Highlights
-
•
The main CT feature of COVID-19 pneumonia is the presence of ground glass opacities.
-
•
The distribution of CT abnormalities is typically peripheral.
-
•
Linear consolidations are frequently observed on CT several days after the onset of disease.
Keywords: COVID-19 pneumonia, Tomography, X-Ray Computed, Cryptogenic Organizing Pneumonia, Pneumonia
Abbreviations: CT, computed tomography; COVID-2019, Coronavirus disease 2019; GGO, ground glass opacities; MIP, maximum intensity projection; RT-PCR, real-time polymerase chain reaction
Abstract
The standard of reference for confirming COVID-19 relies on microbiological tests such as real-time polymerase chain reaction (RT-PCR) or sequencing. However, these tests might not be available in an emergency setting. Computed tomography (CT) can be used as an important complement for the diagnosis of COVID-19 pneumonia in the current epidemic context. In this review, we present the typical CT features of COVID-19 pneumonia and discuss the main differential diagnosis.
1. Introduction
Since March 11, 2020, the World Health Organization has declared Coronavirus disease 2019 (COVID-2019) caused by SARS-CoV-2 to be a pandemic and public health emergency of international concern [1]. As of April 8th, 2020, the epidemic had spread to more than 199 countries and more than one million individuals have contracted the virus worldwide with 81,478 reported deaths, including 82,048 confirmed cases in France and 10,869 deaths [2]. However, these numbers are probably underestimated as not all patients are tested, especially those who are asymptomatic, or with only mild symptoms and no associated comorbidities. The standard of reference for confirming COVID-19 relies on microbiological tests such as real-time polymerase chain reaction (RT-PCR) or sequencing [3]. However, these tests might not be available in an emergency setting and their results are not immediately available. Computed tomography (CT) can be used as an important complement to RT-PCR for diagnosing COVID-19 pneumonia in the current epidemic context [4], [5]. Indeed, when the viral load is insufficient, RT-PCR can be falsely negative while chest CT shows suggestive abnormalities [4], [5]. A large series based on 1014 patients reported a 97% sensitivity of chest CT for the diagnosis of COVID-19, while the mean time interval between initial negative and positive RT-PCR was approximately 5 days [5]. Thus, CT can play a pivotal role in the early detection and management of COVID-19 pneumonia [6], at least for patients who have been symptomatic for more than three days [4]. Indeed, 56% of patients imaged during the first 2 days following symptom onset may have normal CT findings [7].
Given the important role of chest CT, it is important for radiologists to become familiar with the typical CT features associated with this new infection, as well as the imaging criteria for an alternative diagnosis.
In this pictorial review, we present the typical CT features of COVID-19 pneumonia, their changes during follow-up, together with the main differential diagnosis and clues for their recognition.
2. Typical imaging findings
A wide variety of CT findings in COVID-19 have been reported in the different studies [8], [9] [9]. However, all studies indicate that the main CT feature of COVID-19 pneumonia is the presence of ground glass opacities (GGO), typically with a peripheral and subpleural distribution (Fig. 1 ). The involvement of multiple lobes, particularly the lower lobes is reported in the majority of patients with COVID-19 [10]. These areas of GGO may be admixed with areas of focal consolidation (Fig. 2 ) and or associated with superimposed intralobular reticulations, resulting in a crazy paving pattern (Fig. 3 ). Linear consolidations and other signs suggesting organizing pneumonia such as the reverse halo sign (i.e., areas of ground-glass surrounded by peripheral consolidation) are very frequently observed, mostly in patients several days after the onset of disease.
