Abstract
Cellular RNAs can be chemically modified over a hundred different ways. These modifications were once thought to be static, discrete, and utilized to fine-tune RNA structure and function. However, recent studies have revealed that some modifications, like mRNA methylation, can be reversed, and these reversible modifications may play active roles in regulating diverse biological processes. In this perspective, we summarize examples of dynamic RNA modifications that affect biological functions. We further propose that reversible modifications might occur on tRNA, rRNA, and other noncoding RNAs to regulate gene expression analogous to the reversible mRNA methylation.
Main Text
Proteins related to RNA metabolism account for ∼3%–11% coding capacity of the genome in all three domains of life, and RNA modifying enzymes are among the most conserved ones along with proteins involving transcription and translation (Anantharaman et al., 2002). RNA modifications require significant energy from the cell. For example, RNA methylation, a common modification, uses S-adenosylmethionine (SAM) as the methyl donor, and to produce one SAM molecule requires the energy equal to hydrolyzing 12 to 13 ATP molecules (Bakin et al., 1994). Given the significant cellular investment in RNA-modifying processes, RNA modifications are likely to be very important.
There are three main categories of RNA modifications: (1) modifications that enforce certain RNA structures and tune RNA biogenesis, such as modifications on rRNA and small nuclear RNA (snRNA) (Dickmanns and Ficner, 2005); (2) modifications that expand the RNA vocabulary and refine molecular recognition, such as modifications at the decoding region in tRNA; and (3) modifications that code dynamic regulatory information on top of the primary sequence, such as modifications on mRNA. We will briefly summarize the first two categories and then focus primarily on the last category, because the idea that dynamic RNA modifications play active roles in gene regulation has been intensively studied in recent years. Finally, we will extend our discussion to future directions and technique developments in RNA modification research.
Ribosomal RNA Modifications
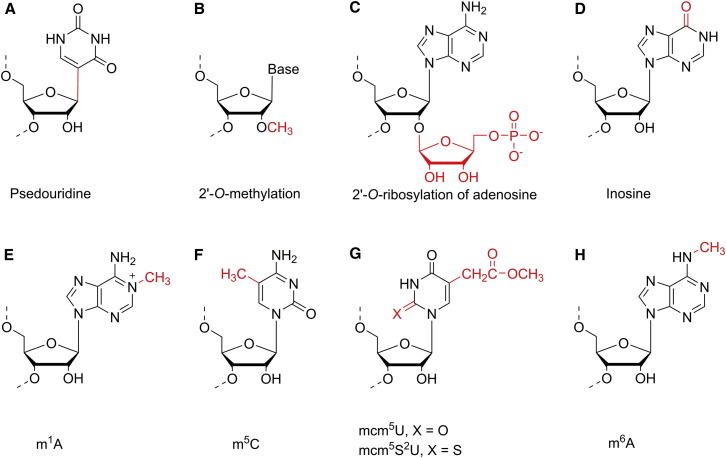
rRNA modifications are concentrated in functional regions such as peptidyl transferase center (PTC) (Decatur and Fournier, 2002). In human ribosomal RNA, there are 91 pseudouridines (Ψ, Figure 1 A), 105 2′-O-methylations on backbone sugars (Figure 1B), and ten methylated bases (Piekna-Przybylska et al., 2008). The biological effects of these modifications on rRNA have remained long-term puzzles. The prevailing hypothesis is that they fine-tune the structure and function of ribosome and perhaps play roles during ribosome biogenesis. Modified nucleotides possess distinctive chemical properties that could alter molecular interactions and conformations. For instance, 2′-O-methyl prevents hydrolysis of the phosphate backbone and causes the ribose sugar to favor the 3′ endo conformation (Kawai et al., 1992), and Ψ promotes base stacking (Davis, 1995). Both modifications enhance the rigidity and stability of certain RNA secondary structures. While individual rRNA modifications seem to be dispensable, these modifications are vital when considered collectively (Decatur and Fournier, 2002). For example, yeast strains with deficiency in rRNA modifications at PTC showed changes in tRNA selection, altered peptidyl transfer rates, reduced translation fidelity, and sensitivity to translation inhibitors (Baxter-Roshek et al., 2007). Therefore, rRNA modifications around PTC are critical for translation accuracy and efficiency of the ribosome, but the exact structural and functional roles still require further investigation.
Figure 1.
Chemical Structures of Selected RNA Modifications
Chemical structures of selected RNA modifications.
Transfer RNA Modifications
tRNAs are the most heavily modified types of RNA. Approximately 15%–25% of all nucleosides in eukaryotic tRNA contain modifications (El Yacoubi et al., 2012). These modifications have been proposed to serve various purposes (Hopper, 2013): (1) tRNA discrimination (e.g., initiator tRNAMet is distinguished from elongator tRNAMet through ribosylation at A64) (Shin et al., 2011) (Figure 1C); (2) translation fidelity, where absence of inosine (I, resulting from deamination of A, Figure 1D) at wobble position 34 causes decoding errors because A only pairs with U while I extends codon-anticodon interaction capability through base pairing with U, A, and C (Gerber and Keller, 1999); and (3) tRNA stability (e.g., m1A58 of tRNAi Met is required for tRNA stability) (Anderson et al., 1998) (Figure 1E).
