Abstract
The efficacy of gaseous disinfection is critical for prevention and treatment of microbial contamination in biotechnological facilities. For an evaluation of gaseous disinfection efficacy, a down-scaled laboratory model was established, using currently available carrier tests and a custom-made dry fog box. A mixture of peroxyacetic acid and hydrogen peroxide (PAA/HP) was investigated as example, at concentrations between 0.4 and 2.9 mL/m3 for up to 3 h for inactivation of a panel of lipid-enveloped and non-lipid-enveloped viruses. The influenza viruses were most sensitive to PAA/HP treatment and minute virus of mice was most resistant. Bovine viral diarrhea virus and reovirus III showed intermediate stability and similar inactivation kinetics. Use of the dry fog box circumvents dedicating an entire lab for the investigation, which renders the generation of data more cost-effective and allows for production of highly reproducible kinetic data.
Keywords: Gaseous disinfection, Dry fog box, Carrier test, Peroxyacetic acid, Hydrogen peroxide, Minute virus of mice
Abbreviations: PAA/HP, peroxyacetic acid/hydrogen peroxide; BVDV, bovine viral diarrhea virus; Reo III, respiratory enteric orphan virus type III; MVM, minute virus of mice; H3N2, influenza A H3N2 virus; H5N1, influenza A H5N1 virus; Flu B, influenza B virus; TCID50, median tissue culture infectious dose 50%; rH, relative humidity
1. Introduction
Gaseous disinfection is a procedure commonly used to inactivate microbes in biotechnological manufacturing facilities and laboratories. The disinfectants should be effective against all relevant microbial agents; however, information supplied by disinfectant manufacturers does not necessarily cover targeted microbes. This lack of information about relevant targets has prompted a variety of past investigations on disinfectant effectiveness, which were typically performed directly in the laboratory environment [1], [2], [3], [4], [5]. The resulting data was rather limited, as the experimental setup did not allow to obtain sequential samples at different times during the inactivation run (kinetic samples) for an assessment of virus inactivation, as requested by regulatory guidance [6], [7]. Additionally, a complete laboratory shutdown was required, which is costly and time consuming. These constraints were circumvented by performing small-scale evaluations using biosafety class III cabinets that were connected to vapor generators [8], [9]. Biosafety class III cabinets however, are only rarely available. As an alternative and more practical approach we established a robust, simple and cost-effective down-scaled investigation procedure, which simulates the practical conditions around biotechnology applications, i.e. in manufacturing facilities as well as laboratory units that allows for kinetic investigation of gaseous disinfectant procedures. Using a custom made Dry Fog box and currently available carrier tests [10], the virus inactivation capacity of the Minncare ® Dry Fog decontamination system that uses a mixture of peroxyacetic acid and hydrogen peroxide (PAA/HP) was investigated with a panel of viruses, including the lipid-enveloped viruses bovine viral diarrhea virus (BVDV) and influenza A and B viruses, as well as the non-lipid-enveloped respiratory enteric orphan virus type III (Reo III) and the parvovirus minute virus of mice (MVM).
2. Materials and methods
2.1. Viruses, cells and infectivity assay
Bovine viral diarrhea virus (BVDV, strain Nadl; ATCC, Rockville, Maryland) was titrated on BT cells (ATCC). Influenza A H3N2 virus (H3N2; strain Victoria; WHO Collaborating Centre for Reference and Research on Influenza, Melbourne, Victoria, Australia) and influenza B virus (Flu B; strain Hubei-Wujiagang; NIBSC, Potters Bar, UK) were titrated on MDCK cells (ATCC). Influenza A H5N1 virus (H5N1; strain Vietnam; CDC, Atlanta, Georgia) and respiratory enteric orphan virus type III (Reo III; strain Dearing; ATCC) were titrated on Vero cells (ATCC). Minute virus of mice (MVM; strain Prototype; ATCC) was titrated on A9 cells (ATCC).
