Abstract
A 5-year-old castrated male domestic longhair cat was presented with neurological signs consistent with a central vestibular lesion and left Horner's syndrome. Computed tomography images revealed hyperattenuating, moderately contrast-enhancing material within the left tympanic bulla, most consistent with left otitis media/interna. Marked neutrophilic pleocytosis was identified on cerebrospinal fluid analysis. Streptococcus equi subspecies zooepidemicus (SEZ) was isolated from the cerebrospinal fluid. Intracranial extension of otitis media/interna is relatively infrequent in small animals. There are no reports of otitis media/interna caused by SEZ in dogs or cats. This is the first report of otitis media/interna and presumptive secondary meningoencephalitis caused by SEZ in a cat.
A 5-year-old castrated male domestic longhair cat was presented to the Ohio State University Veterinary Medical Center for evaluation of a 12-h history of falling over. The cat lived exclusively indoors, and was current on vaccines.
Physical and neurological examination revealed dull mentation, a left-sided head tilt and vestibular ataxia, characterized by falling to the left when walking. Cranial nerve examination revealed miosis, ptosis, enophthalmos and third eyelid protrusion on the left eye (OS), consistent with complete Horner's syndrome. There was also ventral strabismus OS, non-positional, rotary nystagmus of both eyes with fast phase to the right, and absent physiologic nystagmus when turning the head to the left. The remainder of the cranial nerve examination was unremarkable. Postural reactions were mildly delayed in the right thoracic and pelvic limbs. Spinal reflexes and cutaneous trunci were normal. Spinal palpation revealed mild lumbar discomfort. Based on these findings, a multifocal neurolocalization was suspected. A left vestibular lesion with both peripheral and central involvement was considered likely. The presence of concurrent Horner's syndrome OS and left-sided vestibular signs suggested involvement of the left middle/inner ear and the presence of dull mentation was supportive of central disease. Also, extension across midline with involvement of the right side of the brainstem was considered possible, due to the presence of right-sided postural reaction deficits. Differential diagnoses included otitis media/interna (OMI), nasopharyngeal polyp, neoplasia, infectious diseases (toxoplasmosis, cryptococcosis, feline infectious peritonitis (FIP), bacterial) and cerebrovascular event.
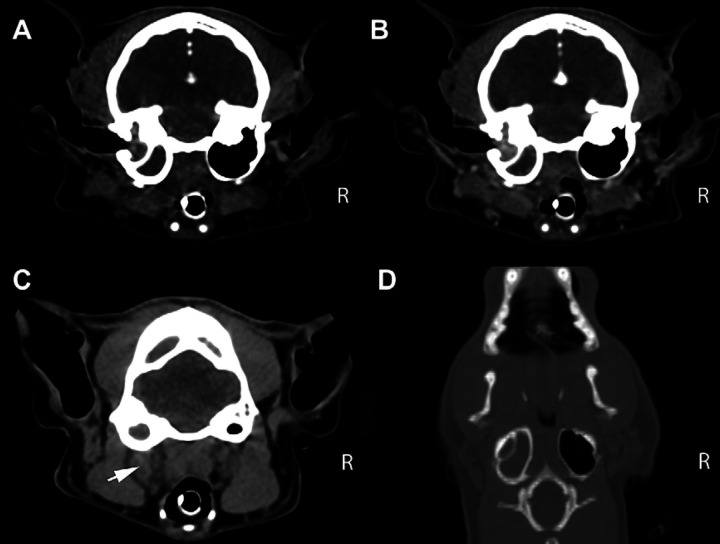
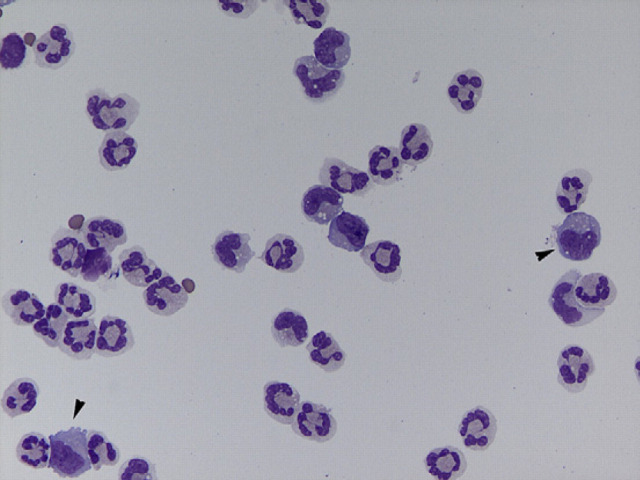
A complete blood count showed leukocytosis (15.8 × 109/l, reference interval (RI) 4.0–14.5 × 109/l), mature neutrophilia (13.1 × 109/l, RI 3–9.2 × 109/l) and monocytosis (1.3 × 109/l, RI 0–0.5 × 109/l), consistent with an inflammatory leukogram. A biochemical profile revealed increased total protein (7.9 g/dl, RI 5.6–7.6 g/dl) due to hyperglobulinemia (4.8 g/dl, RI 3.1–4.1 mg/dl). Enzyme-linked immunosorbent assays (ELISAs) (Idexx, Westbrook, ME, USA) for feline leukemia virus antigen and antibody against feline immunodeficiency virus were negative. Non-invasive Doppler blood pressure and thoracic radiographs were normal. Ophthalmologic examination revealed a normal fundus. Otoscopic examination revealed intact, translucent tympanic membranes bilaterally. Cytology of the external ear canal was normal. The cat was anesthetized for computed tomography (CT) (GE Lightspeed Ultra) of the brain and cerebrospinal fluid (CSF) collection. Anesthesia consisted of intramuscular dexmedetomidine (20 μg/kg), induction with intravenous