Abstract
Background
Little is known about adenovirus infections in adult lung transplant recipients. Because the virus can establish latency, re-activation may be relatively common after transplantation.
Methods
We assessed adenovirus infection in 80 adult lung transplant recipients. Adenovirus polymerase chain reaction (real-time PCR assay; limit of detection ∼25 copies/ml plasma) was done on plasma samples collected at regular intervals until 1 year post-transplant.
Results
Adenovirus DNA was detected in 18 of 80 patients (22.5%) and in 19 of 595 (3.4%) plasma samples up to 12 months post-transplant. Median time to detection of viremia was 134 days post-transplant (range 1 to 370 days). Median viral load was 180 copies/ml plasma (range 50 to 360 copies/ml). Symptoms were evaluated at the time of adenovirus detection: 14 of 18 (78%) patients were asymptomatic; 4 of 18 (22%) patients had otherwise unexplained febrile/flu-like illness that resolved spontaneously. Adenovirus was not found to be a trigger for acute rejection. No detrimental effect on pulmonary function was seen immediately after adenovirus infection.
Conclusions
Adenovirus viremia is common in adult lung transplant recipients. In contrast to findings on adenoviral pneumonitis in lung transplant recipients, isolated episodes of low-level viremia are self-limited and do not trigger acute rejection or a decline in pulmonary function.
Adenoviruses are double-stranded DNA viruses. Over 50 serotypes are known to cause human infections, including respiratory syndromes, gastrointestinal disease and febrile syndromes in immunocompetent hosts.1, 2 After infection, lifelong immunity appears to be serotype-specific and therefore infections with other serotypes may occur. In addition, the virus can establish latency in numerous tissues, including tonsils, adenoids, intestine and urinary tract.2 Therefore, transplant patients may be at risk of adenovirus infection either from new acquisition or from re-activation of latent virus.
In transplant patients, the spectrum of clinical sequelae due adenovirus infections seems to be broad, and may also differ in pediatric and adult populations.3, 4, 5 In children, acquisition of new viruses may be more common, whereas in adults re-activation of endogenous latent virus may be more likely to occur. Severe life-threatening disseminated disease is well described in both adult and pediatric hematopoietic stem cell recipients and solid-organ recipients.5, 6, 7, 8 Overall, lung transplant recipients with adenovirus infection appear to be at high risk for severe necrotizing pneumonitis, resulting in significant graft dysfunction and associated with high mortality.9, 10 Infection of the allograft by adenovirus has also been associated with acute and chronic rejection and graft dysfunction in studies of lung and heart transplant recipients.11, 12 However, recent data have suggested that adenovirus re-activation and viremia may be relatively common in transplant recipients and, in most cases, may not be associated with adverse clinical sequelae, especially if there is no evidence of allograft infection.13 We undertook the present study to assess the incidence, timing and clinical sequelae of adenovirus viremia in lung transplant recipients.
Methods
Patients
Patients in this study were enrolled as part of a clinical trial comparing valganciclovir prophylaxis with ganciclovir (intravenous and oral) as cytomegalovirus (CMV) prophylaxis in D+/R− and R+ lung transplant recipients. The design of the previous study with regard to inclusion and exclusion criteria has been described in detail elsewhere.14 Patients were enrolled at two Canadian transplant centers and were transplanted between 1999 and 2002. The institutional review board at each center approved the study and informed consent was obtained from the patients. The original study evaluated CMV prophylaxis and, as part of this study, patients had received oral ganciclovir, intravenous ganciclovir or oral valganciclovir up to 3 months post-transplant.
Virologic Monitoring
All patients in both groups had plasma samples saved for further testing at regular intervals until 1-year post-transplant. Initially, this was done usually every 2 weeks and then at monthly intervals. All plasma samples underwent adenovirus testing using a quantitative real-time polymerase chain reaction (PCR) assay (see later) and CMV viral load testing by quantitative PCR (Roche Cobas Amplicor Assay, Roche Diagnostic Systems, Branchburg, NJ). The results of viral load testing (both adenovirus and CMV) were not available to the treating physician. No pre-transplant samples were available for testing as part of this study. In the first year, protocol bronchoscopies were performed at only one of the two participating centers. These took place at Weeks 2 and 6 post-transplant and then at Months 3, 6, 9 and 12 post-transplant. Routine microbiologic testing was performed as well as a transbronchial biopsy to assess for rejection.