Recent studies in yeast revealed that certain tRNA modifications can be quite dynamic and adaptive to environment changes. With a highly accurate mass spectroscopic method, it was shown that the spectrum of tRNA modification has signature shift upon exposure to different toxins (Chan et al., 2010). In response to oxidative stress induced by hydrogen peroxide, the C34 at the wobble position (the first residue in the anticodon region) of yeast tRNALeu CAA is modified by tRNA methyltranfserase 4 (Trm4). The m5C-modified tRNALeu CAA enhances the translation of UUG-rich transcripts (Chan et al., 2012) (Figure 1F). The m5C level on tRNAHis (also catalyzed by Trm4) rises in response to nutrient depletion and other growth arrest conditions (Preston et al., 2013). Another yeast tRNA methyltranfsrase, Trm9, completes the formation of mcm5U and mcm5s2U (Figure 1G) at the wobble U34 of tRNAArg UCU and tRNAGlu UUC; this methylation prevents cell death by promoting translation of DNA damage response genes that are enriched with arginine and glutamic acid codons (Begley et al., 2007). Besides methylation, a tRNA isopentenyltransferase enzyme Mod5 catalyzes the formation of N 6-isopentenyladeosine (i6A37) at A37 using dimethylally pyrophosphate. This i6A modification is required for tRNA-mediated nonsense suppression (recognition of a premature stop codon by mutant tRNATyr to suppress protein truncation). In parallel, Erg20 utilizes the same substrate, dimethylally pyrophosphate, to produce an essential precursor of sterols, farnesyl pyrophosphate. Therefore, Mod5 and Erg20 compete for their common substrate. Overexpression of Erg20 elevates the influx of the common substrate to sterol pathway, reducing formation of i6A in tRNATyr, thereby changing translation due to altered nonsense suppression (Benko et al., 2000). The coupling of tRNA modification and sterol biogenesis was further strengthened by the discovery that Mod5 can regulate sterol metabolic pathway via a prion state (Suzuki et al., 2012). Besides enzymatic function, some tRNA modifying enzymes, such as tRNA pesudouridylases TruB, can also function as tRNA chaperone to facilitate maturation of tRNA (Gutgsell et al., 2000).
A mammalian DNA methyltransferase (DNMT), DNMT2, has been shown to actually work on tRNA cytosine methylation (Goll et al., 2006). Several studies on the Drosophila homolog of DNMT2 showed that the DNMT2-mediated methylation protects tRNA against stress-induced fragmentation, which is beneficial because tRNA fragments can inhibit the activity of the small RNA processing enzyme Dicer-2 and cause dysfunction of RNA interference (Schaefer et al., 2010, Durdevic et al., 2013). Dynamic tRNA modifications could directly impact codon selection and the outcome of translation. This is a rich and vibrant research field that should continue to generate surprising discoveries.
mRNA Modifications
mRNA plays a central role in the transduction of biological information from DNA to protein. Because mRNAs encode genetic information, most mRNA modifications need to be nonmutagenic and should not interfere with translation machinery. Therefore, most nucleoside modifications on mRNA are methylations, which minimally perturb the mRNA. There are four primary sites of methylation: N 7-methylguanine (m7G at the 5′ cap), N 6-methyl adenosine (m6A), 5-methylcytosine (m5C), and 2′-O-methylation of ribose (Figure 1). The cap structure has diverse functions; it promotes splicing, regulates mRNA nuclear export, and prevents 5′-3′degradation, and it is crucial during translation initiation, where it is recognized by cap-binding proteins (Cougot et al., 2004, Topisirovic et al., 2011) and also suppresses aberrant translation (Mitchell et al., 2010). While the cap is clearly important, we will focus our discussions on internal (non-cap) mRNA modifications.
Reversible m6A Methylation
The m6A methylation is the most prevalent internal modification on eukaryotic mRNA. It was initially discovered in 1974, together with 5′ cap methylation (Desrosiers et al., 1974, Desrosiers et al., 1975). However, progress on m6A research lagged far behind that of the cap, probably because of the low abundance of mRNA and difficulties in detection. Early studies showed that on average every mammalian mRNA contains three to five m6A within a G(m6A)C (70%) or A(m6A)C (30%) consensus sequence (Wei et al., 1976, Wei and Moss, 1977), but the methylation percentage at each site varies substantially (Kane and Beemon, 1985, Carroll et al., 1990). m6A is posttranscriptionally installed by an m6A methyltransferase complex (Tuck, 1992, Bokar et al., 1994). The identification of a SAM-binding subunit (METTL3) of the complex (Bokar et al., 1997) allowed scientists to examine m6A in model organisms. The resulting work showed that m6A is crucial for yeast meiosis (Shah and Clancy, 1992, Clancy et al., 2002, Schwartz et al., 2013), and for fruit fly (Hongay and Orr-Weaver, 2011) and plant development (Zhong et al., 2008). The m6A methylation also appears to be essential for mammalian cells (Bokar, 2005).
In the last 4 years, the field has witnessed a major revival focusing on functional roles of m6A in eukaryotic mRNA (Fu et al., 2014), initiated by (1) a conceptual conjecture that reversible RNA modification might serve regulatory roles analogous to DNA and histone epigenetic modifications (He, 2010, Yi and Pan, 2011), followed by the subsequent discovery of the first mRNA demethylase FTO (fat mass and obesity-associated protein) that reverses m6A modification (Jia et al., 2011); (2) the development of an antibody-based high-throughput m6A profiling method, m6A-seq (Dominissini et al., 2012) or MeIP-seq (Meyer et al., 2012, Meyer and Jaffrey, 2014); and (3) the discovery and characterization of selective m6A-binding proteins that impact the stability of mRNA (Dominissini et al., 2012, Wang et al., 2014a). As a reversible mark analogous to methylations on DNA and histone tails, m6A on mRNA is installed, erased, and recognized by m6A methyltransferase, demethylase, and m6A-specific binding proteins. We have recently shown that METTL3 forms a stable heterodimer with METTL14 as the enzymatic core of the m6A methyltransferase complex and biochemically reconstituted their methylation activity (Liu et al., 2014, Wang et al., 2014b). In addition, the heterodimer also interacts with a splicing regulator, WTAP, which affects the m6A level inside cells (Liu et al., 2014, Ping et al., 2014). The interaction between WTAP and m6A methyltransferase is also conserved in yeast (Agarwala et al., 2012) and plants (Zhong et al., 2008). Early studies indicated that a large protein complex (200 kDa + 800 kDa) mediates this methylation (Tuck, 1992, Bokar et al., 1994), hence other important protein factors surrounding the enzymatic core remain to be identified. Only a fraction of the all consensus sequences in mammalian mRNA are methylated. The methylation selectivity and its response to various cellular signals and stimuli remain to be elucidated in the future.