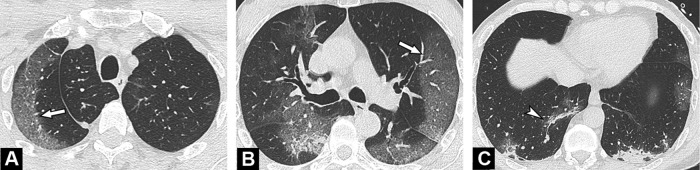
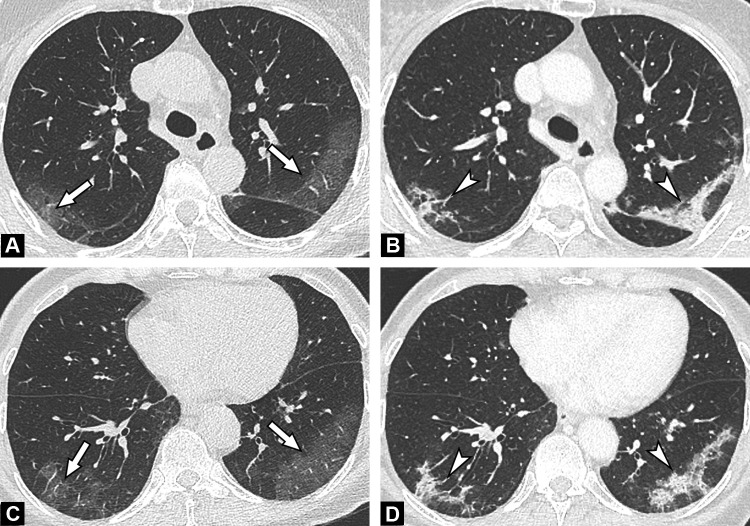
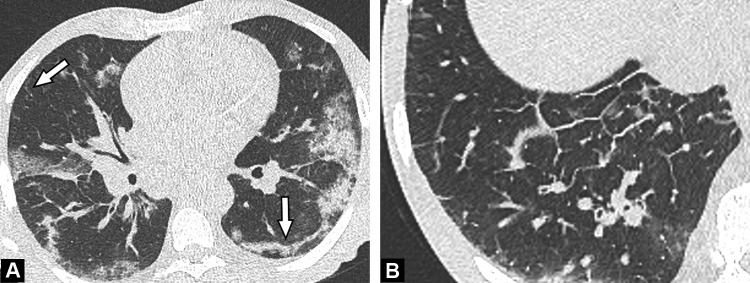
Fig. 1.
Unenhanced CT images show typical findings of COVID-19 pneumonia in a 55-year-old man. Peripheral GGO is seen in the upper portion of both lungs (A, B) (arrows), associated with linear consolidations in the lower lobes (C) (arrowhead). The results of first and second RT-PCRs were negative, with only the third test, repeated in view of CT findings, becoming positive.
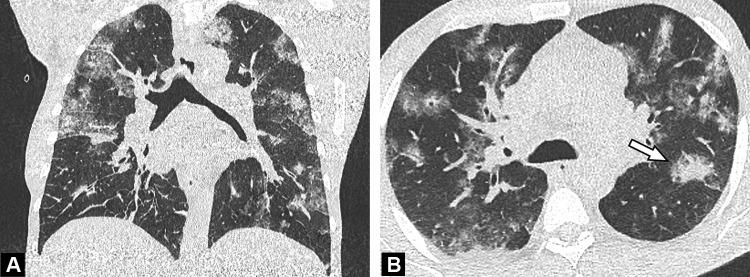
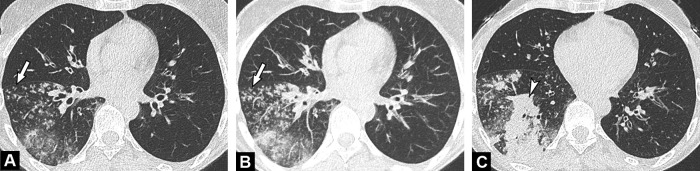
Fig. 2.
Unenhanced CT examination performed 6 days after the onset of symptoms in a 64-year-old-man with COVID-19 pneumonia. Axial (A) and coronal (B) CT images demonstrate bilateral ground glass opacities admixed with patchy areas of consolidation (arrow) in the central and peripheral portions of the lung.
