Abstract
Bartonella henselae has been implicated as a causative agent of chronic uveitis in people and in some cats. The objective of this study was to determine whether Bartonella species seroprevalence or titer magnitude varies among cats with uveitis, cats without ocular diseases recorded and healthy cats, while controlling for age and risk of flea exposure based on state of residence. There was no difference in seroprevalence rates or titer magnitude between cats with uveitis and cats with non-ocular diseases. Healthy cats were more likely to be seropositive for Bartonella species than cats with uveitis. The median Bartonella species titer was 1:64 for all groups, although healthy cats were more likely to have higher titers than cats with uveitis and cats with non-ocular disease. The results suggest that serum antibody tests alone cannot be used to document clinical uveitis associated with Bartonella species infection.
Anterior uveitis in cats can have endogenous or exogenous causes (Powell and Lappin 2001). Exogenous uveitis is the result of an external influence on the eye, such as trauma or surgery, and can generally be diagnosed based upon history and ophthalmic examination. Some causes of endogenous uveitis, such as primary intraocular neoplasia or cataract, also can be diagnosed by ophthalmic examination. However, infectious or immune-mediated causes are not so easily identified because of the lack of adequate diagnostic tests. In these cases, a diagnosis of idiopathic uveitis is made. The infectious agents commonly associated with endogenous uveitis include feline infectious peritonitis virus (Peiffer and Wilcock 1991), feline immunodeficiency virus (FIV) (English et al 1990), feline leukemia virus (FeLV) (Brightman et al 1991), Toxoplasma gondii (Lappin et al 1992), feline herpesvirus type 1 (FHV-1) (Maggs et al 1999), and systemic mycoses (Gerding et al 1994, Medleau et al 1995). However, it is likely that other infectious agents can also cause intraocular inflammation.
Cats are the main reservoir for Bartonella henselae and infection of cats and humans is extremely common (Jackson et al 1993, Nutter et al 2004). The organism replicates in endothelial cells, persists within erythrocytes for months to years and can result in a variety of chronic immune-mediated reactions in the host (Kordick and Breitschwerdt 1995, Resto-Ruiz et al 2003). Bartonella henselae is the most common cause of cat scratch disease in people and has also been associated with ocular inflammation in humans (Rothova et al 1998, Ormerod and Dailey 1999, Cunningham and Koehler 2000, Wade et al 2000).
The first published report of presumptive feline ocular bartonellosis described a cat with chronic anterior uveitis, B henselae antibodies in serum, local (intraocular) production of B henselae antibodies, and clinical improvement following administration of doxycycline (Lappin and Black 1999). In another report, serum and aqueous humor were collected from client-owned cats with uveitis and cats without uveitis within a humane society (Lappin et al 2000). Aqueous humor was tested for anti-Bartonella species IgM and IgG and the results compared to those from the serum to determine whether intraocular production of antibodies against Bartonella species was occurring. In addition, aqueous humor was assayed for DNA from Bartonella species using a conventional polymerase chain reaction (PCR) assay. Cats with uveitis and cats without uveitis had B henselae DNA amplified from the aqueous humor but only cats with uveitis had intraocular production of antibodies against B henselae. In another study, the authors documented serum antibody production against Bartonella species via Western blot immunoassays in cats with uveitis (Ketring et al 2004).
While Bartonella species are now considered by some veterinary ophthalmologists to cause acute and chronic uveitis in cats, the number of cats with proven Bartonella species-associated uveitis is minimal to date. Additional data are needed to determine the importance of this disease syndrome in cats. The purpose of this study was to compare Bartonella species serum antibody test results among groups of cats with or without uveitis.
Materials and methods
Experimental design
An email was posted on the American College of Veterinary Ophthalmologists list-serve requesting submission of serum and aqueous humor samples from cats with endogenous uveitis for free infectious disease agent testing. Samples were collected from client-owned cats between January 1, 2003 and January 1, 2004. Most cats had been examined by a veterinary ophthalmologist and were suspected to have idiopathic or infectious uveitis (group 1: cats with uveitis). Samples were included whether or not treatments had been administered and whether the inflammation involved the anterior or posterior uvea, or both. Samples were shipped by overnight express on cold packs and, upon receipt, were frozen at −70°C until the serum was tested in this study.