Functional understanding of m6A has lagged in part because of limited research on potential reader proteins that can selectively bind the methylated transcripts and mediate biological functions. Potential candidate proteins have been reported in RNA-affinity pull-down experiments using methylated RNA probes (Dominissini et al., 2012). Three members of human YTH domain family proteins (YTHDF1–3) exhibit 5- to 20-fold higher binding affinity for methylated RNAs compared to unmethylated RNA (Wang et al., 2014a). In particular, YTHDF2 has been shown to affect the stability of m6A-containing RNA and localize the methylated mRNA from translatable pool to mRNA decay sites, such as processing bodies in parallel or at a later stage of deadenylation (Wang et al., 2014a). Interestingly, m6A-containing transcripts are enriched with regulatory genes (transcription factors, etc.) and inherently possess shorter half-live than nonmethylated species (Fu et al., 2014), suggesting that the m6A-dependent mRNA turnover serves as a mechanism to dynamically affect expression of these genes (Wang and He, 2014). The m6A methylation has been shown to affect stability of transcriptional regulators in mouse embryonic stem cells (Wang et al., 2014b), and two recent studies on yeast further support the notion that one main function of m6A is likely to affect transcript stability. Mmi1 in fission yeast is homologous to human YTH domain-containing proteins and is responsible for selectively eliminating meiotic mRNA transcripts during vegetative growth (Harigaya et al., 2006, Hiriart et al., 2012). Ydr374c (Pho92 or MRB1), the YTH domain homolog in budding yeast, binds the m6A-containing RNA and seems to regulate the transcript stability of a key gene involved in phosphate signal transduction pathway in response to changing phosphate levels by interacting with the Pop2-Ccr4-Not deadenylation complex (Kang et al., 2014).
The two recently discovered m6A demethylases (FTO and AlkBH5) have distinct physiological functions: FTO is associated with body weight and human diseases (Dina et al., 2007, Frayling et al., 2007, Do et al., 2008, Ho et al., 2010, Keller et al., 2011), while the Alkbh5 knockout mice have impaired fertility (Zheng et al., 2013), demonstrating functional impacts of the removal of m6A and importance of a delicate balance of the m6A methylation/demethylation activities in mammals. As the functional roles of m6A become defined, the mechanisms of the demethylation-based regulation will likely follow.
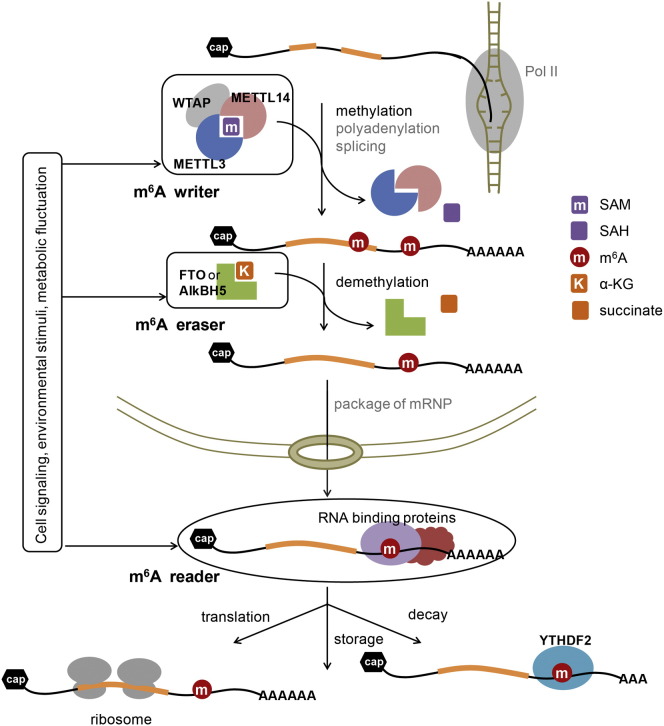
The abundant m6A methylation represents a different regulatory mechanism on top of the primary transcript sequence (Figure 2 ). The discovery and functional elucidation of the writers, erasers, and readers of m6A will continue to reveal functional significance of this methylation. The writer proteins selectively install and set the code of the entire transcriptome at the upstream of information processing. Demethylases balance the methylation stoichiometry of specific mRNAs, perhaps in a pathway- and cell-type-specific manner. The m6A “reader” proteins are at the end of information processing, executing the biological functions of m6A on specific transcripts through rapid and localized reading of the m6A mark. Protein of all three stages can couple with signal transduction pathways via protein-protein interaction or posttranslational modifications, which provide a dynamic and rapid response to cellular signals, environmental stimuli, or programmed biological transformations.
Figure 2.