Infectious virus titers were determined by median tissue culture infectious dose assay (TCID50), using eightfold replicates of serial half-log sample dilutions of virus-containing samples that were titrated on the cell lines indicated above. The cells were incubated at 36 °C, before the cytopathic effect was evaluated by visual inspection under an inverted microscope (Nikon Eclipse TS100). TCID50 titers were calculated according to the Poisson distribution and expressed as log10 [TCID50/mL]. Virus reduction factors were calculated in accordance to the EU Committee for Proprietary Medicinal Products guidance [7], from at least two independent runs.
2.2. Dry fog box and data acquisition
The in-house custom made Dry Fog box consists of an acrylic glass box (Fig. 1 ), which is designed to fit into a common laminar flow bench class II (outer dimensions: 1 m × 0.5 m × 0.5 m). The box has a lateral manipulation opening and three rubber-sealed slots at the bottom front side including three movable carrier slides with twelve sample holders each. Two ventilators inside the box ensure the homogenous distribution of the disinfectant which was confirmed by measurements of relative humidity (rH) that did not vary by more than 3% between the different recorders. Temperature and relative humidity was recorded by four TS Pro2 X logger units and a Tracksense Pro Sky Access Point module. Data monitoring was done with the validated software ValSuitePro (Ellab A/S; Hilleroed, Denmark).
Fig. 1.
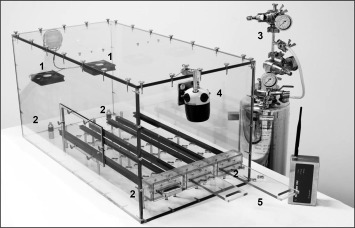
Custom-made dry fog box. Numbers indicate the ventilators (1), the TS Pro2 X Logger –Units (2), the Dry Fog generating unit with its 3 manometers (3), the diffusion head of the Dry Fog unit (4), and the slipcase with sample holders (5).
2.3. Gaseous disinfection
For all runs, 50 μL of virus stock suspension, as described in ASTM standard E2197-11 [10], was dried two to 4 h on the center point of e-polished stainless steel carriers (Ø 2 cm; AISI type 316L; Baumgartner & Co GmbH, Vienna, Austria) and transferred into the Dry Fog box. A Minntech® Dry Fog unit (Cantel Medical Corp./Mar Cor, Little Falls, NJ), was connected to the diffusion head via a magnetic valve for regulation of 10% aqueous Minncare ® Cold Sterilant, containing peroxyacetic acid (PAA, 4.5%) and hydrogen peroxide (HP, 22.0%), that was released into the box. PAA/HP solution was applied in four different run designs (I–IV), at increasing concentrations ranging from 0.4 mL/m3 (run design I) to 2.9 mL/m3 (run design IV). PAA/HP solution concentrations were calculated according to the Excel spreadsheet “Minncare DRY FOG Calculations” provided by Minncare®. During the first 59 min of run design II (0.8 mL PAA/HP solution/m3) re-fogging was performed as soon as the rH decreased by 5%. Kinetic samples were drawn after 5 min, 10 min, 30 min, 59 min by pulling the sample holder out of the Dry Fog box, vortexing the metal carriers at 600 rpm for 30 s in plastic tubes containing sterile glass beads (Ø 0.5 mm) and 1 mL of appropriate cell culture medium, similar to Doerrbecker et al. [11]. For the non-lipid enveloped viruses additional samples were drawn after 114 min and 174 min. The supernatant was immediately used for TCID50 titration.
3. Results
Initially, the recovery of dried virus infectivity from the stainless steel carriers was investigated. In relation to the input virus titer applied to the stainless steel carriers, the recovery titer was reduced by 2.1 and 0.7 log10 TCID50/mL (mean, n = 6) for H5N1 and BVDV respectively. Reo III (n = 6) and MVM (n = 10) remained unchanged. The latter results indicated that virus infectivity could be completely recovered and that the drying on stainless steel had no impact on MVM and Reo III infectivity.