The records' database in the Specialized Infectious Diseases Laboratory at Colorado State University was searched for feline serum samples tested over the same time period and then sequentially selected based on availability of adequate sample volume for additional testing and presence of clinical illness without mention of ocular disease (group 2: clinically ill cats without ocular disease noted). Group 3 (healthy cats) consisted of sequentially selected, healthy cats for which adequate sample was available for additional testing. Samples were collected between April 1, 1998 and April 1, 2000. Age, sex, and geographical location (state in the USA) were recorded for each case. Each sample had been previously submitted for infectious disease testing. The most commonly requested tests included Toxoplasma gondii, FeLV, FIV, and coronavirus. The samples had been stored at −20°C or −80°C until used in this study.
All samples were thawed and assayed in an IgG enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against B henselae (Lappin et al 2000). A 1:64 dilution of positive control, negative control, and suspect sera were each assayed in quadruplicate wells and the mean absorbencies calculated. Mean absorbance values were converted to %ELISA units by use of the following formula: (test sample mean absorbance minus the negative control sample mean absorbance)/(positive control sample mean absorbance minus the negative control sample mean absorbance) multiplied by 100. An individual cat was considered positive for B henselae antibodies if the %ELISA value was greater than the mean %ELISA value plus 3SD of the pre-inoculation samples for a group of 26 specific pathogen-free cats (10 kittens at 8 weeks of age and 16 cats at 3 years of age). Positive suspect serum sample results were converted to estimated titers ranging from 1:64 to 1:4096 by comparing to a standard curve.
Statistical evaluation
Age, flea risk, clinical manifestation and B henselae titer were entered into a spreadsheet and analyzed by use of a commercially available statistical software package (SAS, SAS Institute, Cary, NC, version 9.1). Whether or not cats in this study had been exposed to Ctenocephalides felis was unknown. Therefore, cats were classified as having ‘high’ or ‘low’ flea risk based upon state of origin (Jameson et al 1995). Cats from the following geographic areas (states) were categorized as having low risk of flea exposure: Alaska, Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming. Cats from all other states were considered to have a high risk of flea exposure. Relationships between B henselae serological status (positive or negative) and clinical presentations were first assessed by Fisher's exact test. Additionally, logistic regression was used to assess the influence of serological status, age, and risk of flea exposure on clinical presentation. Cats were grouped into five age classes of approximately equal sample sizes (<2, 2 to <5, 5 to <8, 8 to <11, and ≥11 years of age). Relationships between B henselae titer magnitude and clinical manifestations were assessed by determining median titers for each group and evaluated with Wilcoxon's rank sum test. Significance was defined as P<0.05.
Results
Group 1 consisted of 113 cats with uveitis. Of the cats, 42 were from low flea risk states, 65 were from high flea risk states, and state was unknown for seven cats. Bartonella species seroprevalence was 54.9% (62 of 113 cats) with titers ranging from 0 to 1:512 and a median titer of 1:64.
Group 2 (clinically ill cats without ocular disease noted) consisted of 156 cats. Of the cats, 60 were from low flea risk states, 92 were from high risk states, and state was unknown for four cats. Bartonella species seroprevalence rate was 62.8% (98 of 156 cats) with titers ranging from 0 to 1:1024 and a median titer of 1:64.
Group 3 (healthy cats) consisted of 97 cats. Of the cats, 26 were from low flea risk states, 70 were from high risk states, and state was unknown for one cat. Bartonella species seroprevalence rate was 70.1% (68 of 97 cats) with titers ranging from 0 to 1:8192 and a median titer of 1:64.
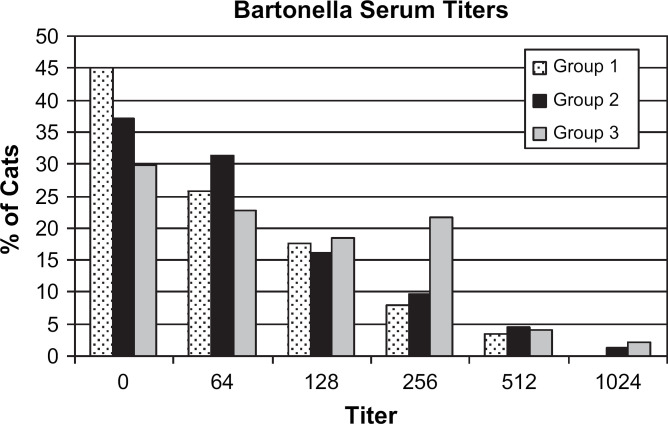
In the initial statistical evaluation, group 3 cats had a greater seropositive rate than group 1 cats (Table 1, P=0.0322). After the addition of variables (age and risk of flea exposure), the effect of group was still significant (P=0.0126). The likelihood of being seropositive for Bartonella species increased with higher flea risk (P<0.0001; odds ratio (OR)=2.731) but decreased as age increased (P=0.0035; OR=0.779) (Table 2). Median titers were equal among groups; however, Wilcoxon's rank sum test revealed that the mean rank of titers in group 3 was significantly greater than that in group 1 (P=0.0024). Figure 1 provides a bar graph of the distribution of Bartonella species antibody titers.