Adenovirus PCR Methods
Two sets of primers and probes were designed from the highly conserved hexon gene of adenovirus Type 2 and 4 using PrimerExpress software (ABI) designed for the detection of all serotypes.15 DNA was extracted from 200 μl of plasma using a Qiagen DNA mini-kit according to the manufacturer’s protocol (Qiagen) and eluted from the column with 50 μl of elution buffer. To quantify adenovirus in plasma, an external standard curve was established with a 10-fold series of dilution from one copy to 1.0E+06 copies using a 375-bp DNA fragment produced from Type 2 adenovirus. Real-time TaqMan PCR was conducted in a closed-tube sequence detection system (Model 7000, ABI-Prism, Applied Biosystems).
Briefly, the PCR was performed in a 25-μl volume containing 12.5 μl of Universal DNA Master Mix (Applied Biosystems), 10 μl DNA, 400 nmol/liter each primer and 200 nmol/liter of each probe. After initial incubation at 50°C for 2 minutes to activate Uracil-N-glycosylase and then at 95°C for 10 minutes for denaturing, PCR amplification was performed with two-step thermal cycles of 94°C for 20 seconds and 60°C for 1 minute by 45 cycles after re-heating at 95°C for 5 minutes. Amplification data were collected and analyzed by computer (Sequence Detection System, version 1.0, Applied Biosystems). The dynamic range of detection of the PCR was 25 to 2.5E+07 copies/ml plasma in normal healthy controls. Adenovirus DNA is not detectable from plasma samples when using this assay.
Clinical Definitions
In patients with detectable adenovirus viremia by PCR, clinical disease was attributed to adenovirus if detectable viremia was accompanied by compatible symptoms (including febrile illnesses, respiratory, gastrointestinal disease or evidence of infection of the allograft) in the absence of another defined etiology. In addition, all complications and microbiologic data during the first year post-transplant were assessed to determine whether adenovirus infection was diagnosed clinically outside the study evaluation. Acute rejection (Grade ≥2) was diagnosed on the basis of a transbronchial biopsy that demonstrated characteristic perivascular lymphocytic infiltrates using criteria defined by the International Society for Heart and Lung Transplantation (ISHLT).16
In patients in whom a biopsy could not be performed, a clinical diagnosis of rejection was permitted (i.e., a deterioration in lung function with no other identifiable etiology that responded to high-dose corticosteroid therapy). Effects on pulmonary function in patients with adenovirus infection were determined based on measurement of forced expiratory volume in 1 second (FEV1). An FEV1 decline of >15% within 3 months of adenovirus infection was considered potentially significant and occurring within a time period in which virally triggered declines in graft function may be playing a role. CMV disease was defined according to standard criteria.17
Informed consent was obtained from all patients for participation in the study. All clinical research was conducted according to institutional guidelines for human experimentation.
Results
Patients
A total of 80 lung transplant recipients were analyzed (63 double-lung and 17 single-lung transplant recipients) (Table 1). A total of 595 plasma samples were tested (mean number of samples per patient = 7.4, range 4 to 12). Mean age was 49.6 years (range 19 to 68), which included 27 women and 53 men. Pre-transplant underlying lung disease included cystic fibrosis (n = 18), emphysema/chronic obstructive pulmonary disease (COPD; n = 28), idiopathic pulmonary fibrosis (n = 21), α1-anti-trypsin deficiency (n = 5) or “other” (n = 8). Maintenance immunosuppression was cyclosporine/prednisone/azathioprine (n = 43), cyclosporine/prednisone/mycophenolate (n = 29) or other (n = 8). A total of 23 of 80 (28.8%) patients received induction anti-lymphocyte globulin. CMV prophylaxis consisted of intravenous ganciclovir for 2 weeks (all patients), followed by 12 weeks of oral ganciclovir (n = 30), intravenous ganciclovir (n = 10) or oral valganciclovir (n = 40).
Table 1.
