Abstract
Objectives
Microbial point-of-care testing (POCT) has potential to revolutionize clinical care. Understanding the prognostic value of microbes identified from the upper respiratory tract (a convenient sampling site) is a necessary first step to understand potential for upper respiratory tract POCTs in assisting antimicrobial treatment decisions for respiratory infections (RTIs). The aim was to investigate the relationship between upper respiratory tract microbial detection and disease prognosis, including effects of antimicrobial use.
Methods
Data sources were the MEDLINE and Embase databases. Study eligibility criteria consisted of quantitative studies reporting microbiological and prognostic data from patients of all age groups presenting with RTI. Patients presenting to healthcare or research settings with RTI participated. Interventions included upper respiratory tract swab. The methods used were systematic review and meta-analysis.
Results
Searches identified 5156 articles, of which 754 were duplicates and 4258 excluded on title or abstract. A total of 144 full texts were screened; 21 articles were retained. Studies reported data for 15 microbes and 26 prognostic measures (390 potential associations). One hundred and seven (27%) associations were investigated statistically, of which 38 (36%) were significant. Most studies reported only prognostic value of test positive results. Meta-analyses suggested hospitalization duration was longer for patients with respiratory syncytial virus than adenovirus and influenza, but significant heterogeneity was observed between studies.
Conclusions
A quarter of potential prognostic associations have been investigated. Of these, a third were significant, suggesting considerable potential for POCT. Future research should investigate prognostic value of positive and negative tests, and interactions between test results, use of antimicrobials and microbial resistance.
Keywords: Antibacterial, Antimicrobial stewardship, Diagnosis, Point-of-care test, Primary care
Introduction
Point-of-care tests used by primary care clinicians to target antimicrobial prescribing could revolutionize the treatment of respiratory tract infections (RTI), improving patient outcomes and reducing drug side-effects and antimicrobial resistance. Primary care clinicians are responsible for the majority of human antibiotic use in the UK, USA and Europe. Paediatric RTI is the most common presentation managed by primary care physicians [1], [2]. Antibiotics are prescribed at up to 67% of RTI consultations [3], yet there is strong evidence that a large proportion of these prescriptions are unnecessary [4], [5]. Antibiotic overprescribing has been partially attributed to uncertainty described by clinicians in identifying patients who may subsequently develop serious illness and require hospital intervention [6]. Policy makers, primary care clinicians and the research community are calling for evidence to help differentiate patients who would benefit from antimicrobials from those who would not [5], [7], [8].
There is currently no way for a primary care clinician to distinguish viral from bacterial aetiology for respiratory infections in a timely manner. The burden placed on primary care means that evidence-based algorithms and tests are actively being sought; for example, an algorithm to identify children at risk of hospitalization has recently been developed in a large observational study [9]. However, this algorithm does not differentiate bacterial from viral infection. Additionally, C-reactive protein blood testing to target prescribing in adults and children is being investigated [10] but is not routinely used in the UK. A third possible strategy, and the subject of this review, is to rapidly test respiratory microbiological samples. Point-of-care test technology is rapidly developing: test devices are now able to detect common respiratory tract microbes in 20 min to 2 hr [11], and could therefore be of value in primary care.
Upper respiratory tract samples are acceptable to patients and are easily obtained in primary care [9]. Recent evidence suggests that specific microbes are weakly associated with clinical characteristics of children with RTI at presentation to primary care and may be aetiological [12]. However, the association between the detection of these microbes and (a) patient prognosis and (b) patient response to antimicrobial treatment is unknown. If detection of specific microbes from the upper respiratory tract was associated with response to antimicrobial treatment, tests for these microbes could be used to target antimicrobial prescribing.
Research question
The aim was to determine whether specific microbes detected from the upper respiratory tract are associated with the prognosis in patients of all ages presenting to all healthcare services with RTIs. Secondary questions were whether prognosis is affected by prescription of antimicrobials or the resistance status of the microbes detected.
Methods
Eligibility criteria
Studies eligible for inclusion were peer-reviewed, quantitative studies reporting microbiological and prognostic data from patients of all age groups presenting to a healthcare service or research team in an Organization for Economic Co-operation and Development member country, with diagnosis or symptoms of RTI. Studies recruiting from primary care, secondary care and community settings such as hospital outpatient or community research clinics were included. Studies were excluded where participants were recruited solely from intensive care or from a population with a high prevalence of pre-existing chronic disease or immune incompetence. Full inclusion and exclusion criteria are given in Table 1 .
Table 1.
