of bacterial infection rather than viral infection due to leucocytosis and high CRP levels.
HAdV-7 was involved in a community outbreak in southern Taiwan from November 1999 to March 2000 [15]. However, over the previous decade, no HAdV-7 outbreaks and only a few HAdV-7 cases during 2008 and 2010 were observed according to surveillance data from CDC-Taiwan [16]. Studies indicated that HAdV-7 was more likely to cause lower respiratory tract involvement and lead to long-term pulmonary sequelae compared with HAdV-3 [28]. This study revealed HAdV-7 re-emergence during the 2011 community outbreak in northern Taiwan.
Compared with those infected with HAdV-2 or HAdV-3, HAdV-7-infected children experienced longer durations of fever and hospital stay and were significantly associated with leucopenia, thrombocytopenia, and impaired liver and renal function. By contrast, HAdV-3 usually leads to leucocytosis and a high serum CRP level [29], [30].
A LRTI was the most common complication associated with HAdV infection during this outbreak. Compared with HAdV-2 and HAdV-3, HAdV-7 was significantly associated with pulmonary complications and intensive care requirements, even in otherwise healthy children. Underlying medical problems, especially neurological diseases, were important risk factors associated with development of respiratory failure and subsequent higher mortality rates. Life-threatening complications were also reported in children with disabilities during an HAdV-7 outbreak in a residential facility for severely disabled children [31]. Lai et al. [17] also reported a total of 45 paediatric patients with HAdV infection that required intensive care in two medical centres, including our hospital, in northern Taiwan in 2010–2011 (16 patients overlapped with this study). Among these, HAdV-7 was the main causative agent (49%) and resulted in higher mortality rates, particularly in those with underlying neurological diseases. Pneumonia was also the most common clinical diagnosis (89%), with half of these patients subsequently developing respiratory failure. A previous study suggested that HAdV-7 is strongly related to severe infections, pneumonia and underlying neurological diseases and is a risk factor for severe HAdV infection [32].
During the 2011 outbreak, HAdV-3 remained the dominant circulating genotype in Taiwan, with >80% of HAdV-3-infected children >3 years old. These children were significantly more likely to manifest upper respiratory tract symptoms. A higher serum CRP level (>40 mg/L) was also noted in nearly 80% of HAdV-3-infected children and mimicked bacterial infection, subsequently resulting in antibiotics prescription for two-thirds of the patients. These findings were consistent with those reported for the 2004 and 2005 HAdV-3 outbreaks in Taiwan [29].
HAdV-7 hexon sequences [25] from Taiwanese patients with clinical symptoms of varying severity were nearly identical with other Taiwanese strains from 2011 and Chinese strains from 2009 and 2015 (Fig. 2). Although one of those Chinese strains caused an outbreak of severe lower respiratory tract disease [13], hexon sequences from 2011 cases were highly conserved [18] and contained only two non-synonymous mutations (S429Y and Q440L) as compared with the Taiwanese strains from 2002 and 2004.
This study has several limitations. First, as a retrospective study, not all clinical information and laboratory data could be collected. Second, nearly all cases (98%) in this study were inpatients, which might result in overestimation of the proportion of HAdV-7 infection and disease severity. Third, co-infection rates might have been underestimated. Virus isolation by tissue culture was used in this study; however, viruses, including human bocavirus, coronavirus and human metapneumovirus, cannot present cytopathogenic effects in selected cells.
Funding
KCT received two grants from Chang Gung Memorial Hospital, Taoyuan, Taiwan (Nos. CLRPG3B0044 and CLRPG3B0045; http://www.cgmh.org.tw), and these two grants partially supported this work. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Transparency declaration
The authors report no conflicts of interest.
Acknowledgements
The authors thank Dr Enzo Emanuele of 2E Science, Italy, for his expert editorial assistance.
Editor: C. Pulcini
Footnotes
Additional Supporting Information may be found in the online version of this article at http://dx.doi.org/10.1016/j.cmi.2016.11.004.
Contributor Information
K.-C. Tsao, Email: kctsao@cgmh.org.tw.
Y.-C. Huang, Email: ychuang@cgmh.org.tw.