Demographics and Characteristics of Patients in the Two Study Arms
| Characteristic | No adenovirus (n = 62) | Positive adenovirus (n = 18) | p-value |
|---|---|---|---|
| Mean age ± SD (years) | 48.8 ± 12.1 | 52.3 ± 13.0 | 0.8 |
| Gender (male/female) | 41 M/21 F | 12 M/6 F | 1.0 |
| Underlying disease | 0.6 | ||
| Cystic fibrosis | 14 (22.6%) | 4 (22.6%) | |
| Emphysema/COPD | 19 (30.6%) | 9 (50.0%) | |
| Idiopathic pulmonary fibrosis | 17 (27.4%) | 4 (22.2%) | |
| Other | 12 (19.4%) | 1 (5.6%) | |
| Immunosuppression | |||
| Cyclosporine/prednisone/azathioprine | 29 (46.8%) | 7 (38.9%) | 0.4 |
| Cyclosporine/prednisone/mycophenolate mofetil | 20 (32.3%) | 8 (44.4%) | |
| Other | 13 (21.0%) | 3 (16.7%) | |
| Any anti-lymphocyte globulin | 19 (30.6%) | 8 (44.4%) | 0.4 |
| Treatment for acute rejection | 34 (54.8%) | 3 (16.7%) | 0.01 |
| CMV viremia | 27 (43.5%) | 8 (44.4%) | 1.0 |
| Symptomatic CMV disease | 11 (17.7%) | 4 (22.2%) | 0.4 |
Adenovirus Viremia
Adenovirus DNA was detected in 18 of 80 patients (22.5%) in the first 12 months post-transplant. Of the total 595 samples tested from these patients, 19 (3.4%) were positive. Quantitative viral load data are shown in Figure 1. The median viral load was 180 copies/ml (range 50 to 410 copies/ml). Viremia was detected on one occasion in 17 of the 18 patients and on two occasions in 1 patient. Timing of viremia post-transplant is shown in Figure 2, and was relatively evenly distributed throughout the first year post-transplant. Median time to detection of first viremia was 134 days post-transplant (range 1 to 370 days). Donors were not specifically tested for adenovirus. Donor chart review for the cases in which virus was detected early in the recipient did not reveal any clinical or radiologic evidence of active infection in the donor. Symptoms were reviewed at the time of viremia. Of the 18 patients with viremia, 14 (78%) had no symptoms at the time of viremia. Four of 18 patients (22%) had symptoms. All symptoms consisted of a febrile, flu-like illness. No patients had clinical or radiologic evidence of pneumonitis at the time of viremia, and adenovirus pneumonitis was not documented in any of the other study patients during the study period.
Figure 1.
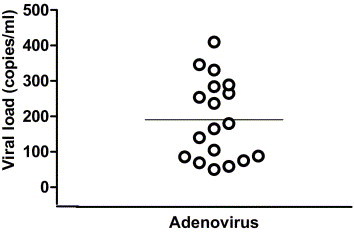
Adenovirus viral loads in patients with infection (viral load in copies per milliliter; solid line indicates viral load). Viral loads in 4 symptomatic patients were 86, 105, 265 and 346 copies/ml, respectively.
Figure 2.
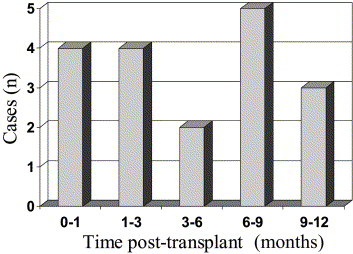
Timing of first detectable viremia in 18 patients with adenovirus infection.
In the 4 patients with viremia and symptoms, no other etiology of symptoms was found (all were negative for CMV pp65 antigenemia). In all cases, symptoms resolved spontaneously without any specific therapy and without any change in immunosuppression. Adenovirus viral load was compared in symptomatic vs asymptomatic patients. Median viral load was 185 copies/ml in patients with symptoms (range 86 to 346) vs 180 copies/ml in asymptomatic patients (range 50 to 410; p = not significant [NS]). Underlying disease in these 4 patients was cystic fibrosis (n = 2), idiopathic pulmonary fibrosis (n = 1) and emphysema (n = 1). Immunosuppression consisted of cyclosporine, prednisone and azathioprine in 3 of the 4 patients. The low viral loads observed in the present study are in contrast to the high viral loads we observed in the clinical setting among transplant patients with documented adenoviral pneumonitis. In the previous respiratory virus season, 2 patients (1 kidney transplant recipient and 1 lung transplant recipient, neither part of this study) were diagnosed with adenovirus pneumonitis. Viral loads in these patients were 6.5 × 105 and 9.5 × 105 copies/ml plasma, respectively.