Reversible m6A Modification Affects Gene Expression Regulation in Mammalian Cells
The m6A writer proteins install the m6A code on the transcriptome in coordination with RNA splicing and processing. The METTL3-METTL14 heterodimer is the enzymatic core of the m6A writer complex, while WTAP and other factors could regulate the methylation process. The m6A eraser proteins (FTO and AlkBH5) further tune the methylation stoichiometry, perhaps in a more pathway-specific manner. The m6A reader proteins (e.g., YTHDF family proteins) recognize the m6A code and execute biological functions. YTHDF2 promotes the decay of the m6A-containing RNA while other reader proteins could potentially affect the translation, storage, or nuclear export of methylated RNA. All these proteins could couple their functions with cellular signaling pathways, responses to environmental stimuli, or programmed biological transformations. S-adenosyl methionine (SAM) is the cofactor of METTL3-METTL14 with S-adenosyl homocysteine (SAH) as the product after the methylation. α-ketoglutarate (α-KG) is the cofactor of FTO and AlkBH5 with succinate as the product.
m5C
5-Methylcytosine is a well-known epigenetic modification in eukaryotic genomic DNA and is known to exist in rRNA and tRNA; however, recent transcriptome-wide mapping of m5C in human RNA has uncovered over ten thousand candidate m5C sites in mRNA and other noncoding RNAs (Squires et al., 2012). In mRNA, these sites are enriched in untranslated regions and around Argonaute binding sites. By using both bisulfite sequencing and immunoprecipitation with anti-m5C antibody followed by sequencing, 14 m5C sites were verified in archaeal (S. solfataricus) mRNA with a consensus motif of AU(m5C)GANGU, similar to the m5C sites on S. solfataricus rRNA, suggesting a shared m5C methyltransferase in the deposition of m5C (Edelheit et al., 2013). Several genes bearing m5C are enzymes involved in energy and lipid metabolism, possibly indicating a regulatory role of m5C in metabolic processes. Several m5C methyltransferases that were thought to work on rRNA and tRNA have binding sites on mRNA, suggesting additional roles that impact mRNA (Zhang et al., 2012, Khoddami and Cairns, 2013, Hussain et al., 2013).
2′-O-Methylation
2′-O-methylation is involved in discrimination of self and nonself mRNA (Daffis et al., 2010). Human and mouse coronavirus mutants lacking 2′-O-methyltransferase activity triggered higher level of type I interferon via the recognition of Mda5, a cytoplasmic protein that senses double-stranded RNA (Züst et al., 2011). Hence, it is not surprising that 2′-O-methylation has been widely incorporated into small interference RNA (siRNA) to optimize the stability and immunogenic properties of siRNA (Judge et al., 2006). Plant microRNAs (miRNA) bear naturally occurring 2′-O-methylation installed by a methyltransferase HEN1 (Yu et al., 2005). The function of such a modification is suggested to protect the 3′ end of miRNA against polyuridylation, thus preventing miRNA from poly(U)-mediated degradation (Li et al., 2005).
Pseudouridine
Pseudourindine, “the fifth base,” was the first known modification, discovered over 60 years ago (Davis and Allen, 1957) (Figure 1A). Pseudouridine modification provides an additional hydrogen-bonding donor that can significantly affect the secondary structure of RNA. Its presence on mRNA could impact translation by affecting the secondary structures of mRNA or recruiting potential reader proteins. A recent inspiring work showed that replacing the first uridine of the stop codon to psedourindine can convert nonsense (stop) codon to sense codon, thus raising the possibility of expanding the genetic codon or recoding transcripts by introducing RNA modifications on mRNA (Karijolich and Yu, 2011). More recently, transcriptome-wide mapping has uncovered hundreds of naturally occurring psedourindine sites in yeast and human mRNA. These pseudouridine sites are responsive to nutrition starvation and heat shock (Carlile et al., 2014, Schwartz et al., 2014), suggesting mRNA pseudouridylation as a potential mechanism to rapidly adapt the translation landscape to environmental stress.
Modifications of Other Noncoding RNAs
Long noncoding RNAs (lncRNAs) are known to have diverse roles in chromatin remodeling, transcription, and mRNA processing (Mercer et al., 2009, Rinn and Chang, 2012, Lee and Bartolomei, 2013). It has been shown that some lncRNAs, such as MALAT1, TUG1, and NEAT1, contain multiple m6A sites (Dominissini et al., 2012, Meyer et al., 2012, Liu et al., 2013). The roles and potential reversibility of these m6A sites on lncRNAs are still unclear. Another major class of noncoding RNAs are the U snRNAs as the well-established RNA components of the spliceosome (U1, U2, U4, U5, and U6, etc.). The 5′-terminal capping is essential to the exportation of U snRNAs to cytoplasm from nucleus (Dickmanns and Ficner, 2005). snRNAs also contain internal modifications such as Ψ, 2′-O-methylation, and m6A. It is unclear if these modifications are dynamic, and their functional roles remain to be fully elucidated.
Prospects
We propose several emerging themes in the rapidly developing field of RNA modifications.
Identifying and Characterizing RNA Modifying/Demodifying Enzymes and Binding Proteins
The discovery of the first mRNA demethylase FTO stimulated the study of reversible RNA modifications. Much needs to be done to understand factors like FTO and others that have already been identified. Indeed, important questions remain. For example, how does the writer complex achieve selectivity? And how does the function of the writer complex relate to transcription and splicing?
Beyond the known factors, it is likely other writer, easer, and reader proteins for the RNA m6A methylation exist. Identifying and characterizing these proteins, present in either the cytoplasm or nucleus, will be critical for understanding and expanding the biological roles of m6A. We know that m6A affects mRNA stability through the YTHDF2-mediated decay pathway (Wang et al., 2014a, Wang and He, 2014), but other proteins might read m6A differently, leading to effects on RNA transport, storage, and translation in response to signals and cellular stress.
Other RNA modifications might be written and erased like m6A. If they are, the SAM-binding methyltransferases and α-KG-dependent dioxygenases (including AlkB family that FTO and AlkBH5 belong to) are promising groups of methyltransferases and demethylases worth studying. The methyl group of m6A can be further oxidized to N 6-hydroxymethyladenosine (hm6A) and N 6-formyladenosine (f6A) in vitro and in vivo (Fu et al., 2013), and future work should establish the functional importance of these changes.