Appendix A. Supporting information
The following supplementary materials are available for this article:
Data S1 Materials and methods
Table S1 Primer and probe designs for the human adenoviruses types 2, 3 and 7 hexon genes
Table S2 Virus types from 1598 culture-confirmed cases
Table S3 Distribution of clinical specimens obtained from 632 culture-confirmed adenovirus-infected children
Table S4 Multivariate logistic regression of risk factors for human adenovirus-related lower respiratory tract infections and paediatric intensive care unit admission
Table S5 Clinical diagnosis of children with human adenovirus infections stratified by genotype.
References
- 1.Allen C.W., Alexander S.I. Adenovirus associated haematuria. Arch Dis Child. 2005;90:305–306. doi: 10.1136/adc.2003.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox J.P., Hall C.E., Cooney M.K. The seattle virus watch. Vii. Observations of adenovirus infections. Am J Epidemiol. 1977;105:362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko H., Suzutani T., Aoki K., Kitaichi N., Ishida S., Ishiko H. Epidemiological and virological features of epidemic keratoconjunctivitis due to new human adenovirus type 54 in Japan. Br J Ophthalmol. 2011;95:32–36. doi: 10.1136/bjo.2009.178772. [DOI] [PubMed] [Google Scholar]
- 4.Krajden M., Brown M., Petrasek A., Middleton P.J. Clinical features of adenovirus enteritis: a review of 127 cases. Pediatr Infect Dis J. 1990;9:636–641. [PubMed] [Google Scholar]
- 5.Paduch D.A. Viral lower urinary tract infections. Curr Urol Rep. 2007;8:324–335. doi: 10.1007/s11934-007-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhnoo I., Wadell G., Svensson L., Johansson M.E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984;20:365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson C.M., Singh G., Lee J.Y., Dehghan S., Rajaiya J., Liu E.B. Molecular evolution of human adenoviruses. Sci Rep. 2013;3:1812. doi: 10.1038/srep01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girouard G., Garceau R., Thibault L., Oussedik Y., Bastien N., Li Y. Adenovirus serotype 14 infection, New Brunswick, Canada, 2011. Emerg Infect Dis. 2013;19:119–122. doi: 10.3201/eid1901.120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong J.Y., Lee H.J., Piedra P.A., Choi E.H., Park K.H., Koh Y.Y. Lower respiratory tract infections due to adenovirus in hospitalized korean children: epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001;32:1423–1429. doi: 10.1086/320146. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y.C., Huang S.L., Chen S.P., Huang Y.L., Huang C.G., Tsao K.C. Adenovirus infection associated with central nervous system dysfunction in children. J Clin Virol. 2013;57:300–304. doi: 10.1016/j.jcv.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Lewis P.F., Schmidt M.A., Lu X., Erdman D.D., Campbell M., Thomas A. A community-based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J Infect Dis. 2009;199:1427–1434. doi: 10.1086/598521. [DOI] [PubMed] [Google Scholar]
- 12.Munoz F.M., Piedra P.A., Demmler G.J. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis. 1998;27:1194–1200. doi: 10.1086/514978. [DOI] [PubMed] [Google Scholar]
- 13.Tang L., Wang L., Tan X., Xu W. Adenovirus serotype 7 associated with a severe lower respiratory tract disease outbreak in infants in Shaanxi Province, China. Virol J. 2011;8:23. doi: 10.1186/1743-422X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S.Y., Luo Y.P., Huang D.D., Fan H., Lu Q.B., Wo Y. Fatal pneumonia cases caused by human adenovirus 55 in immunocompetent adults. Infect Dis. 2016;48:40–47. doi: 10.3109/23744235.2015.1055585. [DOI] [PubMed] [Google Scholar]
- 15.Lin K.H., Lin Y.C., Chen H.L., Ke G.M., Chiang C.J., Hwang K.P. A two decade survey of respiratory adenovirus in Taiwan: the reemergence of adenovirus types 7 and 4. J Med Virol. 2004;73:274–279. doi: 10.1002/jmv.20087. [DOI] [PubMed] [Google Scholar]