propofol (2 mg/kg) and maintenance with inhalatory isofluorane and oxygen using mechanical ventilation. The CT study consisted of 1.3 mm contiguous transverse acquisitions, pre- and post-contrast administration (iohexol, Omnipaque 240 mg/ml, dose: 2 ml/kg IV). Hyperattenuating material was noted completely filling the left tympanic bulla, with mild contrast enhancement after iohexol administration (Fig 1). The left retropharyngeal lymph node was mildly enlarged (Fig 1C). No brain parenchyma abnormalities were noted. However, CT has inherent limitations when imaging soft tissues, so the lack of brain parenchyma abnormalities in this case may have been related to the limitations of this imaging modality. 1 The differentials considered were left OMI or a polyp, with neoplasia considered less likely based on the presence of only mild contrast enhancement and no lytic lesions. CSF was collected from the cerebellomedullary cistern. The fluid was colorless and slightly hazy, with a total protein of 19.9 mg/dl (RI<25 mg/dl), a white blood cell (WBC) count of 1368 cells/μl (RI<5 cells/μl) and a red blood cell (RBC) count of 99/μl (RI<5 cells/μl). Cytology revealed 61% non-degenerate neutrophils, 27% large mononuclear cells and 12% lymphocytes (Fig 2). The large mononuclear cells were vacuolated and interpreted as reactive. The lymphocytes were small and well differentiated. No evidence of hemosiderin, erythrophagia, etiologic agents or neoplastic cells was seen. The findings were consistent with a neutrophilic pleocytosis with mild blood contamination. Based on the combination of the CT images and CSF results, a bacterial meningoencephalitis secondary to extension of OMI was considered the most likely presumptive diagnosis. Other differentials included cryptococcosis, viral infection (feline infectious peritonitis) and toxoplasmosis.
Fig 1.
(A) Transverse pre-contrast image at the level of the tympanic bulla. Note the hyperattenuating material completely filling the left tympanic bulla. No lytic lesions were noted. No abnormalities were noted involving the brain parenchyma. (B) Transverse post-contrast image, obtained at the same level as image (A), which shows mild contrast enhancement of the material present within the left tympanic bulla. (C) Transverse pre-contrast image at the most caudal level of the tympanic bulla. Note the enlargement of the left retropharyngeal lymph node (white arrow). (D) Dorsal pre-contrast reconstruction at the level of the tympanic bulla, showing hyperattenuating material completely filling the left tympanic bulla.
Fig 2.
Cerebrospinal fluid cytology obtained from the cerebellomedullary cistern. Note the predominant cell population of non-degenerate neutrophils. Occasional large mononuclear cells with vacuolated cytoplasm were also present (arrowheads). No etiologic agents are seen (Cytocentrifugation and Diff Quick staining, 50×).
Cerebrospinal fluid was cultured on trypticase soy agar with 5% sheep's blood (TSAII, Becton-Dickenson, NJ, USA) and incubated at 35°C in 5% CO2; reduced thioglycolate broth (Becton-Dickenson, NJ, USA) was inoculated to recover fastidious organisms and anaerobes. A polymerase chain reaction (PCR) was performed for Toxoplasma gondii, feline coronavirus and feline leukemia virus.
Streptococcus equi subspecies zooepidemicus (SEZ) was isolated from the CSF in high numbers in pure culture. The organism was speciated using Lancefield grouping (Streptocard, Becton-Dickenson, NJ, USA) and conventional biochemicals (API-20 STREP System, Biomerieux, MO, USA). PCR results were negative. Cryptococcus species antigen enzyme immunoassay was negative.