Table 1.
Relationships between Bartonella henselae seroprevalence rates among cats with uveitis (group 1), ill cats without recorded ocular disease (group 2), and healthy cats (group 3)
| Seroprevalence number of positives (%) | P value * | |
|---|---|---|
| Group 1 (n=113) | 62 (54.9%) | |
| Group 2 (n=156) | 98 (62.8%) | 0.2094 |
| Group 3 (n=97) | 68 (70.1%) | 0.0322 |
Fisher's exact test was used to compare group 1 to group 2 and group 1 to group 3.
Table 2.
Influence of clinical presentation (group), risk of flea exposure and age on serological status
| Effect | P value |
| Group | 0.0126 |
| Flea-risk | <0.0001 |
| Age class * | 0.0035 |
Cats were grouped into five age classes of approximately equal number: <2, 2 to <5, 5 to<8, 8 to <11 and ≥11 years of age.
Fig 1.
Distribution of Bartonella species antibody titers in cats with or without uveitis. Group 1: cats with uveitis, group 2: ill cats without ocular disease noted, and group 3: healthy cats. The serum Bartonella species titer for one cat in group 3 was 1:8192. This value was not included in the graph.
Discussion
Due to its retrospective nature, there are several limitations to this study. Samples were submitted by multiple veterinary clinicians (both board certified ophthalmologists and general practitioners) and the motivation to submit sera for testing differed among groups. Thus one of the major weaknesses of this study is the lack of a consistent ophthalmic examination among cases. It is possible that the eyes of some cats in the control groups were not extensively examined and may have had uveitis. This difference between groups may be heightened as cats in group 1 were presented for ophthalmic disease and were examined by a board certified ophthalmologist, whereas those in groups 2 and 3 were not. In addition, the complete medical record was not available; therefore, the ophthalmic findings could not be used to accurately classify cats as having anterior uveitis, posterior uveitis, or panuveitis. Due to the association between flea infestation and B henselae, cats in this study were classified into high or low flea risk categories by geographic location (state) and it was shown that presence of Bartonella species antibodies was associated with high flea risk. However, knowledge of the physical presence of fleas would have been more accurate as flea prevalence can vary within states and can also be dependent upon the housing status of individual cats (indoor vs outdoor). Lastly, because there is serological cross-reactivity between B henselae and other Bartonella species, results of the antibody test in this study cannot be used to document specific exposure to B henselae. However, in most studies that have used culture or genetic sequencing to evaluate the Bartonella species causing feline infection in the United States, B henselae is the most common (Guptill et al 2004, Lappin et al 2006).
Although most people that are exposed to B henselae are subclinically infected, cat scratch disease occurs in a small percentage. Cat scratch disease was estimated at 9.3 cases per 100,000 people per year in the United States (Jackson et al 1993). Approximately 5–10% of people with cat scratch disease are reported to have ocular manifestations such as Parinaud's oculoglandular syndrome, optic neuritis, vitritis, retinitis or anterior uveitis (Ormerod and Dailey 1999, Wade et al 2000). It is not known why some people develop ocular bartonellosis and others do not. It may relate to the host response to the organism (Resto-Ruiz et al 2003). However, it also may relate to the strain of B henselae as it appears that some strains are more pathogenic than others (Woestyn et al 2004). Co-infection with other agents may also play a role in some cases. However, cats experimentally coinfected with T gondii, B henselae, and FHV-1 did not develop ocular bartonellosis (Powell et al 2002).