Reversible Modifications on tRNA, rRNA, and Other Noncoding RNAs
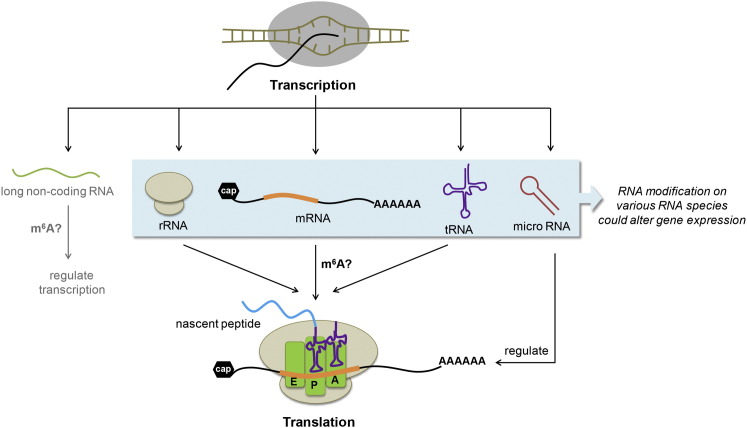
In 2010, when we proposed reversible RNA modification, or “RNA epigenetics,” as a means to effect genetic information akin to DNA and histone modifications. Reversible methylation, as recently discovered, may not be restricted to mRNA. tRNA, rRNA, and other noncoding RNAs could also be targets for methylation and demethylation (Figure 3 ). Indeed, pri-microRNAs and lncRNA are known to contain m6A (Dominissini et al., 2012, Liu et al., 2013), which might be reversible and affect their cellular localization and functions.
Figure 3.
Dynamic RNA Modifications in Gene Expression Regulation
RNA modifications in various RNA species (rRNA, mRNA, tRNA, lncRNA, and miRNA) could be reversible and play active roles in regulating transcription and posttranscriptional gene expression.
tRNA and rRNA are enriched with diverse chemical modifications, including various methylations. Reversible modifications on rRNA could affect biogenesis of rRNA and functions of ribosome. On tRNA, reversible modifications could rapidly and broadly impact cellular protein production. We know that tRNA methylation can be quite dynamic in mammalian cells (Chan et al., 2010, Saikia et al., 2010, Fu et al., 2010). It will be hard to believe the simple reversible methylation chemistry, already known to occur on mRNA, is not harnessed by nature via evolution to directly affect translation through tRNA and/or rRNA. Additional discoveries and future research in these directions could reveal new mechanisms of biological regulation.
Elucidating the Functions of RNA Modification in Dynamic Biological Processes
In contrast to regulatory information encoded by the primary sequence, reversible RNA modification is dynamic and may affect biological processes involving major transformations of cell states, such as gametogenesis, embryonic development, neuronal differentiation, and immune responses, without affecting the coding sequence. For example, m6A has been suggested to act as a pacesetter of the mammalian circadian clock (Fustin et al., 2013) and a switch for yeast meiotic entrance (Shah and Clancy, 1992, Agarwala et al., 2012). As discussed above, various RNA modifications are responsive to changes in nutrient and metabolite levels, probably because the modifications require energy to produce and because cofactors like SAM, iron, and α-KG are shared by RNA-modifying enzymes and metabolic enzymes (Figure 2). We expect that future studies will reveal further connections between RNA modifications and cell metabolism, and they may connect RNA modifications to human diseases like cancer, in part, through effects on metabolism. Because the functions of mRNA, rRNA, and tRNA directly connect to translation, modifications on these RNA species could significantly determine the outcome of protein production, localized and general, to affect states of the cell in various contexts, such as localized translation in neurons.
The DNA and histone epigenetic modifications are not only reversible and affect gene expression regulation, but the effects can also be heritable. Could reversible RNA modification be heritable too? Several promising directions could be explored to address this question. RNA modification might be involved in maternal effect. At the early stage of embryo development, transcription is inactivated and protein expression is dominated by the translation of prestored maternal mRNA (Tadros and Lipshitz, 2009). Therefore, any shift in the modification pattern of maternal mRNA would have a profound effect on zygotic development, and if such a change is remembered at the posttranscriptional or transcriptional level, it could be passed on to the next generation. Similarly, RNA modifications could mark different transcripts during meiosis and/or mitosis to affect transfer of inheritable information between generations.
Detection Techniques
The lack of accurate and sensitive detection methods has limited the study of RNA modifications in low abundance RNA species (e.g., mRNA). Traditional techniques involve isotope labeling and lipid or thin-plate chromatography, which are tedious, semiquantitative, low-throughput, and require a large amount of starting materials. The recent developments in highly sensitive technologies have revolutionized research on RNA modifications, in particular on low-abundance RNA species. These developments include (1) liquid chromatography-tandem mass spectroscopy followed by mass fingerprinting (LC-MS/MS), which enables accurate quantification of modified nucleosides with unambiguous chemical identity with fmol (10−15) sensitivity, and (2) next-generation sequencing coupled with modification-specific antibodies, which enables transcriptome-wide profiling of RNA modification sites. While LC-MS/MS provides quantification for a population of RNA and sequencing qualitatively locates candidate modification sites, there has been a lack of a high-throughput method available to determine precise modification sites and the stoichiometry for each modification at those sites.
The study of m5C in DNA/RNA has greatly benefited from bisulfate sequencing capable of determining methylation percentage at each site. However, similar chemistry is not always available for other base modifications. For example, the dynamic m6A methylation is nonstoichiometric on mRNA, and the balance of methyltransferase/demethylase activities indeed has physiological consequences. So far, SCARLET (site-specific cleavage and radioactive labeling followed by ligation-assisted extraction and thin-layer chromatography) is the only reported method to directly determine the presence and fraction of m6A at single-nucleotide resolution (Liu et al., 2013), but it is low-throughput. The use of m6A-sensitive reverse transcription enzymes to detect the kinetic delay or stall at the m6A site (Vilfan et al., 2013, Harcourt et al., 2013) and the protein-modified nanopore sequencing (Laszlo et al., 2013, Schreiber et al., 2013) might be promising, but they are still not ready to be used in real applications. A method that could detect modifications with limited input materials (e.g., RNA isolated from a single cell) is also lacking. The dynamics in each individual cell and effects on early developmental events of RNA modifications can be learned with new technologies. Since every RNA modification has its own unique biochemical/chemical property, the solution to efficiently detect and distinguish each of them remains challenging for chemists, biochemist, and biologists.
