Abstract
Background
Upper respiratory tract infection symptoms are a common cause of morbidity. Herbal preparations of the plant Echinacea purpurea have immune-enhancing properties.
Objective
To compare the frequency of upper respiratory tract symptoms in individuals receiving E purpurea capsules and those receiving placebo to evaluate the preventive efficacy of echinacea.
Methods
In a randomized, double-blind clinical trial, 90 volunteers recruited from hospital personnel were randomly assigned to receive 3 capsules twice daily of either placebo (parsley) or E purpurea for 8 weeks during the winter months. Upper respiratory tract symptoms were reported weekly during this period.
Results
Fifty-eight individuals were included in the final data analysis: 28 in the echinacea group and 30 in the placebo group. Individuals in the echinacea group reported 9 sick days per person during the 8-week period, whereas the placebo group reported 14 sick days (z = −0.42; P = .67). Mild adverse effects were noted by 8% of the echinacea group and 7% of the placebo group (P = .24).
Conclusion
Prophylactic treatment with commercially available E purpurea capsules did not significantly alter the frequency of upper respiratory tract symptoms compared with placebo use.
INTRODUCTION
Upper respiratory tract infections are a common cause of morbidity, general discomfort, and missed days of work. Symptoms are more frequently encountered during the winter “flu season.” More than 200 different viruses can cause common colds in adults, including rhinovirus (the most frequent cause), coronavirus, adenoviruses, respiratory syncytial virus, and parainfluenza viruses. 1 In the United States, on average, adults develop 2 to 4 colds and children 6 to 8 colds each year. 2
Herbal preparations of the leaves and root of the plant Echinacea purpurea increase phagocytic cells in the spleen and bone marrow, acting as phytoimmune modulators or immune system enhancers. 3, 4, 5 In vitro studies support claims of immune modulation and indicate that this effect may be mediated by a modification of the activity of polymorphonuclear neutrophil granulocytes and macrophages. 6, 7, 8, 9 Echinacea-containing preparations are extensively used for the treatment and prevention of infections and, apart from allergic reactions and an increased incidence of rash, are reported to be generally safe. 10, 11, 12, 13, 14
Clinical trials 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 testing the ability of echinacea to prevent colds and ameliorate symptoms have had mixed results. A Cochrane Review 26 found that most available studies reported benefits from echinacea use, but variations in the preparations of echinacea used and methodological quality limited the conclusions. A recent meta-analysis 27 found that standardized echinacea extracts were effective in the prevention of symptoms of the common cold after clinical inoculation. Methodological shortcomings in studies of herbal medicines, including echinacea, have been noted by other researchers as well. 28 A meta-analysis 10 of studies published since 1997 suggested that echinacea was more effective at treating colds than at preventing them.
Although the efficacy of echinacea has not been conclusively demonstrated, there were more than 2.5 million prescriptions for echinacea preparations in Germany in 1993. 8 Use of nonprescription herbal remedies is undoubtedly higher, as almost 40% of patients in a US health maintenance organization indicated using herbal remedies, and echinacea ranks fifth in herbal medicine sales. 12, 29 To address the methodological shortcomings identified in previous echinacea studies, we used a randomized, double-blind, placebo-controlled clinical trial designed to answer the following question: Does a commonly used echinacea preparation prevent or reduce upper respiratory tract infection symptoms?
METHODS
Sampling
This study was approved by the University of California San Francisco Institutional Review Board. This research was completed as part of the requirements for completion of the Family Medicine Residency Program at the University of California San Francisco-Fresno. For this reason, a convenience sample of healthy adults working in the University Medical Center Family Health Center, including residents, staff, faculty, and nursing staff, served as the participants in this study. This population was expected to have more equitable exposure to cold/influenza. Recruitment consisted of flyers distributed at the University Medical Center and the Family Health Center. Participation was voluntary, and participants were not reimbursed.
Ninety volunteers aged 18 to 65 years were recruited from hospital personnel in November and December 1998. The project was described, and written informed consent was obtained from each participant. Persons with known immune dysfunction, those undergoing immunosuppressive therapy, pregnant or lactating women, and persons with allergies to echinacea or parsley were excluded. Individual characteristics, such as age, race/ethnicity, tobacco use, presence of allergic rhinitis, use of other upper respiratory tract infection preventive measures (herbs, vitamins, or medications), and administration of the influenza vaccine, were recorded. Individuals currently using echinacea were not considered for the study, whereas those using other upper respiratory tract infection preventive measures were allowed to continue their use. Each participant was given an 8-week supply of medicine on enrollment.