As many as 93% of cats in some geographical regions are seropositive for B henselae with the highest prevalence in warm, humid climates that are well suited to support flea populations (Nutter et al 2004). Due to the high prevalence of serum antibodies in naturally exposed cats, it is difficult to determine how many cats develop clinical disease due to Bartonella species and it is difficult to confirm the diagnosis in individual cats. For example, in one study of clinically ill cats in North Carolina, multiple clinical syndromes were assessed for association with the presence of Bartonella species antibodies. No association was demonstrated for most abnormalities, including ocular disease (Breitschwerdt et al 2005). These results indicate that, as with other causative agents of uveitis (eg, T gondii, coronaviruses, FHV-1), the presence of serum antibodies to B henselae does not correlate with the precise cause of the intraocular inflammation in individual cats (Peiffer and Wilcock 1991). Confirmation of B henselae as the cause of uveitis has been attempted by locating the organism's DNA within the eye by PCR and by detecting local antibody production within the eye (Lappin et al 2000). As Bartonella species is an intraerythrocytic bacterium and most cats with uveitis have some degree of at least microscopic hyphema, the detection of B henselae DNA in aqueous humor does not prove ocular infection. In addition, the organism in erythrocytes could enter the eye via hemorrhage induced during anterior chamber paracentesis. Detection of local antibody production within the eye can be used as an indirect method of diagnosing local tissue infection and has been previously used with T gondii (Lappin et al 1992), FHV-1 (Maggs et al 1999), and B henselae (Lappin et al 2000). Further studies of this type are needed on greater numbers of cats with and without uveitis in order to determine if an epidemiologic association between B henselae and anterior uveitis exists. It is also possible that comparison of serum and aqueous humor B henselae antigen recognition patterns between cats with uveitis and those without ocular disease could identify improved diagnostic parameters (Freeland et al 1999).
Using this study design, there was no difference in seroprevalence between cats with uveitis and cats with non-ocular diseases; however, healthy cats were more likely to be seropositive for Bartonella species than cats with uveitis. Although there was overlap in the magnitude of serum titers and the median serum titers were identical (1:64) in all three groups, the healthy cats were more likely to have higher titers than cats with uveitis. These results suggest that Bartonella species antibody titer magnitude is unlikely to correlate with the presence of disease. Previous studies have found an increasing proportion of cats seropositive for Bartonella species with increasing age which may be related to an increased chance of exposure over time (Chomel et al 1995, Guptill et al 2004). In this study, the likelihood of being seropositive for Bartonella species decreased with increasing age. It is unclear why there are differences between these studies; they may merely be due to sample selection. For example, it is possible that older cats in this study were more likely to be administered antibiotics or flea control products.
In summary, the results of this study document that the presence or magnitude of Bartonella species serum antibodies cannot be used alone to document ocular bartonellosis in cats. Until more sensitive and specific diagnostic tests are identified, one can only suspect B henselae as a possible cause of uveitis if B henselae DNA or antibodies directed against the organism are detected in blood or aqueous humor, if other known causes of uveitis are eliminated, and if their clinical signs resolve following administration of appropriate antibiotic therapy.
Acknowledgments
The authors express appreciation to the veterinarians who submitted samples for this research and to Jennifer Hawley, Arianne Morris, and Melissa Brewer for their technical assistance.
References
- Breitschwerdt E.B., Levine J.F., Radulovic S., Hanby S.B., Kordick D.L., LaPerle K.M.D. Bartonella henselae and Rickettsia seroreactivity in a sick cat population from North Carolina, International Journal of Applied Research in Veterinary Medicine 3, 2005, 287–302. [Google Scholar]
- Brightman A.H., Ogilvie G.K., Tompkins M. Ocular disease in FeLV positive cats: 11 cases (1981–1986), Journal of the American Veterinary Medical Association 198, 1991, 1049–1051. [PubMed] [Google Scholar]
- Chomel B.B., Abbott R.C., Kasten R.W., Floyd-Hawkins K.A., Kass P.A., Glaser C.A., Pedersen N.C., Koehler J.E. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers, Journal of Clinical Microbiology 33, 1995, 2445–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham E.T., Koehler J.E. Ocular bartonellosis, American Journal of Ophthalmology 130, 2000, 340–349. [DOI] [PubMed] [Google Scholar]
- English R.V., Davidson M.G., Nasisse M.P., Jamieson V.E., Lappin M.R. Intraocular disease associated with feline immunodeficiency virus infection in cats, Journal of the American Veterinary Medical Association 196, 1990, 1116–1119. [PubMed] [Google Scholar]