The cat recovered uneventfully from anesthesia. Treatment was initiated with ampicillin–sulbactam (30 mg/kg IV q 8 h, Unasyn; Pfizer), enrofloxacin (5 mg/kg IV q 24 h, Baytril; Bayer), dexamethasone-sodium phosphate (0.15 mg/kg IV q 24 h for 2 days) and famotidine (0.5 mg/kg PO q 12 h), pending culture results. The day after presentation the cat developed severe hypersensitivity to light, touch and sound, and self-inflicted multiple bite wounds to his limbs. The cat was started on a dexmedetomidine constant rate infusion (3 μg/kg/h IV) for sedation, which was slowly weaned off over the next 8 h. The intravenous catheter was removed on the third day of hospitalization because of poor patient tolerance. After obtaining the culture results, the cat was started on trimethoprim–sulfamethoxazole (TMS–SMZ, 15 mg/kg PO q 12 h), as treatment for the meningoencephalitis, and amoxicillin–clavulanic acid (62.5 mg PO q 12 h, Clavamox; Pfizer) to prevent infection from the self-inflicted bite wounds. The neurologic status of the patient improved gradually. A left ventral bulla osteotomy was performed 5 days after presentation. A large amount of purulent material was removed from the bulla. Histopathology revealed marked suppurative and lymphoplasmacytic otitis media with no signs of a polyp. No etiologic agents were noted but the inflammation was suggestive of a chronic bacterial infection. Aerobic, anaerobic and Mycoplasma species cultures of the material removed from the bulla were negative. A second cerebellomedullary cistern CSF sample was obtained at the time of surgery. The fluid was colorless and clear, with a total protein of 8.0 mg/dl (RI<25 mg/dl), a WBC count of 7 cells/μl (RI<5 cells/μl) and an RBC count of 3/μl (RI<5 cells/μl). Cytology showed 2% non-degenerate neutrophils, 3% large mononuclear cells and 95% lymphocytes. The results of the second CSF showed marked improvement (7 versus 1368 WBC/μl) in the magnitude of the pleocytosis. The cat recovered uneventfully from surgery. A severe left Horner's syndrome was noted postoperatively, which along with the rest of the neurological signs, gradually improved and resolved over the following weeks. The total duration of TMS–SMZ therapy was 8 weeks. Upon last contact with the owners 8 months after diagnosis, the cat remained neurologically normal.
Central nervous system (CNS) complications of OMI have been recognized in animals, although they are considered uncommon. 1–3 In people, the incidence of these complications has decreased with the wider availability of antibiotics; however, they are still associated with mortality rates ranging from 5 to 31%. 4–7 The case reported here made a full recovery. A variety of organisms have been isolated from the few feline cases of intracranial extension of OMI reported to date, including Pasteurella multocida, Escherichia coli, Enterococcus species, Staphylococcus aureus, Mycoplasma species, and Streptococcus canis. 1
Streptococcus equi subsp zooepidemicus is considered a commensal organism of the mucous membranes and skin of various animals, notably horses. 8–11 It frequently acts opportunistically in horses, causing respiratory infections, wound infections, endometritis, and abortion. 8,10 This bacterium is not regarded as a component of the commensal flora of neither dogs nor cats. 10,12 Over the last few years, SEZ has been reported as an emerging pathogen in dogs, associated with severe hemorrhagic pneumonia in shelter dogs. 9,13 Only recently, two reports have documented infections caused by SEZ in cats. 10,11 One report described an outbreak of respiratory disease in a cattery. 11 Four of the cats necropsied showed signs of pyogranulomatous meningoencephalitis. 11 The other report described two cases of rhinitis and meningitis caused by SEZ in two cats housed in separate shelters. 10 Neither of the two cats or their attendants had any known exposure to horses. 10 In our case, no exposure to horses or farm animals was identified upon questioning the owner. As there were no clinical signs or history of otitis externa, it is likely that the route of infection into the middle/inner ear was via the oral mucosa and/or the nasopharynx. Negative bacterial culture from the tympanic bulla is likely due to the 5 days of antimicrobial therapy given to the cat between the original CSF collection and the bulla osteotomy.
Infection with SEZ is a rare cause of meningitis in humans with only 22 cases reported so far. 14,15 The majority of these cases were caused by contact with animals (mostly horses) or ingestion of unpasteurized dairy products. The reported mortality rate was 24%. 14
In this case, antimicrobial therapy using TMS–SMZ was elected. This is a bactericidal drug that penetrates both normal and inflamed meninges and achieves therapeutic levels in the CSF. 16 Two doses of intravenous dexamethasone were also administered, starting with the first dose of antimicrobials. In spite of the controversy regarding the use of steroids in bacterial meningitis, 1,2,17 we elected to use it in our patient following the most current recommendations for treatment of acute bacterial meningitis in people. 17 A recent meta-analysis, which reviewed 24 randomized controlled trials of corticosteroids use for acute bacterial meningitis in people, revealed a lower rate of short-term neurologic sequelae and a trend toward lower mortality in the corticosteroid-treated group in adults in high-income countries. 17
To the authors’ knowledge, this is the first report of OMI and secondary meningoencephalitis caused by SEZ in a cat. Clinicians should be aware of the rare zoonotic disease potential of this agent, which seems to be an emerging pathogen in small animal companion species.