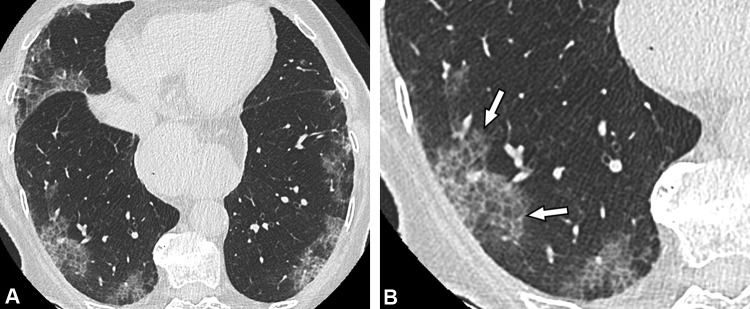
Fig. 3.
Unenhanced CT images of an 86-year-old woman with a crazy-paving pattern due to COVID-19 pneumonia. (a) CT examination performed 4 days after symptom onset (dry cough and chest pain) demonstrates moderate disease extent (10–25%). (b) Peripheral ground-glass opacities with superimposed intralobular reticulations (arrows) resulting in a crazy-paving pattern, are seen in both lower lobes.
In the study by Salehi et al., the frequencies of the different CT abnormalities were as follows: GGO was observed in 88.0% of patients, consolidation in 31.8%, bilateral involvement in 87.5% and peripheral distribution in 76.0% of patients [8].
3. Various forms of severity
Patients with COVID-19 pneumonia present with variable disease extent, ranging from mild involvement, affecting less than 10% of the lung parenchyma (Fig. 4 ) to severe disease extent with a “white lung” appearance on CT (Fig. 5 ). Yuan et al. evaluated imaging findings associated with mortality and reported that the frequency of consolidations as well as the median CT score were both higher in the group of patients who died at the hospital, as compared to patients who could be discharged [11]. The CT score in this study was calculated as follows: CT attenuation was graded using a 3-point scale, with 1 for normal attenuation, 2 for ground glass and 3 for consolidation. Then the degree of lung involvement was evaluated for 6 lung regions: upper, middle and lower lung on each side and graded using a 5-point scale: 0 no involvement, 1 less than 25%, 2: 25–50%, 3: 50–75% and 4 > 75%. The maximal CT score was 72. Using a cut-off value of 24.5, the CT score predicted mortality with a sensitivity of 85.6% and a specificity of 84.5% [11]. Other predictive factors for mortality included older age and higher comorbidity rate [12]. In the series by Li et al., the incidence of consolidation, linear opacities and crazy-paving pattern in severe/critical patients was significantly higher than that observed in non-severe (e.g., ordinary) patients [13]. This series based on 83 patients also confirmed that severe/critical patients were older and with more co-morbidities [13].
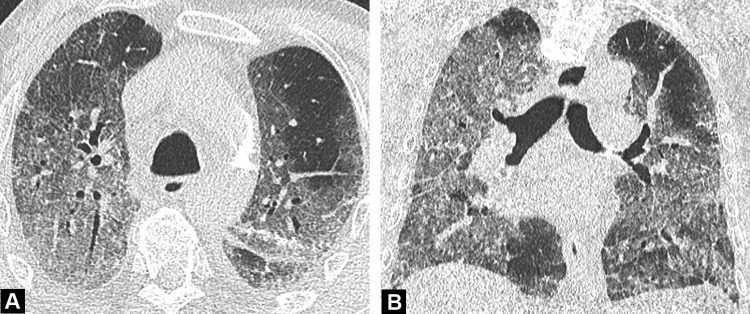
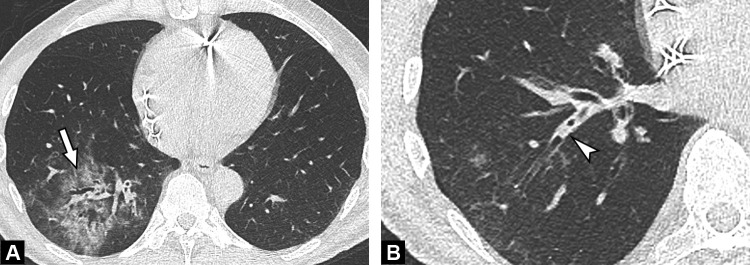
Fig. 4.