Procedure
Participants were randomly assigned to either the experimental or the control group from a list generated using the random-number generator in a spreadsheet program (Microsoft Excel; Microsoft Corp, Redmond, Washington). Participants were asked to take 3 capsules 2 times daily for 8 weeks of either E purpurea, 300 mg per capsule, or parsley, 300 mg per capsule. This dose of echinacea was similar to that recommended by the manufacturer. Both types of capsules were provided by the same company and were indistinguishable in size, color, and smell. Capsules were provided in containers marked “A” or “B.” Participants, the main investigator, and all persons involved in the study remained blinded to the identity of each group until data analyses were completed.
Participants were contacted by telephone once a week by a trained research assistant and asked to report the number of days during that week in which they experienced sore throat, runny nose, headache, hoarseness, nasal congestion, muscle aches, cough, and fever. Participants with symptoms were also asked about the number of days missed from work and any medications used to treat symptoms, (eg, aspirin, acetaminophen, vitamins, and cold formulas). The number of capsules missed that week and the perceived adverse effects were also recorded. Participants were defined as nonadherent and were excluded from data analysis if they missed more than one-third of the recommended dosage for 2 or more weeks. None of the persons in this study experienced serious adverse effects requiring disclosure of group assignment. Participants were told at the completion of data analyses whether they were in the experimental or placebo group.
Statistical Analysis
Data were entered into a database program (Microsoft Access; Microsoft Corp) and were analyzed using statistical analysis software (SAS for Windows, release 6.12; SAS Institute Inc, Cary, North Carolina). A prospective power analysis was calculated. Based on the assumption of an effect size of 14%, a sample size of 80 was calculated to provide sufficient power (0.80) to detect a difference of 30% (cold frequency SD = 0.5) to 60% (cold frequency SD = 1.0) between the 2 groups at α = .05.
Missing data from dropped participants precluded an intention-to-treat analysis. A per-protocol analysis was performed. 30 Means and proportions were calculated for individual characteristics. χ2 and t tests were used to test for differences between the groups after randomization. The median number of days with each individual symptom was reported owing to the nonnormal distribution of this information. The total number of days with symptoms was calculated by summing the maximum number of days reported with the most prevalent symptom in each week. Thus, the maximum possible total number of symptom days was 56. A Wilcoxon rank sum test was used to compare the treatment and placebo groups for each of the 8 symptoms and the total symptom days. We also compared the 2 groups each week separately for persons reporting symptoms of any kind vs no symptoms at all. χ2 or Fisher exact tests were used to compare these data.
RESULTS
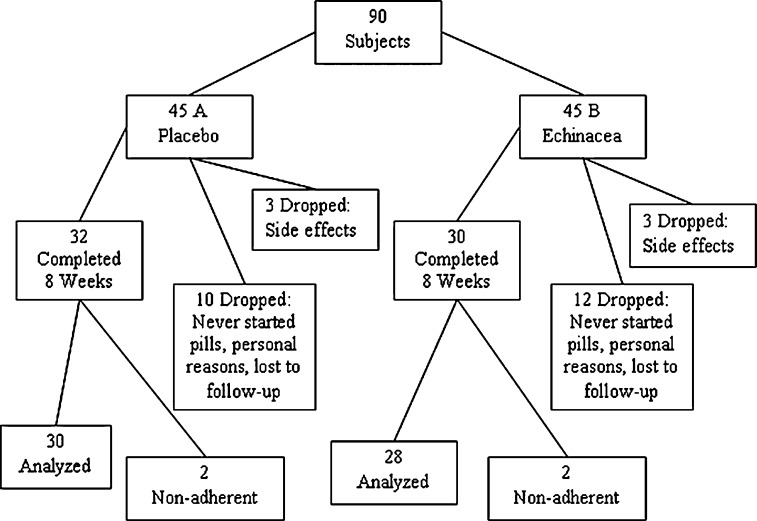
Ninety individuals were recruited for this study and randomly assigned to either the placebo or the echinacea group. Fifteen individuals in the echinacea group and 13 in the placebo group chose to leave the study, a 31% dropout rate (Fig 1) . Six individuals leaving the study (3 from each group) reported adverse effects that contributed to their study discontinuation. Each group also had 2 nonadherent individuals. There were no significant differences in individual characteristics, reason for dropout, or adherence between the 2 groups after randomization (data not shown). Fifty-eight individuals were included in the final analyses, 28 in the echinacea group and 30 in the placebo group. Table 1 has the individual characteristics for these groups and for individuals randomized but not analyzed. There were no significant differences between the echinacea and placebo groups in their demographic characteristics. There were significant differences between individuals who dropped out or were nonadherent and those who completed the study. Persons not included in the final analysis used fewer vitamins and herbs (P < .01) and reported fewer allergies (P = .03).