Dry Fog disinfection using a PAA/HP solution at 0.4 mL/m3 (run design I) revealed rapid inactivation of all influenza viruses investigated (H5N1, H3N2 and Flu B), to a titer below the detection limit after 5 min of treatment in 8 out of 12 sample series of the virus inactivation runs performed (Table 1 A). In contrast, BVDV was inactivated at the same disinfectant concentration after 59 min treatment by merely 1.6 log10 (Table 1A, Fig. 2 A). Doubling the amount of PAA/HP solution (0.8 mL/m3, run design II) resulted in inactivation of BVDV by 2.6 log10 after the total inactivation time of 59 min (Table 1A, Fig. 2A). Similar inactivation kinetics were found for Reo III, of which 1.9 log10 virus infectivity were inactivated after 59 min treatment with run design I and 1.6 log10 at the higher disinfectant concentration (run design II; Table 1B, Fig. 2B). After the complete inactivation period of 174 min Reo III was inactivated by 2.3 log10 in run design I and 2.1 log10 in run design II, respectively (Table 1B, Fig. 2B). MVM was only inactivated by 1.1 log10 at the low PAA/HP solution amount in run design I after 174 min (Table 1B, Fig. 2C). Application of a PAA/HP solution amount of 0.8 mL/m3 including repeated re-fogging to maintain a nearly constant PAA/HP concentration during the first 59 min treatment showed inactivation of 1.6 log10 MVM (Fig. 2C). Inactivation to below the detection limit could be achieved with MVM after 174 min treatment with 2.9 mL/m3 PAA/HP solution (run design IV; Table 1B, Fig. 2C).
Table 1.
Virus inactivation by PAA/HP treatment: Mean virus titers and 95% C.I. values are given in log10 (TCID50/mL). “Virus Control” is the recovered virus titer obtained after the drying phase. (a) Titers at this sampling stage were used for calculation of the log10 reduction factor. (b) For the influenza viruses H5N1, H3N2 and FLU B the virus titer calculated from successive negative samples is given where no viral infectivity was detected in successive kinetic samples up until the final sample, the volume of all successive negative samples were taken into account for calculation of the assay detection limit. A) lipid-enveloped viruses, B) non-enveloped viruses. n = number of sample series; n.a. = not applicable.