References
- 1. Sturges B.K., Dickinson P.J., Kortz G.D., et al. Clinical signs, magnetic resonance imaging features, and outcome after surgical and medical treatment of otogenic intracranial infection in 11 cats and 4 dogs, J Vet Intern Med 20, 2006, 648–656. [DOI] [PubMed] [Google Scholar]
- 2. Spangler E.A., Dewey C.W. Meningoencephalitis secondary to bacterial otitis media/interna in a dog, J Am Anim Hosp Assoc 36, 2000, 239–243. [DOI] [PubMed] [Google Scholar]
- 3. Iverson W.O., Popp J.A. Meningoencephalitis secondary to otitis in a gorilla, J Am Vet Med Assoc 173, 1978, 1134–1136. [PubMed] [Google Scholar]
- 4. Penido N.O., Borin A., Iha L.C.N., et al. Intracranial complications of otitis media: 15 years of experience in 33 patients, Otolaryngol Head Neck Surg 132, 2005, 37–42. [DOI] [PubMed] [Google Scholar]
- 5. Dubey S.P., Larawin V. Complications of chronic suppurative otitis media and their management, Laryngoscope 117, 2007, 264–267. [DOI] [PubMed] [Google Scholar]
- 6. Dubey S.P., Larawin V., Molumi C.P. Intracranial spread of chronic middle ear suppuration, Am J Otolaryngol Head Neck Med Surg 31, 2010, 73–77. [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim S.I., Cheang P.P., Nunez D.A. Incidence of meningitis secondary to suppurative otitis media in adults, J Laryngol Otol 124, 2010, 1158–1161. [DOI] [PubMed] [Google Scholar]
- 8. Acke E., Abbott Y., Pinilla M., Markey B.K., Leonard F.C. Isolation of Streptococcus zooepidemicus from three dogs in close contact with horses, Vet Rec 167, 2010, 102–103. [DOI] [PubMed] [Google Scholar]
- 9. Pesavento P.A., Hurley K.F., Bannasch M.J., Artiushin S., Timoney J.F. A clonal outbreak of acute fatal hemorrhagic pneumonia in intensively housed (shelter) dogs caused by Streptococcus equi subsp zooepidemicus , Vet Pathol 45, 2008, 51–53. [DOI] [PubMed] [Google Scholar]
- 10. Britton A.P., Davies J.L. Rhinitis and meningitis in two shelter cats caused by Streptococcus equi subspecies zooepidemicus , J Comp Path 143, 2010, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blum S., Elad D., Zukin N., et al. Outbreak of Streptococcus equi subsp zooepidemicus infections in cats, Vet Microbiol 144, 2010, 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Devriese L.A., Colque J.I. Cruz, De Herdt P., Haesebrouck F. Identification and composition of the tonsillar and anal enterococcal and streptococcal flora of dogs and cats, J Appl Bacteriol 73, 1992, 421–425. [DOI] [PubMed] [Google Scholar]
- 13. Priestnall S.L., Erles K., Brooks H.W., et al. Characterization of pneumonia due to Streptococcus equi subsp zooepidemicus in dogs, Clin Vaccine Immunol 17, 2010, 1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eyre D.W., Kenkre J.S., Bowler J.W., McBride S.J. Streptococcus equi subspecies zooepidemicus – a case report and review of the literature, Eur J Clin Microbiol Infect Dis 29, 2010, 1459–1463. [DOI] [PubMed] [Google Scholar]
- 15. Minces L.R., Brown P.J., Veldkamp P.J. Human meningitis from Streptococcus equi subsp zooepidemicus acquired as zoonoses, Epidemiol Infect 139, 2011, 406–410. [DOI] [PubMed] [Google Scholar]
- 16. Kent M. Bacterial infections of the central nervous system. Greene C.E. Infectious diseases of the dog and cat, 3rd edn, 2006, Elsevier: St Louis, 962–974. [Google Scholar]
- 17. Brouwer M.C., McIntyre P., de Gans J., Prasad K., van de Beek D. Corticosteroids for acute bacterial meningitis, Cochrane Database Syst Rev 8, 2010, CD004405 [DOI] [PubMed] [Google Scholar]