Figure 1.
Randomization and participation results.
Table 1.
Demographic Characteristics of the 90 Study Participants
 |
Table 2 summarizes the symptom information for the 2 groups during the 8-week period. Each of the 8 symptoms was compared separately for the 2 groups. No significant differences were found. The median total number of sick days was 9.0 for the echinacea group and 14.0 for the placebo group (z = −0.42; P = .67). Mild adverse effects were noted by 8% of the echinacea group and 7% of the placebo group (P = .24) (data not shown).
Table 2.
Incidence of Upper Respiratory Tract Symptoms in 8 Weeks by Treatment Group
 |
We compared the 2 groups each week for those reporting symptoms of any kind. Approximately 40% of the participants reported having symptoms most weeks, with no differences between the 2 groups (Fig 2) . Of individuals in either group who reported symptoms, less than half treated those symptoms, and even fewer missed work because of them. The placebo group consistently reported symptoms during any given week more than the echinacea group and treated themselves more often. However, none of these differences were statistically significant.
Figure 2.
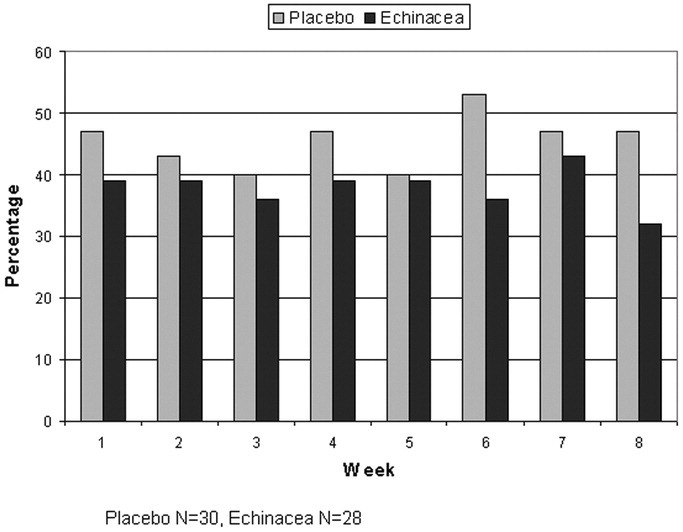
Participants with any respiratory problems.
DISCUSSION
We found no difference in total symptom days or individual respiratory symptoms for patients taking prophylactic echinacea for 8 weeks compared with those taking parsley capsules. These findings are consistent with other recent studies that do not demonstrate the effectiveness of echinacea in preventing upper respiratory tract infections.
It is conceivable that echinacea actually reduces the total number of symptom days and that this study was simply too small to detect the underlying difference. Assuming the same variance as we observed, at a significance level of 5% and 80% power, group sizes of 28 and 30 participants provided numbers sufficient to detect only a prophylactic echinacea effect of 10 total symptom days. If we had retained and analyzed all the enrolled individuals, this detectable difference would have been reduced to 8 total symptom days. We saw an actual difference of 5 days in the median total number of symptom days. The total number of symptom days ranged from 0 to 49 (of 56) in the echinacea group and from 0 to 41 in the placebo group. Assuming an actual reduction of 5 total symptom days with echinacea use, a per-group sample size of 111 would be required to detect this reduction at the same 80% power and 5% significance.
The relatively large number of unanalyzed individuals who dropped out or took less than one-third of the scheduled doses (32 of 90) may have biased the results. Excluded individuals were also different from study participants because they reported less herbal remedy use and fewer allergy symptoms. These differences suggest that the dropouts are people who are less afflicted with respiratory symptoms and less preoccupied with prevention. One might speculate that the burden of adherence to the protocol was excessive for this group. Dropouts and nonadherent participants were evenly distributed between the control and intervention groups.
Another limitation of this study is the use of health care professionals as participants, which makes it difficult to generalize these findings to other groups. We did not record participant sex or characterize the findings by this variable. Neither did we control for preventive measures that may have been taken by study participants and possibly biased the findings. Outcomes were not collected from those who dropped out, precluding an intention-to-treat analysis. However, the equal number of dropouts in both arms, coupled with the finding of no effect on symptom days in those taking echinacea, suggests that an intention-to-treat analysis would likely not have changed the conclusions.