Risk factors for viremia were also analyzed (Table 1). Maintenance immunosuppression regimens were similar in viremic vs non-viremic patients, with cyclosporine/prednisone/azathioprine and cyclosporine/prednisone/mycophenolate mofetil the most commonly used combinations (viremic patients: 7 of 18 [38.9%] and 8 of 18 [44.4%] on these regimens, respectively; non-viremic patients: 29 of 62 [46.8%] and 20 of 62 [32.3%] on these regimens, respectively; p = NS for all comparisons). Anti-lymphocyte globulin (ALG) therapy (either for induction or as treatment for acute rejection) was associated with a slightly higher incidence of adenovirus, although this was not statistically significant (adenovirus patients: 8 of 18 [44.4%] had previous ALG; non-adenovirus patients: 19 of 62 [30.6%] had previous ALG; p = 0.42). No difference in age and gender of viremic patients was observed (Table 1). No specific epidemiologic links were observed between patients with viremia, and no evidence of seasonality was apparent.
Indirect Effects
Adenovirus infection was not found to be a trigger for acute rejection (clinically treated or biopsy proven). The overall incidence of one or more acute rejection episodes in patients with adenovirus viremia was 3 of 18 (16.7%) vs 34 of 62 (54.8%) in non-viremic patients (p = 0.01). In only 1 case did an episode of acute rejection develop within the 3 months after viremia, whereas, in 2 cases, the rejection episode occurred before viremia. In all 18 patients with viremia, pulmonary function tests (FEV1) remained stable in the immediate 3 months after detection of viremia. No interaction with the development of CMV viremia or symptomatic CMV disease was observed. CMV viremia occurred in 8 of 18 (44.4%) patients with adenovirus vs 27 of 62 (43.5%) patients without adenovirus (p = NS). Symptomatic CMV disease occurred in 4 of 18 (22.2%) patients with adenovirus vs 11 of 62 (17.7%) patients without adenovirus (p = NS). In patients with both adenovirus and CMV viremia (n = 8), CMV preceded adenovirus in 5 patients and occurred after adenovirus in 3 patients. Mean peak CMV viral load in patients with adenovirus was 8,630 copies/ml (range undetectable to 56,000 copies/ml) vs 8,645 copies/ml (range undetectable to 81,900 copies/ml) in patients without adenovirus (p = NS). CMV prophylaxis did not influence detection of adenovirus viremia. The number of patients who were positive while on anti-CMV prophylaxis was 8 of 18 (45%), similar to the rate of positivity after discontinuation of prophylaxis.
Discussion
We have shown that adenovirus viremia is very common in lung transplant recipients, and was detected in 18 of 80 (22.5%) of the patients during the first year post-transplant. The majority of infections were not associated with any symptoms. In 4 of 18 (22%) patients, symptoms likely attributable to adenovirus were present and consisted of a febrile, flu-like illness. In all patients with symptoms, resolution occurred without use of anti-viral therapy or reduction of immunosuppression. Viral loads were generally low in these patients (range 86 to 346 copies/ml), and viremia was not sustained. However, this did represent true replicative infection rather than detection of latent virus, because only cell-free samples (i.e., plasma) were used for viral detection. Adenovirus viremia was not associated with any evidence of indirect effects on graft function or presence of acute rejection.
Our results are in contrast to previous reports of adenovirus infection in lung transplant recipients. For example, in a study of pediatric lung and heart–lung recipients, specimens (swabs, bronchoalveolar lavage fluid) were prospectively tested for respiratory viruses by culture, PCR and antigen detection.12 In 16 patients adenovirus was identified in the transplanted lungs of 8 patients. Of these patients, 2 appeared to have donor-transmitted early fulminant adenovirus pneumonitis. Overall, adenovirus infection of the transplanted lung was significantly associated with graft loss, obliterative bronchiolitis (OB) and death due to respiratory failure.12
In another study, biopsy and autopsy specimens were analyzed for adenovirus by immunohistochemistry and in situ hybridization in 308 lung transplant recipients.9 Adenovirus pneumonitis was identified in 4 patients (1.3%). All cases had severe necrotizing pneumonia and were fatal. Three of the 4 cases were in pediatric transplant recipients and all cases occurred within 45 days of transplant, suggesting either donor transmission or early acquisition/re-activation in the recipient.9 Late fatal adenovirus pneumonitis occurring several years after the lung transplant has also been described.10 The PCR assay we used had a sensitivity of 25 copies/ml. This would be expected to be significantly more sensitive than the culture or direct antibody staining methods commonly used for respiratory specimens. Also, we assessed blood samples as opposed to respiratory specimens. Both of these factors may have partially accounted for the higher rate of infection observed compared with other studies.