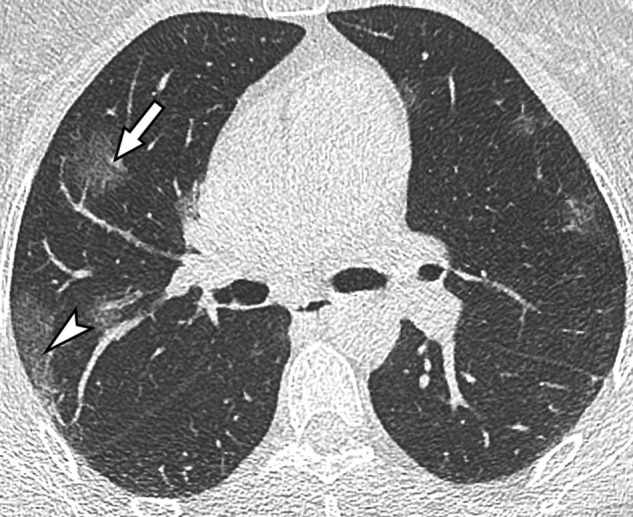
Unenhanced CT image in a 50-year-old woman with a mild form of COVID-19. She has asthma with no respiratory symptoms except fever. Her husband has been recently diagnosed with COVID-19. CT demonstrates rounded ground-glass opacities in both upper lobes, some in the sub pleural region (arrowhead) and others more centrally distributed (arrow).
Fig. 5.
Unenhanced CT images of a “white lung” appearance in an 89-year-old man with respiratory distress due to COVID-19 pneumonia. Axial (A) and coronal (B) CT images, performed before admission in ICU demonstrate extensive ground glass opacities, with more than 75% of the lung involved.
4. Evolution during follow-up
COVID-19 pneumonia CT features change over time, with different presentations according to the phase and severity of lung infection. Pan et al. investigated lung changes by time in patients who recovered from COVID-19 [14]. They classified the evolution of lung abnormalities into four stages (early 0–4 days, progressive 5–8 days, peak 9–13 days, and absorption ≥ 14 days) according to time periods. They visually quantified the extent of CT abnormalities. Each of the 5 lung lobes was visually scored from 0 to 5 as follows: 0, no involvement; 1, < 5% involvement; 2, 25% involvement; 3, 26%–49% involvement; 4, 50%–75% involvement; and 5, > 75% involvement [14]. The total CT score ranged from 0 to a maximal value of 25. They found that the total CT score increased until about 10 days after symptom onset and then gradually decreased. Regarding the category of CT abnormalities, stage 2 was characterized by an increase of GGO extent, with a crazy-paving pattern more frequently observed [8]. On the opposite, in stage 3, consolidation was the main feature with a decreased GGO ratio. In a series of 919 patients, Salehi et al. confirmed this observation and reported that CT findings in the intermediate stage of the disease were characterized by an increase in the number and size of GGOs, a progressive transformation of GGO into multifocal consolidation, with septal thickening and development of a crazy-paving pattern [8]. The transformation of GGO into linear consolidation is typical for an evolution towards organizing pneumonia, which is a nearly universal response to lung injury whether it is focal or diffuse, due to infection, radiation therapy or following drug-induced pneumonitis (Fig. 6 ) [15]. Wang et al. also evaluated longitudinal changes and confirmed that pure ground glass was the most common observation after symptoms onset, whereas a mixed pattern combining ground glass with irregular linear opacity peaked on illness days 6-11 [10].