| A | ||||||
|---|---|---|---|---|---|---|
| Sampling stage | Virus |
H5N1 |
H3N2 |
Flu B |
BVDV |
|
| Run design (PAA/HP [mL/m3]; n) | I (0.4; 4) | I (0.4; 4) | I (0.4; 4) | I (0.4; 4) | II (0.8; 2) | |
| Virus control (a) | 4.3 ± 0.3 | 3.7 ± 0.3 | 3.7 ± 0.3 | 5.5 ± 0.3 | 5.8 ± 0.3 | |
| 5 min | <1.1 + 0.6 | 1.1 ± 0.9 | <1.1 + 0.6 | 5.2 ± 0.3 | 5.3 ± 0.3 | |
| 10 min | 0.6 ± 0.9 | 1.1 ± 0.9 | <1.1 + 0.6 | 4.8 ± 0.3 | 5.1 ± 0.3 | |
| 30 min | <0.6 + 0.6 | 1.2 ± 0.9 | <1.1 + 0.6 | 4.0 ± 0.3 | 4.1 ± 0.3 | |
| 59 min | <0.6 + 0.6 | <1.1 + 0.6 | <1.1 + 0.6 | 3.9 ± 0.3(a) | 3.1 ± 0.3(a) | |
| Virus titer calculated from the cumulative volume of successive negative samples (a) (b) |
<0.2 + 0.6 |
<0.8 + 0.6 |
<0.5 + 0.6 |
n.a. |
n.a. |
|
| Virus reduction factor (95% C.I./SD) | >4.1 (−0.6/0.0) | >2.9 (−0.6/0.0) | >3.2 (−0.7/0.0) | 1.6 (±0.4/0.2) | 2.6 (±0.4/0.1) | |
| B | |||||||
|---|---|---|---|---|---|---|---|
| Sampling stage | Virus |
Reo III |
MVM |
||||
| Run design (PAA/HP [mL/m3]; n) | I (0.4; 4) | II (0.8; 2) | I (0.4; 4) | II (0.8; 2) | III (1.4; 2) | IV (2.9; 2) | |
| Virus control (a) | 5.9 ± 0.3 | 5.8 ± 0.3 | 6.7 ± 0.3 | 6.1 ± 0.3 | 6.1 ± 0.3 | 6.1 ± 0.2 | |
| 5 min | 4.6 ± 0.3 | 4.6 ± 0.3 | 6.7 ± 0.3 | 5.7 ± 0.3 | 5.6 ± 0.3 | 5.3 ± 0.3 | |
| 10 min | 4.2 ± 0.3 | 4.2 ± 0.3 | 6.4 ± 0.2 | 5.7 ± 0.3 | 5.2 ± 0.3 | 4.8 ± 0.3 | |
| 30 min | 3.9 ± 0.3 | 4.0 ± 0.3 | 6.3 ± 0.3 | 5.0 ± 0.3 | 4.1 ± 0.3 | 3.7 ± 0.3 | |
| 59 min | 4.0 ± 0.3 | 4.2 ± 0.3 | 6.0 ± 0.3 | 4.7 ± 0.3 | 4.0 ± 0.3 | 2.5 ± 0.4 | |
| 114 min | 3.8 ± 0.3 | 3.9 ± 0.3 | 5.8 ± 0.3 | 4.7 ± 0.2 | 3.1 ± 0.3 | 1.8 ± 0.6 | |
| 174 min (a) |
3.6 ± 0.3 |
3.7 ± 0.3 |
5.7 ± 0.3 |
4.5 ± 0.3 |
3.1 ± 0.3 |
<1.6 + 0.6 |
|
| Virus reduction factor (95% C.I./SD) | 2.3 (±0.4/0.2) | 2.1 (±0.4/0.2) | 1.1 (±0.4/0.1) | 1.6 (±0.4/0.1) | 3.0 (±0.4/0.1) | >4.6 (−0.7/0.0) | |
Fig. 2.
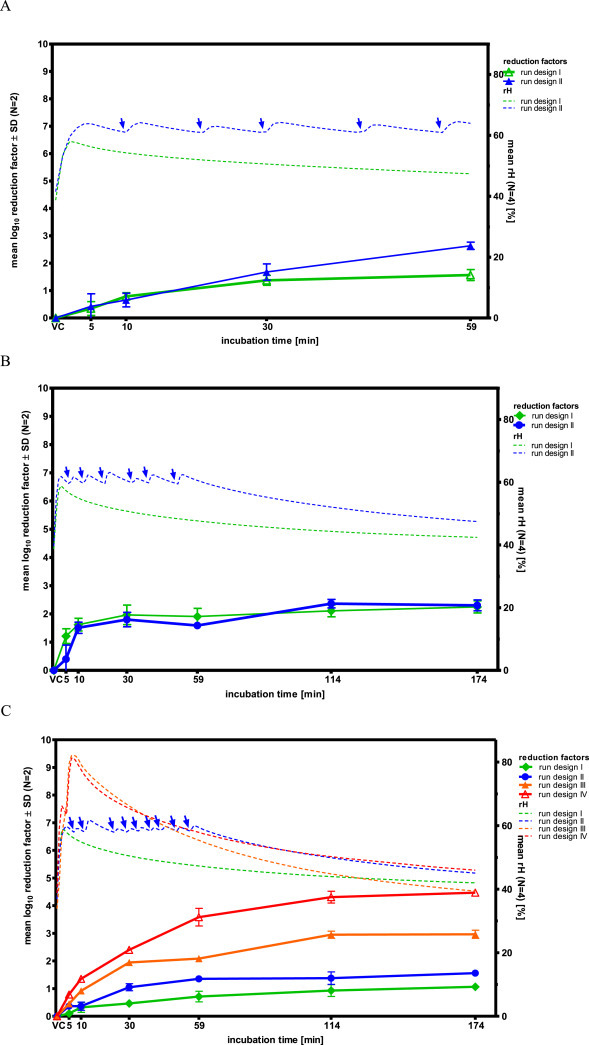
Virus reduction kinetics and relative humidity during PAA/HP treatment: A) BVDV, B) Reo III, C) MVM. Blue arrows indicate re-fogging during run design II. VC indicates “Virus control”, i.e. 0 min incubation.