Respiratory viral infections in adult lung transplant recipients have long been suspected as potential triggers for allograft rejection and the development of obliterative bronchiolitis or its clinical equivalent, bronchiolitis obliterans syndrome (BOS). Taking into account the limitations of recent studies (most with small, retrospective sample sizes), the literature supports an association between respiratory virus infection and BOS.18 For example, Khalifah et al19 retrospectively reviewed 259 lung transplant recipients (respiratory samples for cultures and antigen detection), and found 21 respiratory viral infections (including 2 cases of adenovirus respiratory tract infection); these patients were at increased risk for BOS and death.
Kumar et al20 prospectively studied 100 lung transplant patients, including 50 patients with respiratory virus infection and 50 controls. For respiratory specimens, molecular detection methods (PCR) were used in addition to standard viral techniques. Adenovirus was not identified in any of the patients, but the most common etiologies of viral infection were rhinovirus, coronavirus, respiratory syncytial virus (RSV) and influenza. Viral infection was associated with acute rejection and a significant decline in FEV1 over time, consistent with the development of BOS. There is evidence from other organ recipients that adenovirus infection of the allograft may be associated with indirect effects.
Shirali et al11 performed viral PCR on endomyocardial biopsies from pediatric heart transplant recipients and found that adenovirus was the most commonly detected virus found in 24 of 149 (16.1%) patients. Viral detection was associated with an increased risk of coronary vasculopathy and graft loss.
Overall, the data on adenovirus infection in lung transplant recipients suggest that infections are severe, especially in pediatric patients, and are associated with necrotizing pneumonia and result in a decline in pulmonary function. However, our data suggest that, in adults, infections are very common and often asymptomatic or associated with mild self-limited symptoms. This discrepancy may be due to several factors. First, we assessed viremia rather than infection within the allograft. It may be that the two sites of infection behave very differently in terms of symptomatology and clinical consequences. Second, used active molecular surveillance techniques, which are more likely to pick up sub-clinical cases, as compared with symptom-based diagnostic evaluation. Third, we did not assess pediatric patients, who may be more likely to have newly acquired adenovirus in the absence of pre-existing immunity. Therefore, it should be emphasized that, although no significant clinical effects of low-level viremia were observed, patients with higher viral loads may well have more severe disease or an association with rejection.
It is possible that most cases of adenovirus we detected may have been due to re-activation of a previously latent virus. However, the possibility of newly acquired adenovirus due to exogenous exposure cannot be excluded. Our results are consistent with recently published molecular surveillance data in other organ transplant recipients.13 In a study of 263 adult kidney (and kidney–pancreas), liver and heart transplant recipients, who had testing of blood samples at regular intervals, the incidence of detectable viremia was 7.2%. In 58% of these patients viremia was asymptomatic, whereas in the remaining patients viremia was associated with self-limited respiratory or gastrointestinal symptoms. In that study, the investigators did not detect an association with subsequent allograft rejection.13
Limitations of our study include the small sample size and the fixed testing intervals. For example, testing may not have necessarily been carried out at all time-points at which patients may have had compatible symptoms (e.g., respiratory or gastrointestinal symptoms). It is therefore possible that the incidence of infection may have been even higher. No additional cases of adenovirus were clinically diagnosed other than the patients who were positive by our surveillance; however, specific PCR testing for adenovirus was not routinely performed for clinical purposes during the study period. Finally, this study was considered a sub-study, part of a CMV prophylaxis study, and so adenovirus surveillance was not the primary objective.
In conclusion, adenovirus viremia is very common in adult lung transplant recipients. Such infections may be different from those in which the allograft is primarily involved. Viremic episodes were either asymptomatic or associated with only self-limited febrile syndromes despite no change in immunosuppression or specific anti-viral therapy. No association with acute rejection episodes or with a decline in lung function was observed after infection. At present, routine PCR surveillance after lung transplantation would not be indicated based on the data presented, although further studies are required.
Acknowledgments
The authors thank Jayne Fenton and Kate Toogood for assistance with data collection and sample preparation.
Footnotes
Supported in part by an unrestricted grant from Roche Pharmaceuticals.