Fig. 6.
Initial and follow-up CT images in a 71-year-old woman with COVID-19 pneumonia. Unenhanced initial CT performed before RT-PCR confirmation (A,C) shows bilateral peripheral ground-glass in the dorsal segment of upper (a) and lower lobes (C) (arrows). Contrast-enhanced CT (B,dD) performed 6 days later to rule out pulmonary embolism demonstrates linear consolidations typical for an organizing pneumonia pattern (arrowheads).
In patients with clinical worsening not explained by an extension of lung opacities on CT, pulmonary embolism should be suspected and a contrast-enhanced CT examination should be performed if possible, taking into consideration the clinical severity and the renal function. Of note, patients with severe COVID-19 pneumonia have a marked elevation of d-dimers, so that this d-dimer levels do no help identify those who have superimposed pulmonary embolism [16].
5. CT features suggesting pneumonia of other cause
Among differential diagnosis, one major differential is pneumonia from bacterial origin. Community-acquired pneumonia is usually characterized by an airspace consolidation in one segment or lobe, limited by the pleural surfaces. CT may additionally show ground glass attenuation, centrilobular nodules, bronchial wall thickening and mucoid impactions (Fig. 7, Fig. 8 ) [17]. COVID-19 pneumonia presentation is very different, with an absence of centrilobular nodules and no mucoid impactions in the absence of superinfection.
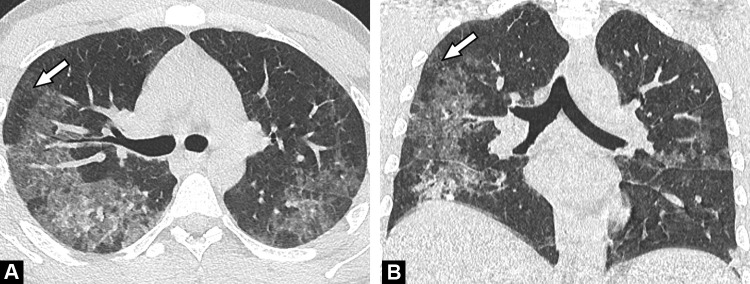
Fig. 7.
Unenhanced CT images in a 55-year-old-patient with bacterial bronchopneumonia. Centrilobular nodules (A) with a tree-in-bud pattern better seen on MIP reformatted images (B) (arrows) are seen in the right lower lobe, together with a segmental consolidation (arrowhead) (C).
Fig. 8.
Unenhanced CT images of a 45-year-old man with bacterial pneumonia. Ground glass opacities (arrow) limited to the posterior and lateral segment of the right lower lobe are demonstrated (A), associated with endobronchial secretions (arrowhead) (B) more proximally.
Pneumocystis Jiroveci pneumonia is another infectious cause of diffuse ground glass on CT, but occurs in immunocompromised patients. Even though GGO is the main CT feature, its distribution within the lung parenchyma is not similar to that observed in patients with COVID-19, it is more diffusely distributed with a tendency to spare the subpleural regions (Fig. 9 ) [18].
Fig. 9.
Unenhanced CT images of a 30-year-old man with Pneumocystis Jiroveci infection. Bilateral ground glass opacities with right lung predominance are demonstrated. Note the relative subpleural sparing (arrows) on both axial (A) and coronal (B) CT images.
It is much more difficult to distinguish COVID-19 from pneumonia due to other viral causes. CT features largely overlap, even though it has been reported that CT abnormalities in COVID-19 pneumonia more frequently exhibit a peripheral predominance, with less frequent pleural effusion and lymphadenopathy (Fig. 10 ) [19]. It is mainly the high current epidemic context which suggests COVID-19 as the cause of GGO in patients with fever and respiratory symptoms.
Fig. 10.