For an assessment of the accuracy of the experimental setup, the variability of results was evaluated by comparing distinct reduction factors obtained with the same virus and run design, respectively. The variability of reduction factors ranged between 0.1 log10 (Reo III, run design II (n = 2)) and 0.4 log10 (BVDV, run design I (n = 4)), and the mean overall reduction factor variability was 0.2 log10 (n = 7).
4. Discussion
The panel of viruses for this study were chosen to represent on one hand target viruses, i.e. the three influenza strains, due to their importance as human/animal pathogens in general and on the other hand model viruses, i.e. BVDV, Reo III and MVM, which are recommended by regulatory guidelines for virus clearance studies [6], [7]. Since Reo III and MVM both have already been reported to have contaminated biotechnological processes, they are relevant target viruses, too [12], [13].
Gaseous disinfection of different viruses dried on stainless steel using PAA/HP showed highly variable virus inactivation efficiency: the influenza viruses were most sensitive and MVM the least sensitive against PAA/HP treatment. However, the lipid-enveloped BVDV and non-enveloped Reo III were comparably stable, indicating that non-enveloped viruses might not necessarily be more stable than lipid-enveloped viruses, as often assumed [14], [15].
Within this study, PAA/HP solution concentrations between 0.4 and 2.9 mL/m3 were applied for virus inactivation for up to 174 min, covering the manufacturer's recommendations to use 1.5 mL/m3 PAA/HP solution for 60 min [16], i.e. run design III in this study. Data on virus inactivation provided by the manufacturer show effective inactivation of poliovirus, human coronavirus and human immunodeficiency virus by use of suspension tests only [17], which are however not meaningful regarding the application of dry fog, underlining the requirement for the conduct of relevant disinfectant efficacy studies. We found that already lower PAA/HP concentrations (run design I) were sufficient for inactivation of influenza viruses, but MVM could be only partially inactivated during run design III; inactivation to below the detection limit (i.e. > 4.6 log10) required a 174 min treatment and elevated PAA/HP solution concentration (run design IV: 2.9 mL/m3). If MVM was the target virus, i.e. after contamination of a biotechnology facility with specifically this virus [12], an extended disinfection period would have to be considered. An earlier investigation had shown inactivation of Reo III and MVM to below the detection limit after 60 min exposure time, applying only 2 mL/m3 PAA/HP solution of roughly a 25% higher concentration [1]. These data are generally comparable to our findings, however a clear comparison is not possible as drawing of kinetic samples was not feasible within the limitations of the whole laboratory fogging set-up chosen, and consequently safety margins of the inactivation approach could not be assessed.
Conflict of interest
All authors are employees of Baxalta. JM and TRK have stock interest in Baxalta.
Acknowledgments
The contributions of the entire Pathogen Safety team, most notably Markus Weber (data acquisition and monitoring), Veronika Sulzer, Sabrina Brandtner, Sonja Kurzmann, Cornelia Lackner (cell culture, virus propagation), Christian Medek, Eva Ha, and Florian Kaiser, (equipment) are herewith gratefully acknowledged.
The supplier of the Minntech® Dry Fog unit (Cantel Medical Corp./Mar Cor) was not involved in this study.
Contributor Information
Simone Knotzer, Email: simone.knotzer@baxalta.com.
Johanna Kindermann, Email: johanna.kindermann@baxalta.com.
Jens Modrof, Email: jens.modrof@baxalta.com.
Thomas R. Kreil, Email: thomas.kreil@baxalta.com.