Because this study used a dried plant extract, it can be argued that another echinacea formulation might have been more efficacious, but systematic reviews have failed to identify therapeutic distinctions between echinacea preparations. 18, 19 This study involved the use of readily available over-the-counter products widely used by the public. Although none are suspected, if parsley has effects on respiratory symptoms, we could expect that findings might be different for the use of a truly inert placebo. A MEDLINE search of parsley and respiratory tract infections yielded no results.
Allopathic medicine has much to learn about alternative therapies. However, until further research is conducted that has greater power to detect differences in outcomes, that accounts for dropout rates, and that standardizes dose effects, the findings from this randomized controlled trial suggest that echinacea does not have a meaningful effect on respiratory tract infection symptoms.
ACKNOWLEDGMENTS
We thank Sean Schafer, MD, research director of the University of California San Francisco Fresno Family Medicine Residency while this research was conducted, for his valuable contributions in the revision process of the manuscript.
Footnotes
Disclosures: Authors have nothing to disclose.
Funding Sources: This study was supported by grant 5 D39 HP 00023-09 from the Health Resources and Services Administration Border Health Education and Training Center (Ms Hughes). The medication used in this original research was donated by Natures Resource, Mission Hills, California.
REFERENCES
- 1.Mossad SB, Macknin ML, Medendorp SV, Mason P. Zinc gluconate lozenges for treating the common cold: a randomized, double-blind, placebo-controlled study. Ann Intern Med. 1996;125:81–88. doi: 10.7326/0003-4819-125-2-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Gwaltney JM, Jr, Hendley JO, Simon G, Jordan WS., Jr Rhinovirus infections in an industrial population, I: the occurrence of illness. N Engl J Med. 1966;275:1261–1268. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- 3.Sun LZ, Currier NL, Miller SC. The American coneflower: a prophylactic role involving nonspecific immunity. J Altern Complement Med. 1999;5:437–446. doi: 10.1089/acm.1999.5.437. [DOI] [PubMed] [Google Scholar]
- 4.Wustenberg P, Henneicke-von Zepelin HH, Kohler G, Stammwitz U. Efficacy and mode of action of an immunomodulator herbal preparation containing Echinacea, wild indigo, and white cedar. Adv Ther. 1999;16:51–70. [PubMed] [Google Scholar]
- 5.Percival SS. Use of echinacea in medicine. Biochem Pharmacol. 2000;60:155–158. doi: 10.1016/s0006-2952(99)00413-x. [DOI] [PubMed] [Google Scholar]
- 6.Luettig B, Steinmuller C, Gifford GE, Wagner H, Lohmann-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst. 1989;81:669–675. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- 7.Roesler J, Emmendorffer A, Steinmuller C, Luettig B, Wagner H, Lohmann-Matthes ML. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to test subjects mediates activation of the phagocyte system. Int J Immunopharmacol. 1991;13:931–941. doi: 10.1016/0192-0561(91)90046-a. [DOI] [PubMed] [Google Scholar]
- 8.Melchart D, Linde K, Worku F. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. J Altern Complement Med. 1995;1:145–160. doi: 10.1089/acm.1995.1.145. [DOI] [PubMed] [Google Scholar]
- 9.Goel V, Lovlin R, Chang C. A proprietary extract from the echinacea plant (Echinacea purpurea) enhances systemic immune response during a common cold. Phytother Res. 2005;19:689–694. doi: 10.1002/ptr.1733. [DOI] [PubMed] [Google Scholar]
- 10.Giles JT, Palat CT, III, Chien SH, Chang ZG, Kennedy DT. Evaluation of echinacea for treatment of the common cold. Pharmacotherapy. 2000;20:690–697. doi: 10.1592/phco.20.7.690.35173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mengs U, Clare CB, Poiley JA. Toxicity of Echinacea purpurea: acute, subacute and genotoxicity studies. Arzneimittelforschung. 1991;41:1076–1081. [PubMed] [Google Scholar]