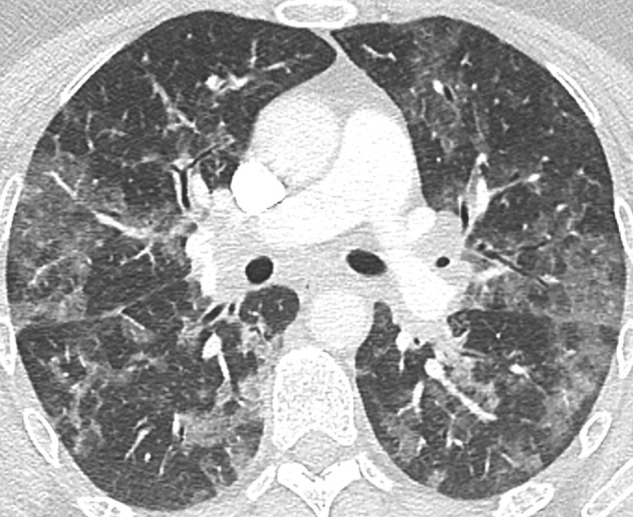
Unenhanced CT image in a 45-year-old-patient with Influenza virus-associated pneumonia. Bilateral diffuse ground glass opacities are demonstrated. Differential diagnosis with COVID-19 pneumonia is not possible and relies on RT-PCR results, even though peripheral predominance is less common. RT-PCR result for SARS-Cov-2 was negative but positive for influenza A.
6. Non-infectious causes of acute GGO
Pulmonary edema is a very common cause of diffuse GGO, but is characterized by a central predominance with sparing of the peripheral portions of the lung contrary to COVID-19. It is associated with other suggestive signs such as septal lines, pleural effusion, large pulmonary veins and mediastinal lymphadenopathy. It has been reported that COVID-19 might be responsible for acute myocarditis [20]. This diagnosis should be suspected if there are signs of interstitial pulmonary edema such as septal lines in younger patients (Fig. 11 ). Intra alveolar hemorrhage due to small vessel vasculitis is also characterized by diffuse GGO, but patients usually present with mild hemoptysis and acute renal failure is associated especially in Goodpasture syndrome. There is no subpleural predominance contrary to that seen in COVID-19 (Fig. 12 ) [21]. Drug-induced pneumonitis manifesting as nonspecific interstitial pneumonia is another cause of ground glass. Subpleural sparing, history of drug exposure help diagnosis. Those manifesting as organized pneumonia have similarities to COVID-19, but are associated with GGO and occur in a very different context [22].
Fig. 11.
Unenhanced CT images of a 64-year-old man with COVID-19 and pulmonary edema. Ground glass opacities admixed with patchy consolidation are seen in both lungs and associated with linear consolidation in the subpleural region of the left lower lobe (arrow) (A). The associated smooth thickening of the interlobular septa (B), indicating pulmonary edema, suggests left ventricular failure and potentially associated myocarditis.
Fig. 12.
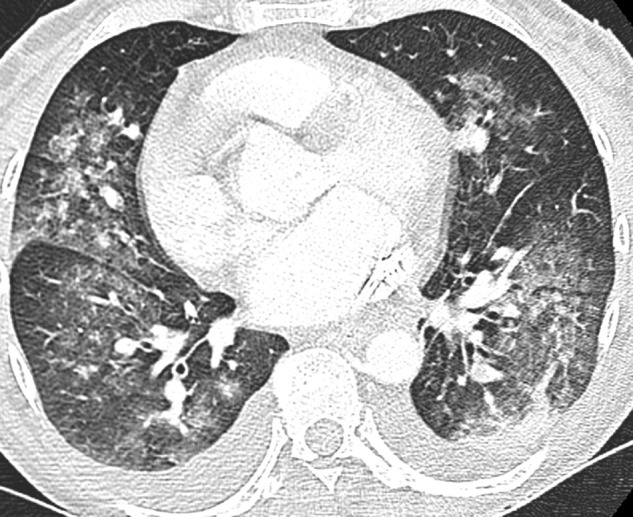
Unenhanced CT performed in a 48-year-old woman with Goodpasture syndrome. CT image shows bilateral ground glass opacities with central predominance, associated with pleural effusion, which is very uncommon in COVID-19 pneumonia. The patient presented with mild hemoptysis related to intra alveolar hemorrhage and acute renal failure. Histopathological analysis of tissues samples obtained from renal biopsy confirmed Goodpasture syndrome.