References
- 1.Gregersen J.P., Roth B. Inactivation of stable viruses in cell culture facilities by peracetic acid fogging. Biol J Int Assoc Biol Stand. 2012;404:282–287. doi: 10.1016/j.biologicals.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Neighbor N.K., Newberry L.A., Bayyari G.R., Skeeles J.K., Beasley J.N., McNew R.W. The effect of microaerosolized hydrogen peroxide on bacterial and viral poultry pathogens. Poult Sci. 1994;7310:1511–1516. doi: 10.3382/ps.0731511. [DOI] [PubMed] [Google Scholar]
- 3.Ide P.R. The sensitivity of some avian viruses to formaldehyde fumigation. Can J Comp Med. 1979;432:211–216. [PMC free article] [PubMed] [Google Scholar]
- 4.Tuladhar E., Terpstra P., Koopmans M., Duizer E. Virucidal efficacy of hydrogen peroxide vapour disinfection. J Hosp Infect. 2012;802:110–115. doi: 10.1016/j.jhin.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Kahnert A., Seiler P., Stein M., Aze B., McDonnell G., Kaufmann S.H. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Lett Appl Microbiol. 2005;406:448–452. doi: 10.1111/j.1472-765X.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- 6.ICH . 23-9-1999. ICH harmonised tripartite guideline viral safety evaluation of biotechnology products derived from cell lines of human or animal origin Q5A(R1) [PubMed] [Google Scholar]
- 7.European Medicines Agency . 1996. CPMP note for guidance on virus validation studies: the design, contribution and interpretation of studies validating the inactivation and removal of viruses. CPMP/BWP/268/95/rev. [Google Scholar]
- 8.Bentley K., Dove B.K., Parks S.R., Walker J.T., Bennett A.M. Hydrogen peroxide vapour decontamination of surfaces artificially contaminated with norovirus surrogate feline calicivirus. J Hosp Infect. 2012;802:116–121. doi: 10.1016/j.jhin.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Pottage T., Richardson C., Parks S., Walker J.T., Bennett A.M. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J Hosp Infect. 2010;741:55–61. doi: 10.1016/j.jhin.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 10.ASTM - American Society for Testing and Materials . 2011. ASTM E2197–11 standard quantitative disk carrier test method for determining bactericidal, virucidal, fungicidal, mycobactericidal, and sporicidal activities of chemicals. [Google Scholar]
- 11.Doerrbecker J., Friesland M., Ciesek S., Erichsen T.J., Mateu-Gelabert P., Steinmann J. Inactivation and survival of hepatitis C virus on inanimate surfaces. J Infect Dis. 2011;20412:1830–1838. doi: 10.1093/infdis/jir535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moody M., Alves W., Varghese J., Khan F. Mouse minute virus (MMV) contamination–a case study: detection, root cause determination, and corrective actions. PDA J Pharm Sci Technol. 2011;65:580–588. doi: 10.5731/pdajpst.2011.00824. [DOI] [PubMed] [Google Scholar]
- 13.Nims R.W. Detection of adventitious viruses in biologicals–a rare occurrence. Dev Biol (Basel) 2006;123:153–164. [PubMed] [Google Scholar]
- 14.US Pharmacopoeia . 2009. USP United States Pharmacopoeia - reagents: test solutions (TS) general chapters: 1045 biotechnology-derived articles and 1057 biotechnology-derived articles - total protein assay. [Google Scholar]
- 15.Robert Koch Institute (RKI) “Virus Disinfection” Expert Committee of German Association for the control of Viral Diseases (DVV), disinfectants Commission of German Society for Hygiene and Microbiology (DGHM). Testing and labeling of disinfectant activity against viruses. Bundesgesundheitsblatt. 2004;47:62–66. [Google Scholar]
- 16.Mar Cor . 2014. Research report: compatibility of Minncare® Dry Fog™ and common cleanroom materials. P/N: 3028725 Rev.B. [Google Scholar]
- 17.Mar Cor . 2005. Technical report: testing of Minncare® cold sterilant for virus inactivation. P/N: 50096–306/A. [Google Scholar]