Inclusion and exclusion criteria
Inclusion criteria
|
Exclusion criteria
|
Search strategy
Our search strategy was designed to identify studies and systematic reviews that reported the relationships between microbes sampled from the upper respiratory tract in patients with respiratory tract infection, and prognostic outcomes. MEDLINE and Embase databases were searched using the OVID platform to 15 March 2018.
The MEDLINE search strategy is presented in Appendix I and used combinations of MeSH (Medical Subject Headings) terms and text words for clinical diagnoses of respiratory infection; 20 different microbes implicated in respiratory tract infection (identified by consultant microbiologists and used in previously published work) [13]; and MeSH terms and text words for prognosis. The search excluded papers focusing on cystic fibrosis and tuberculosis. This search strategy was developed, extensively tested and refined using an iterative process with input from the University of Bristol subject librarian and search expert, and was subsequently adapted for use in Embase. The search was limited to humans, and no time restrictions were applied. Reference lists of all included full-text articles were also screened.
Study selection
Titles and abstracts of all identified studies were assessed for eligibility by one author (H.T.) and those which did not fulfil the inclusion/exclusion criteria were excluded. Full-text copies of included articles were independently reviewed. Dual screening was performed for 20% of all records by three authors (I.L., A.B. and C.H.) and eligibility disagreements resolved by discussion.
Data extraction and quality assessment
Data were extracted from full texts using a purpose-designed Access form. Descriptive variables were country of recruitment; study setting (e.g. primary/secondary care), study design, anatomical respiratory tract sampling location, laboratory methods, microbes identified, diagnoses of participants, number of participants, participant age inclusion criteria, type of prognostic outcomes reported, and whether results were stratified by antibiotic prescribing or consumption. Outcome data extracted were any measure of prognosis, including but not limited to symptom duration, hospitalization and length of hospital stay. The number of outcomes reported by studies for each microbe, and any association found between microbe and outcome, was recorded and reported in a ‘vote count’ table. Where the same outcome was reported for the same microbe by three or more studies, with means and standard deviations, random-effects meta-analysis was carried out using STATA (Stata Statistical Software, Release 13; StataCorp LP, College Station, TX, USA) using the ‘metan’ command. Quality assessment was conducted for all studies included in the review using the QUIPS tool [14].
Results
Ascertainment
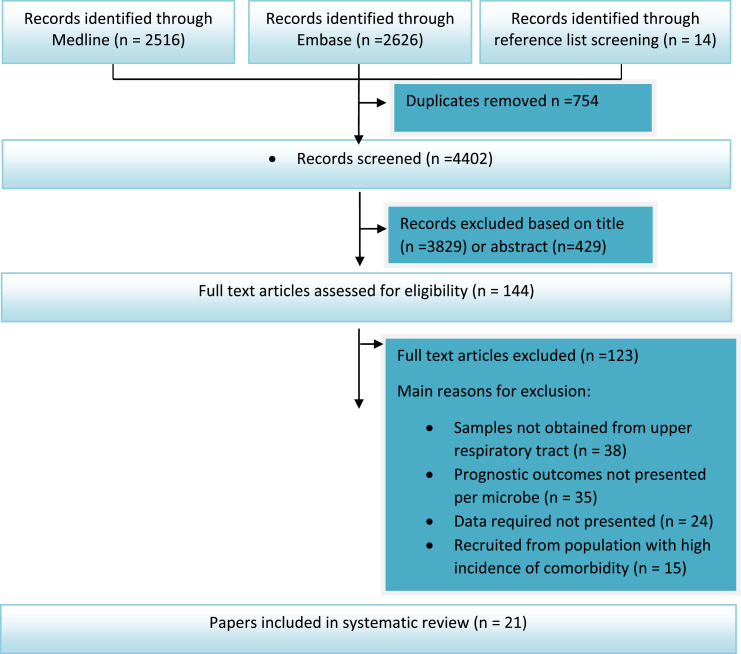
Our search identified 5156 articles of which 754 were duplicates (Fig. 1 ). Of the 4402 remaining, 3829 were excluded on the basis of title and a further 429 on the basis of abstract. Full texts of 144 articles were screened and 21 were eligible for inclusion in the review.
Fig. 1.
Flow chart: exclusion stages for studies in the review.
Study characteristics and microbiological data
Characteristics of the 21 studies included in this review are summarized in Table 2 . The most common recruitment setting was hospital inpatients (13 studies; 62%), followed by hospital outpatient/community research clinics or primary care centres (five studies; 24%) and emergency departments (two studies; 10%). One study recruited in both primary care centres and an emergency department. The majority of studies (16; 76%) recruited only children, with eight recruiting children aged less than 2 years.
Table 2.