7. Conclusion
In this epidemic situation, CT undoubtedly plays an important role, for early identification of COVID-19 pneumonia. Typical CT features include peripheral GGOs with multifocal distribution, and a progressive evolution towards organizing pneumonia patterns. CT may be used for prognosis purposes, with poorer outcome for patients having important disease extent and more consolidative forms and also to early detect complications in patients who require further mechanical ventilation [23]. Centrilobular nodules, mucoid impactions and unilateral segmental or lobar consolidations suggest a bacterial origin of pneumonia, or superinfection. RT-PCR remains needed for final confirmation but its positivity can be delayed, with the need to repeat the test if the CT features are suggestive.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020 n.d. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.(accessed March 22, 2020).
- 2.Coronavirus Update (Live): 629,450 Cases and 28,963 Deaths from COVID-19 Virus Outbreak - Worldometer n.d. https://www.worldometers.info/coronavirus/.(accessed March 28, 2020).
- 3.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.20202004.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zu Z.Y., Jiang M.D., Xu P.P., Chen W., Ni Q.Q., Lu G.M. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020 doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Chest C.T. findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.20202004.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 Patients. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Z., Lu Y., Cao Q., Qin L., Pan Z., Yan F. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.22959. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Dong C., Hu Y., Li C., Ren Q., Zhang X. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020 doi: 10.1148/radiol.43.20202008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–893. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li K., Wu J., Wu F., Guo D., Chen L., Fang Z. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020 doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan F., Ye T., Sun P., Gui S., Liang B., Li L. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) Pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kligerman S.J., Franks T.J., Galvin J.R. From the Radiologic Pathology Archives: Organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33:1951–1975. doi: 10.1148/rg.337130057. [DOI] [PubMed] [Google Scholar]
- 16.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka N., Matsumoto T., Kuramitsu T., Nakaki H., Ito K., Uchisako H. High resolution CT findings in community-acquired pneumonia. J Comput Assist Tomogr. 1996;20:600–608. doi: 10.1097/00004728-199607000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Kuhlman J.E., Kavuru M., Fishman E.K., Siegelman S.S. Pneumocystis carinii pneumonia: spectrum of parenchymal CT findings. Radiology. 1990;175:711–714. doi: 10.1148/radiology.175.3.2343118. [DOI] [PubMed] [Google Scholar]
- 19.Bai H.X., Hsieh B., Xiong Z., Halsey K., Choi J.W., Tran T.M.L. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C., Zhou Y., Wang D.W. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020 doi: 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordier J.-F., Cottin V. Alveolar hemorrhage in vasculitis: primary and secondary. Seminars in Respiratory and Critical Care Medicine. 2011;32:310–321. doi: 10.1055/s-0031-1279827. [DOI] [PubMed] [Google Scholar]
- 22.Rossi S.E., Erasmus J.J., McAdams H.P., Sporn T.A., Goodman P.C. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. 2000;20:1245–1259. doi: 10.1148/radiographics.20.5.g00se081245. [DOI] [PubMed] [Google Scholar]
- 23.Langlet B., Dournes G., Laurent F. CT features of pulmonary interstitial emphysema. Diagn Interv Imaging. 2019;100:825–826. doi: 10.1016/j.diii.2019.04.004. [DOI] [PubMed] [Google Scholar]