- 16.Tsou T.P., Tan B.F., Chang H.Y., Chen W.C., Huang Y.P., Lai C.Y. Community outbreak of adenovirus, Taiwan, 2011. Emerg Infect Dis. 2012;18:1825–1832. doi: 10.3201/eid1811.120629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai C.Y., Lee C.J., Lu C.Y., Shao P.L., Wu E.T., Wang C.C. Adenovirus serotype 3 and 7 infection with acute respiratory failure in children in Taiwan, 2010–2011. PloS One. 2013;8:e53614. doi: 10.1371/journal.pone.0053614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung K.H., Lin L.H. Adenovirus pneumonia complicated with acute respiratory distress syndrome: a case report. Medicine. 2015;94:e776. doi: 10.1097/MD.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu X., Trujillo-Lopez E., Lott L., Erdman D.D. Quantitative real-time PCR assay panel for detection and type-specific identification of epidemic respiratory human adenoviruses. J Clin Microbiol. 2013;51:1089–1093. doi: 10.1128/JCM.03297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong S., Pabbaraju K., Pang X.L., Lee B.E., Fox J.D. Detection of a broad range of human adenoviruses in respiratory tract samples using a sensitive multiplex real-time PCR assay. J Med Virol. 2008;80:856–865. doi: 10.1002/jmv.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto D., Okamoto M., Lupisan S., Suzuki A., Saito M., Tamaki R. Impact of human adenovirus serotype 7 in hospitalized children with severe fatal pneumonia in the Philippines. Jpn J Infect Dis. 2014;67:105–110. doi: 10.7883/yoken.67.105. [DOI] [PubMed] [Google Scholar]
- 22.Lu X., Erdman D.D. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch Virol. 2006;151:1587–1602. doi: 10.1007/s00705-005-0722-7. [DOI] [PubMed] [Google Scholar]
- 23.Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W. Fast, scalable generation of high-quality protein multiple sequence alignments using CLUSTAL omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa M., Kishino H., Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S., Stecher G., Tamura K. Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvo C., Garcia-Garcia M.L., Sanchez-Dehesa R., Román C., Tabares A., Pozo F. Eight year prospective study of adenoviruses infections in hospitalized children. Comparison with other respiratory viruses. PloS One. 2015;10:e0132162. doi: 10.1371/journal.pone.0132162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y., Liu F., Wang C., Zhao M., Deng L., Zhong J. Molecular identification and epidemiological features of human adenoviruses associated with acute respiratory infections in hospitalized children in southern China, 2012–2013. PloS One. 2016;11:e0155412. doi: 10.1371/journal.pone.0155412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callaway Z., Kim S.H., Kim J.Y., Kim D.W., Kim C.K. Adenovirus infection with serious pulmonary sequelae in korean children. Clin Respir J. 2011;5:92–98. doi: 10.1111/j.1752-699X.2010.00204.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang S.Y., Lee C.N., Lin P.H., Huang H.H., Chang L.Y., Ko W. A community-derived outbreak of adenovirus type 3 in children in Taiwan between 2004 and 2005. J Med Virol. 2008;80:102–112. doi: 10.1002/jmv.21045. [DOI] [PubMed] [Google Scholar]
- 30.Lin C.H., Huang Y.C., Chiu C.H., Huang C.G., Tsao K.C., Lin T.Y. A cluster of adenovirus serotype 3 infections in children in northern Taiwan: clinical features and laboratory findings. J Microbiol Immunol Infect. 2007;40:302–309. [PubMed] [Google Scholar]
- 31.Ghanaiem H., Averbuch D., Koplewitz B.Z., Yatsiv I., Braun J., Dehtyar N. An outbreak of adenovirus type 7 in a residential facility for severely disabled children. Pediatr Infect Dis J. 2011;30:948–952. doi: 10.1097/INF.0b013e31822702fe. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J.L., Peng C.C., Chiu N.C., Weng L.C., Chiu Y.Y., Chang L. Risk factor analysis and molecular epidemiology of respiratory adenovirus infections among children in northern Taiwan, 2009–2013. J Microbiol Immunol Infect. 2015 doi: 10.1016/j.jmii.2015.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.