- 12.Gallo M, Koren G. Can herbal products be used safely during pregnancy? focus on echinacea. Can Fam Physician. 2001;47:1727–1728. [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst E. The risk-benefit profile of commonly used herbal therapies: ginkgo, St. John's wort, ginseng, echinacea, saw palmetto, and kava. Ann Intern Med. 2002;136:42–53. doi: 10.7326/0003-4819-136-1-200201010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Taylor JA, Weber W, Standish L. Efficacy and safety of echinacea in treating upper respiratory tract infections in children: a randomized controlled trial. JAMA. 2003;290:2824–2830. doi: 10.1001/jama.290.21.2824. [DOI] [PubMed] [Google Scholar]
- 15.Lindenmuth GF, Lindenmuth EB. The efficacy of echinacea compound herbal tea preparation on the severity and duration of upper respiratory and flu symptoms: a randomized, double-blind placebo-controlled study. J Altern Complement Med. 2000;6:327–334. doi: 10.1089/10755530050120691. [DOI] [PubMed] [Google Scholar]
- 16.Henneicke-von Zepelin H, Hentschel C, Schnitker J, Kohnen R, Kohler G, Wustenberg P. Efficacy and safety of a fixed combination phytomedicine in the treatment of the common cold (acute viral respiratory tract infection): results of a randomised, double blind, placebo controlled, multicentre study. Curr Med Res Opin. 1999;15:214–227. doi: 10.1185/03007999909114094. [DOI] [PubMed] [Google Scholar]
- 17.Turner RB, Riker DK, Gangemi JD. Ineffectiveness of echinacea for prevention of experimental rhinovirus colds. Antimicrob Agents Chemother. 2000;44:1708–1709. doi: 10.1128/aac.44.6.1708-1709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulten B, Bulitta M, Ballering-Bruhl B, Koster U, Schafer M. Efficacy of Echinacea purpurea in patients with a common cold: a placebo-controlled, randomised, double-blind clinical trial. Arzneimittelforschung. 2001;51:563–568. doi: 10.1055/s-0031-1300080. [DOI] [PubMed] [Google Scholar]
- 19.Grimm W, Muller HH. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am J Med. 1999;106:138–143. doi: 10.1016/s0002-9343(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous Echinacea for prevention and treatment of upper respiratory infections. Med Lett Drugs Ther. 2002;44:29–30. [PubMed] [Google Scholar]
- 21.Weber W, Taylor JA, Stoep AV, Weiss NS, Standish LJ, Calabrese C. Echinacea purpurea for prevention of upper respiratory tract infections in children. J Altern Complement Med. 2005;11:1021–1026. doi: 10.1089/acm.2005.11.1021. [DOI] [PubMed] [Google Scholar]
- 22.Barrett BP, Brown RL, Locken K, Maberry R, Bobula JA, D'Alessio D. Treatment of the common cold with unrefined Echinacea: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:939–946. doi: 10.7326/0003-4819-137-12-200212170-00006. [DOI] [PubMed] [Google Scholar]
- 23.Goel V, Lovlin R, Barton R. Efficacy of a standardized echinacea preparation (Echinilin) for the treatment of the common cold: a randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. 2004;29:75–83. doi: 10.1111/j.1365-2710.2003.00542.x. [DOI] [PubMed] [Google Scholar]
- 24.Yale SH, Liu K. Echinacea purpurea therapy for the treatment of the common cold: a randomized, double-blind, placebo-controlled clinical trial. Arch Intern Med. 2004;164:1237–1241. doi: 10.1001/archinte.164.11.1237. [DOI] [PubMed] [Google Scholar]
- 25.Sperber SJ, Shah LP, Gilbert RD, Ritchey TW, Monto AS. Echinacea purpurea for prevention of experimental rhinovirus colds. Clin Infect Dis. 2004;38:1367–1371. doi: 10.1086/386324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD000530. CD000530. [DOI] [PubMed] [Google Scholar]
- 27.Schoop R, Klein P, Suter A, Johnston SL. Echinacea in the prevention of induced rhinovirus colds: a meta-analysis. Clin Ther. 2006;28:174–183. doi: 10.1016/j.clinthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Linde K, Jonas WB, Melchart D, Willich S. The methodological quality of randomized controlled trials of homeopathy, herbal medicines and acupuncture. Int J Epidemiol. 2001;30:526–531. doi: 10.1093/ije/30.3.526. [DOI] [PubMed] [Google Scholar]
- 29.Bennett J, Brown CM. Use of herbal remedies by patients in a health maintenance organization. J Am Pharm Assoc (Wash) 2000;40:353–358. doi: 10.1016/s1086-5802(16)31082-8. [DOI] [PubMed] [Google Scholar]
- 30.Heritier SR, Gebski VJ, Keech AC. Inclusion of patients in clinical trial analysis: the intention-to-treat principle. Med J Aust. 2003;179:438–440. doi: 10.5694/j.1326-5377.2003.tb05627.x. [DOI] [PubMed] [Google Scholar]