The development of in situ detection methods of RNA modification is another challenge. Fluorescence in situ hybridization is a main method used to visualize mRNA within cell or tissue specimen. For nonstoichiometric RNA modifications, RNA modification could impact spatial localization information among a heterogeneous population of target RNA. In order to fully reveal the biological functions of RNA modifications, it would be valuable to directly visualize and report the modification status on specific transcripts.
In summary, with modern technologies available, RNA modifications can be studied in a more quantitative manner. We propose that reversible RNA modifications occur in different RNA species and broadly influence gene expression as a previously unappreciated layer of posttranscriptional regulation.
Acknowledgments
C.H. is an investigator of the Howard Hughes Medical Institute. We thank Ms. T. Shpidel for editing the manuscript.
References
- Agarwala S.D., Blitzblau H.G., Hochwagen A., Fink G.R. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 2012;8:e1002732. doi: 10.1371/journal.pgen.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman V., Koonin E.V., Aravind L. Comparative genomics and evolution of proteins involved in RNA metabolism. Nucleic Acids Res. 2002;30:1427–1464. doi: 10.1093/nar/30.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Phan L., Cuesta R., Carlson B.A., Pak M., Asano K., Björk G.R., Tamame M., Hinnebusch A.G. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–3662. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin A., Lane B.G., Ofengand J. Clustering of pseudouridine residues around the peptidyltransferase center of yeast cytoplasmic and mitochondrial ribosomes. Biochemistry. 1994;33:13475–13483. doi: 10.1021/bi00249a036. [DOI] [PubMed] [Google Scholar]
- Baxter-Roshek J.L., Petrov A.N., Dinman J.D. Optimization of ribosome structure and function by rRNA base modification. PLoS ONE. 2007;2:e174. doi: 10.1371/journal.pone.0000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley U., Dyavaiah M., Patil A., Rooney J.P., DiRenzo D., Young C.M., Conklin D.S., Zitomer R.S., Begley T.J. Trm9-catalyzed tRNA modifications link translation to the DNA damage response. Mol. Cell. 2007;28:860–870. doi: 10.1016/j.molcel.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko A.L., Vaduva G., Martin N.C., Hopper A.K. Competition between a sterol biosynthetic enzyme and tRNA modification in addition to changes in the protein synthesis machinery causes altered nonsense suppression. Proc. Natl. Acad. Sci. USA. 2000;97:61–66. doi: 10.1073/pnas.97.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokar J.A. The biosynthesis and functional roles of methylated nucleosides in eukaryotic mRNA. In: Grosjean H., editor. Fine-Tuning of RNA Functions by Modiðcation and Editing. Sprinter; New York: 2005. pp. 141–177. [Google Scholar]
- Bokar J.A., Rath-Shambaugh M.E., Ludwiczak R., Narayan P., Rottman F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J. Biol. Chem. 1994;269:17697–17704. [PubMed] [Google Scholar]
- Bokar J.A., Shambaugh M.E., Polayes D., Matera A.G., Rottman F.M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- Carlile T.M., Rojas-Duran M.F., Zinshteyn B., Shin H., Bartoli K.M., Gilbert W.V. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014 doi: 10.1038/nature13802. Published online September 5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S.M., Narayan P., Rottman F.M. N6-methyladenosine residues in an intron-specific region of prolactin pre-mRNA. Mol. Cell. Biol. 1990;10:4456–4465. doi: 10.1128/mcb.10.9.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.T., Dyavaiah M., DeMott M.S., Taghizadeh K., Dedon P.C., Begley T.J. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010;6:e1001247. doi: 10.1371/journal.pgen.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.T., Pang Y.L., Deng W., Babu I.R., Dyavaiah M., Begley T.J., Dedon P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy M.J., Shambaugh M.E., Timpte C.S., Bokar J.A. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 2002;30:4509–4518. doi: 10.1093/nar/gkf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougot N., van Dijk E., Babajko S., Séraphin B. ‘Cap-tabolism’. Trends Biochem. Sci. 2004;29:436–444. doi: 10.1016/j.tibs.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Daffis S., Szretter K.J., Schriewer J., Li J., Youn S., Errett J., Lin T.Y., Schneller S., Zust R., Dong H. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D.R. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res. 1995;23:5020–5026. doi: 10.1093/nar/23.24.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F.F., Allen F.W. Ribonucleic acids from yeast which contain a fifth nucleotide. J. Biol. Chem. 1957;227:907–915. [PubMed] [Google Scholar]
- Decatur W.A., Fournier M.J. rRNA modifications and ribosome function. Trends Biochem. Sci. 2002;27:344–351. doi: 10.1016/s0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R.C., Friderici K.H., Rottman F.M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5′ terminus. Biochemistry. 1975;14:4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Dickmanns A., Ficner R. Role of the 5′-cap in the biogenesis of spliceosomal snRNPs. In: Grosjean H., editor. Fine-Tuning of RNA Functions by Modiðcation and Editing. Sprinter; New York: 2005. pp. 179–204. [Google Scholar]
- Dina C., Meyre D., Gallina S., Durand E., Körner A., Jacobson P., Carlsson L.M.S., Kiess W., Vatin V., Lecoeur C. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- Do R., Bailey S.D., Desbiens K., Belisle A., Montpetit A., Bouchard C., Pérusse L., Vohl M.C., Engert J.C. Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes. 2008;57:1147–1150. doi: 10.2337/db07-1267. [DOI] [PubMed] [Google Scholar]
- Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- Durdevic Z., Mobin M.B., Hanna K., Lyko F., Schaefer M. The RNA methyltransferase Dnmt2 is required for efficient Dicer-2-dependent siRNA pathway activity in Drosophila. Cell Rep. 2013;4:931–937. doi: 10.1016/j.celrep.2013.07.046. [DOI] [PubMed] [Google Scholar]
- Edelheit S., Schwartz S., Mumbach M.R., Wurtzel O., Sorek R. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet. 2013;9:e1003602. doi: 10.1371/journal.pgen.1003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi B., Bailly M., de Crécy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R.B., Elliott K.S., Lango H., Rayner N.W. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Dai Q., Zhang W., Ren J., Pan T., He C. The AlkB domain of mammalian ABH8 catalyzes hydroxylation of 5-methoxycarbonylmethyluridine at the wobble position of tRNA. Angew. Chem. Int. Ed. Engl. 2010;49:8885–8888. doi: 10.1002/anie.201001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Jia G.F., Pang X.Q., Wang R.N., Wang X., Li C.J., Smemo S., Dai Q., Bailey K.A., Nobrega M.A. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Dominissini D., Rechavi G., He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat. Rev. Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- Fustin J.M., Doi M., Yamaguchi Y., Hida H., Nishimura S., Yoshida M., Isagawa T., Morioka M.S., Kakeya H., Manabe I., Okamura H. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Gerber A.P., Keller W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science. 1999;286:1146–1149. doi: 10.1126/science.286.5442.1146. [DOI] [PubMed] [Google Scholar]
- Goll M.G., Kirpekar F., Maggert K.A., Yoder J.A., Hsieh C.L., Zhang X., Golic K.G., Jacobsen S.E., Bestor T.H. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- Gutgsell N., Englund N., Niu L., Kaya Y., Lane B.G., Ofengand J. Deletion of the Escherichia coli pseudouridine synthase gene truB blocks formation of pseudouridine 55 in tRNA in vivo, does not affect exponential growth, but confers a strong selective disadvantage in competition with wild-type cells. RNA. 2000;6:1870–1881. doi: 10.1017/s1355838200001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt E.M., Ehrenschwender T., Batista P.J., Chang H.Y., Kool E.T. Identification of a selective polymerase enables detection of N(6)-methyladenosine in RNA. J. Am. Chem. Soc. 2013;135:19079–19082. doi: 10.1021/ja4105792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya Y., Tanaka H., Yamanaka S., Tanaka K., Watanabe Y., Tsutsumi C., Chikashige Y., Hiraoka Y., Yamashita A., Yamamoto M. Selective elimination of messenger RNA prevents an incidence of untimely meiosis. Nature. 2006;442:45–50. doi: 10.1038/nature04881. [DOI] [PubMed] [Google Scholar]
- He C. Grand challenge commentary: RNA epigenetics? Nat. Chem. Biol. 2010;6:863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- Hiriart E., Vavasseur A., Touat-Todeschini L., Yamashita A., Gilquin B., Lambert E., Perot J., Shichino Y., Nazaret N., Boyault C. Mmi1 RNA surveillance machinery directs RNAi complex RITS to specific meiotic genes in fission yeast. EMBO J. 2012;31:2296–2308. doi: 10.1038/emboj.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.J., Stein J.L., Hua X., Lee S., Hibar D.P., Leow A.D., Dinov I.D., Toga A.W., Saykin A.J., Shen L., Alzheimer’s Disease Neuroimaging Initiative A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc. Natl. Acad. Sci. USA. 2010;107:8404–8409. doi: 10.1073/pnas.0910878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongay C.F., Orr-Weaver T.L. Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc. Natl. Acad. Sci. USA. 2011;108:14855–14860. doi: 10.1073/pnas.1111577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A.K. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae. Genetics. 2013;194:43–67. doi: 10.1534/genetics.112.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S., Sajini A.A., Blanco S., Dietmann S., Lombard P., Sugimoto Y., Paramor M., Gleeson J.G., Odom D.T., Ule J., Frye M. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.G., He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge A.D., Bola G., Lee A.C., MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Kane S.E., Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol. Cell. Biol. 1985;5:2298–2306. doi: 10.1128/mcb.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.J., Jeong S.J., Kim K.N., Baek I.J., Chang M., Kang C.M., Park Y.S., Yun C.W. A novel protein, Pho92, has a conserved YTH domain and regulates phosphate metabolism by decreasing the mRNA stability of PHO4 in Saccharomyces cerevisiae. Biochem. J. 2014;457:391–400. doi: 10.1042/BJ20130862. [DOI] [PubMed] [Google Scholar]
- Karijolich J., Yu Y.T. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature. 2011;474:395–398. doi: 10.1038/nature10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai G., Yamamoto Y., Kamimura T., Masegi T., Sekine M., Hata T., Iimori T., Watanabe T., Miyazawa T., Yokoyama S. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2′-hydroxyl group. Biochemistry. 1992;31:1040–1046. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- Keller L., Xu W.L., Wang H.X., Winblad B., Fratiglioni L., Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. J. Alzheimers Dis. 2011;23:461–469. doi: 10.3233/JAD-2010-101068. [DOI] [PubMed] [Google Scholar]