References
- 1.Kojaoghlanian T., Flomenberg P., Horwitz M.S. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 2.Baum S.G. Adenovirus. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and practice of infectious diseases. 6th ed. Elsevier, Churchill Livingstone; Philadelphia: 2005. pp. 1835–1841. [Google Scholar]
- 3.Green M., Ljungman P., Michaels M.G. Adenovirus, parvovirus B19, papilloma virus, and polyomaviruses after hemopoietic stem cell or solid organ transplantation. In: Bowden R.A., Ljungman P., Paya C., editors. Transplant infections. 2nd ed. Lippincott, Williams and Wilkins; Philadelphia: 2003. pp. 399–411. [Google Scholar]
- 4.McLaughlin G.E., Delis S., Kashimawo L. Adenovirus infection in pediatric liver and intestinal transplant recipients: utility of DNA detection by PCR. Am J Transplant. 2003;3:224–228. doi: 10.1034/j.1600-6143.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 5.McGrath D., Falagas M.E., Freeman R. Adenovirus infection in adult orthotopic liver transplant recipients: incidence and clinical significance. J Infect Dis. 1998;177:459–462. doi: 10.1086/517375. [DOI] [PubMed] [Google Scholar]
- 6.La Rosa A.M., Champlin R.E., Mirza N. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–876. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 7.Ardehali H., Volmar K., Roberts C., Forman M., Becker L.C. Fatal disseminated adenoviral infection in a renal transplant patient. Transplantation. 2001;71:998–999. doi: 10.1097/00007890-200104150-00029. [DOI] [PubMed] [Google Scholar]
- 8.Walls T., Shankar A.G., Shingadia D. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect Dis. 2003;3:79–86. doi: 10.1016/s1473-3099(03)00515-2. [DOI] [PubMed] [Google Scholar]
- 9.Ohori N.P., Michaels M.G., Jaffe R., Williams P., Yousem S.A. Adenovirus pneumonia in lung transplant recipients. Hum Pathol. 1995;26:1073–1079. doi: 10.1016/0046-8177(95)90268-6. [DOI] [PubMed] [Google Scholar]
- 10.Simsir A., Greenebaum E., Nuovo G., Schulman L.L. Late fatal adenovirus pneumonitis in a lung transplant recipient. Transplantation. 1998;65:592–594. doi: 10.1097/00007890-199802270-00027. [DOI] [PubMed] [Google Scholar]
- 11.Shirali G.S., Ni J., Chinnock R.E. Association of viral genome with graft loss in children after cardiac transplantation. N Engl J Med. 2001;344:1498–1503. doi: 10.1056/NEJM200105173442002. [DOI] [PubMed] [Google Scholar]
- 12.Bridges N.D., Spray T.L., Collins M.H., Bowles N.E., Towbin J.A. Adenovirus infection in the lung results in graft failure after lung transplantation. J Thorac Cardiovasc Surg. 1998;116:617–623. doi: 10.1016/S0022-5223(98)70168-0. [DOI] [PubMed] [Google Scholar]
- 13.Humar A., Kumar D., Mazzulli T. A surveillance study of adenovirus infection in adult solid organ transplant recipients. Am J Transplant. 2005;5:2555–2559. doi: 10.1111/j.1600-6143.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 14.Humar A., Kumar D., Preiksaitis J. A trial of valganciclovir prophylaxis for cytomegalovirus prevention in lung transplant recipients. Am J Transplant. 2005;5:1462–1468. doi: 10.1111/j.1600-6143.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee B.E., Robinson J.L., Khurana V., Pang X.L., Preiksaitis J.K., Fox J.D. Enhanced identification of viral and atypical bacterial pathogens in lower respiratory tract samples with nucleic acid amplification tests. J Med Virol. 2006;78:702–710. doi: 10.1002/jmv.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousem S.A., Berry G.J., Cagle P.T. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 17.Humar A., Michaels M., AST ID Working Group on Infectious Disease Monitoring American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 18.Vilchez R.A., Dauber J., Kusne S. Infectious etiology of bronchiolitis obliterans: the respiratory viruses connection—myth or reality? Am J Transplant. 2003;3:245–249. doi: 10.1034/j.1600-6143.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 19.Khalifah A.P., Hachem R.R., Chakinala M.M. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 20.Kumar D., Erdman D., Keshavjee S. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5:2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