- Freeland R.L., Scholl D.T., Rohde K.R., Shelton L.J., O'Reilly K.L. Identification of Bartonella-specific immunodominant antigens recognized by the feline humoral immune system, Clinical and Diagnostic Laboratory Immunology 6, 1999, 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding P.A., Jr., Morton L.D., Dye J.A. Ocular and disseminated candidiasis in an immunosuppressed cat, Journal of the American Veterinary Medical Association 204, 1994, 1635–1638. [PubMed] [Google Scholar]
- Guptill L., Wu C.C., HogenEsch H., Slater L.N., Glickman N., Dunham A., Syme H., Glickman L. Prevalence, risk factors, and genetic diversity of Bartonella henselae infections in pet cats in four regions of the United States, Journal of Clinical Microbiology 42, 2004, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.A., Perkins B.A., Wenger J.D. Cat scratch disease in the United States: an analysis of three national databases, American Journal of Public Health 83, 1993, 1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson P.H., Greene C.E., Regnery R.L., Dryden M., Marks A., Brown J., Cooper J., Glaus B., Greene R. Prevalence of Bartonella henselae antibodies in pet cats throughout regions of North America, Journal of Infectious Diseases 172, 1995, 1145–1149. [DOI] [PubMed] [Google Scholar]
- Ketring K.L., Zuckerman E.E., Hardy W.D., Jr. Bartonella: a new etiological agent of feline ocular disease, Journal of the American Animal Hospital Association 40, 2004, 6–12. [DOI] [PubMed] [Google Scholar]
- Kordick D.L., Breitschwerdt E.B. Intraerythrocytic presence of Bartonella henselae, Journal of Clinical Microbiology 33, 1995, 1655–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M.R., Black J.C. Bartonella spp. infection as a possible cause of uveitis in a cat, Journal of the American Veterinary Medical Association 214, 1999, 1205–1207. [PubMed] [Google Scholar]
- Lappin M.R., Griffin B., Brunt J., Riley A., Burney D., Hawley J., Brewer M.M., Jensen W.A. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States, Journal of Feline Medicine and Surgery 8, 2006, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M.R., Kordick D.L., Breitschwerdt E.B. Bartonella spp. antibodies and DNA in aqueous humour of cats, Journal of Feline Medicine and Surgery 2, 2000, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M.R., Roberts S.M., Davidson M.G., Powell C.C., Reif J.S. Enzyme-linked immunosorbent assays for the detection of Toxoplasma gondii-specific antibodies and antigens in the aqueous humor of cats, Journal of the American Veterinary Medical Association 201, 1992, 1010–1016. [PubMed] [Google Scholar]
- Maggs D.J., Lappin M.R., Nasisse M.P. Detection of feline herpesvirus 1 specific antibodies and DNA in the aqueous humor of cats, American Journal of Veterinary Research 60, 1999, 932–936. [PubMed] [Google Scholar]
- Medleau L., Jacobs G.J., Marks M.A. Itraconazole for the treatment of cryptococcosis in cats, Journal of Veterinary Internal Medicine 9, 1995, 39–42. [DOI] [PubMed] [Google Scholar]
- Nutter F.B., Dubey J.P., Levine J.F., Breitschwerdt E.B., Ford R.B., Stoskopf M.K. Seroprevalences of antibodies against Bartonella henselae and Toxoplasma gondii and fecal shedding of Cryptosporidium spp., Giardia spp., and Toxocara cati in feral and domestic cats, Journal of the American Veterinary Medical Association 235, 2004, 1394–1398. [DOI] [PubMed] [Google Scholar]
- Ormerod L.D., Dailey J.P. Ocular manifestations of cat-scratch disease, Current Opinion in Ophthalmology 10, 1999, 209–216. [DOI] [PubMed] [Google Scholar]
- Peiffer R., Wilcock B. Histopathologic study of uveitis in cats: 139 cases (1978–1988), Journal of the American Veterinary Medical Association 198, 1991, 135–138. [PubMed] [Google Scholar]
- Powell C.C., Kordick D.L., Lappin M.R. Inoculation with Bartonella henselae followed by feline herpesvirus 1 fails to activate ocular toxoplasmosis in chronically infected cats, Journal of Feline Medicine and Surgery 4, 2002, 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C.C., Lappin M.R. Causes of feline uveitis, Compendium on Continuing Education for the Practicing Veterinarian 23, 2001, 128–141. [Google Scholar]
- Resto-Ruiz S., Burgess A., Anderson B.E. The role of the host immune response in pathogenesis of Bartonella henselae, DNA and Cell Biology 22, 2003, 431–440. [DOI] [PubMed] [Google Scholar]
- Rothova A., Kerkoff F., Hooft H.J., Ossewaarde J.M. Bartonella serology for patients with intraocular inflammatory disease, Journal of Retinal and Vitreous Diseases 18, 1998, 348–355. [DOI] [PubMed] [Google Scholar]
- Wade N.K., Jones M.R., Bhisitkul R., Fine L., Cunningham E.T., Jr. Optic disk edema associated with peripapillary serous retinal detachment: an early sign of systemic Bartonella henselae infection, American Journal of Ophthalmology 130, 2000, 327–334. [DOI] [PubMed] [Google Scholar]
- Woestyn S., Olive N., Bigaignon G., Avesani V., Delmee M. Study of genotypes and virB4 secretion gene of Bartonella henselae strains from patients with clinically defined cat scratch disease, Journal of Clinical Microbiology 42, 2004, 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]