- Khoddami V., Cairns B.R. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat. Biotechnol. 2013;31:458–464. doi: 10.1038/nbt.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo A.H., Derrington I.M., Brinkerhoff H., Langford K.W., Nova I.C., Samson J.M., Bartlett J.J., Pavlenok M., Gundlach J.H. Detection and mapping of 5-methylcytosine and 5-hydroxymethylcytosine with nanopore MspA. Proc. Natl. Acad. Sci. USA. 2013;110:18904–18909. doi: 10.1073/pnas.1310240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.T., Bartolomei M.S. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Li J., Yang Z., Yu B., Liu J., Chen X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr. Biol. 2005;15:1501–1507. doi: 10.1016/j.cub.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Parisien M., Dai Q., Zheng G.Q., He C., Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19:1848–1856. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L., Jia G., Yu M., Lu Z., Deng X. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer T.R., Dinger M.E., Mattick J.S. Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- Meyer K.D., Jaffrey S.R. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 2014;15:313–326. doi: 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.F., Walker S.E., Algire M.A., Park E.H., Hinnebusch A.G., Lorsch J.R. The 5′-7-methylguanosine cap on eukaryotic mRNAs serves both to stimulate canonical translation initiation and to block an alternative pathway. Mol. Cell. 2010;39:950–962. doi: 10.1016/j.molcel.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekna-Przybylska D., Decatur W.A., Fournier M.J. The 3D rRNA modification maps database: with interactive tools for ribosome analysis. Nucleic Acids Res. 2008;36(Database issue):D178–D183. doi: 10.1093/nar/gkm855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping X.L., Sun B.F., Wang L., Xiao W., Yang X., Wang W.J., Adhikari S., Shi Y., Lv Y., Chen Y.S. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M.A., D’Silva S., Kon Y., Phizicky E.M. tRNAHis 5-methylcytidine levels increase in response to several growth arrest conditions in Saccharomyces cerevisiae. RNA. 2013;19:243–256. doi: 10.1261/rna.035808.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia M., Fu Y., Pavon-Eternod M., He C., Pan T. Genome-wide analysis of N1-methyl-adenosine modification in human tRNAs. RNA. 2010;16:1317–1327. doi: 10.1261/rna.2057810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Pollex T., Hanna K., Tuorto F., Meusburger M., Helm M., Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J., Wescoe Z.L., Abu-Shumays R., Vivian J.T., Baatar B., Karplus K., Akeson M. Error rates for nanopore discrimination among cytosine, methylcytosine, and hydroxymethylcytosine along individual DNA strands. Proc. Natl. Acad. Sci. USA. 2013;110:18910–18915. doi: 10.1073/pnas.1310615110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Agarwala S.D., Mumbach M.R., Jovanovic M., Mertins P., Shishkin A., Tabach Y., Mikkelsen T.S., Satija R., Ruvkun G. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Bernstein D.A., Mumbach M.R., Jovanovic M., Herbst R.H., Leon-Ricardo B.X., Engreitz J.M. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014 doi: 10.1016/j.cell.2014.08.028. Published online September 10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J.C., Clancy M.J. IME4, a gene that mediates MAT and nutritional control of meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992;12:1078–1086. doi: 10.1128/mcb.12.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin B.S., Kim J.R., Walker S.E., Dong J., Lorsch J.R., Dever T.E. Initiation factor eIF2γ promotes eIF2-GTP-Met-tRNAi(Met) ternary complex binding to the 40S ribosome. Nat. Struct. Mol. Biol. 2011;18:1227–1234. doi: 10.1038/nsmb.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires J.E., Patel H.R., Nousch M., Sibbritt T., Humphreys D.T., Parker B.J., Suter C.M., Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G., Shimazu N., Tanaka M. A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science. 2012;336:355–359. doi: 10.1126/science.1219491. [DOI] [PubMed] [Google Scholar]
- Tadros W., Lipshitz H.D. The maternal-to-zygotic transition: a play in two acts. Development. 2009;136:3033–3042. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- Topisirovic I., Svitkin Y.V., Sonenberg N., Shatkin A.J. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip. Rev. RNA. 2011;2:277–298. doi: 10.1002/wrna.52. [DOI] [PubMed] [Google Scholar]
- Tuck M.T. Partial purification of a 6-methyladenine mRNA methyltransferase which modifies internal adenine residues. Biochem. J. 1992;288:233–240. doi: 10.1042/bj2880233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilfan I.D., Tsai Y.C., Clark T.A., Wegener J., Dai Q., Yi C.Q., Pan T., Turner S.W., Korlach J. Analysis of RNA base modification and structural rearrangement by single-molecule real-time detection of reverse transcription. J. Nanobiotechnology. 2013;11:8. doi: 10.1186/1477-3155-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., He C. Reading RNA methylation codes through methyl-specific binding proteins. RNA Biol. 2014;11:669–672. doi: 10.4161/rna.28829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D., Fu Y., Parisien M., Dai Q., Jia G. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Y., Toth J.I., Petroski M.D., Zhang Z., Zhao J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C.M., Moss B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry. 1977;16:1672–1676. doi: 10.1021/bi00627a023. [DOI] [PubMed] [Google Scholar]
- Wei C.M., Gershowitz A., Moss B. 5′-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry. 1976;15:397–401. doi: 10.1021/bi00647a024. [DOI] [PubMed] [Google Scholar]
- Yi C., Pan T. Cellular dynamics of RNA modification. Acc. Chem. Res. 2011;44:1380–1388. doi: 10.1021/ar200057m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Yang Z., Li J., Minakhina S., Yang M., Padgett R.W., Steward R., Chen X. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu Z., Yi J., Tang H., Xing J., Yu M., Tong T., Shang Y., Gorospe M., Wang W. The tRNA methyltransferase NSun2 stabilizes p16INK4 mRNA by methylating the 3′-untranslated region of p16. Nat. Commun. 2012;3:712. doi: 10.1038/ncomms1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J., Vågbø C.B., Shi Y., Wang W.L., Song S.H. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Li H., Bodi Z., Button J., Vespa L., Herzog M., Fray R.G. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Züst R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., Szretter K.J., Baker S.C., Barchet W., Diamond M.S. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]