Characteristics of studies included in the review
| Author, year | Country | Recruitment location | Study design | Study size | Eligible age group | Diagnoses of participants | Sample type | Laboratory methods | Microbe(s) | Prognostic outcome(s) reported | Clear swab ‘control’ group? | Results stratified by antibiotic use? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bamberger 2012 [21] | Israel | Inpatient | Prospective observational | 366 | <24 mo | Acute bronchiolitis | NPA | PCR | RSV | Duration of hospitalization: categories <3 d, 4–7 d, 7+d; mean PICU stay; supplemental oxygen duration <3 d: yes/no | No | No |
| Bennett 2007 [22] | USA | A&E | Prospective observational | 101 | <24 mo | Bronchiolitis | Nasal wash | Viral culture, monoclonal antibody, stain | RSV | Duration of illness: median; hospitalization: yes/no | Yes | No |
| Chan 2007 [23] | Hong Kong | Inpatient | Retrospective case review | 561 | ≤3 y | Acute respiratory infection | NPA | Assay and immunofluorescence | RSV and flu A and B (combined) | Duration of fever & duration of hospitalization: mean (SD), PICU admission y/n | No | No |
| Chiu 2010 [24] | Hong Kong | Inpatient | Prospective observational | 1031 | <18 y | Febrile upper respiratory tract infection | NPA | Immunofluorescence | RSV, PIV, Adv | Duration of hospitalization: mean (SD) | No | No |
| Cohen 2015 [25] | France and Turkey | Community clinic and A&E | Prospective observational | 774 | Any age | Laboratory-confirmed influenza A or B | Rhino-pharyngeal swab | RT-PCR | Flu A and B | Hospitalization: yes/no; illness duration split by age group: odds ratio | No | No |
| Foshaug 2015 [26] | Norway | Primary care | Retrospective case–control | 414 | Adult | ‘Airway infections’ | NPS | PCR | M. pneumoniae | Admission to hospital (yes/no) | Yes | No |
| Franz 2010 [27] | Germany | Inpatient | Prospective observational | 404 | 0–16 y | Lower respiratory tract infections | NPA | RT-PCR | RSV, RV, HBoV, adenoviruses | Duration of hospitalization: median | No | No |
| Garcia-Garcia 2017 [28] | Spain | Inpatient | Prospective observational | 3906 | <14 y | Acute respiratory tract infection | NPA | RT-PCR | HMPV, RSV, RV, HBoV, adeno | Duration of fever and duration of hospitalization: mean (SD) | No | No |
| Güllü 2017 [29] | Turkey | Inpatient | Prospective observational | 361 | <2 y | Viral lower RTI | NP swab | Rapid antigen detection test | RSV | Duration of hospitalization: mean and SD | Yes | No |
| Iwane 2011 [30] | USA | Inpatient | Prospective observational | 1867 | <5 y | Acute respiratory tract infection | NS and TS | RT-PCR | RV | Hospital stay >3 d: yes/no; duration of hospitalization: median (IQR) | No | No |
| Lambert 2007 [31] | Australia | Community clinic | Prospective observational | 234 | <5 y | Acute respiratory infection | Combined NS and TS | PCR | hMPV, coronavirus, picornaviruses (pooled), PIV, ADV, RSV, influenza A | Hospitalization: yes/no; ED presentation: yes/no; symptom duration: mean and median | Yes | No |
| Lau 2006 [32] | Hong Kong | Inpatient | Prospective observational | 4181 | Any age | ‘Respiratory tract infections’ | NPA | RT-PCR | HCoV, Flu A and B, Adv, parainfluenzaviruses, RSV, hMPV | Duration of fever and duration of hospitalization: mean and SD | No | No |
| Laundy 2003 [16] | UK | Primary care centre and A&E | Prospective observational | 51 | <5 y | Community-acquired pneumonia | NPA | Indirect immunofluorescence, PCR | RSV, influenza A | Duration of hospitalization, fever and illness duration: median, mean and range | No | No |
| Mansbach 2008 [33] | USA | A&E | Prospective observational | 277 | <2 y | Bronchiolitis | NPA | PCR | RSV, RV | Symptom duration: median, IQR; relapse within two weeks: yes/no; days of activity limitation post hospital visit: median (IQR) | No | No |
| Marguet 2009 [34] | France | Inpatient | Prospective observational | 209 | 1 mo–1 y | First episode acute bronchiolitis | NPA | RT-PCR | RSV, RV, hMPV | Duration of hospitalization: median (IQR) | Yes | No |
| Mullins 2011 [35] | USA | University health clinic | Prospective observational | 60 | Adult | Influenza-like illness | NPS | PCR | Influenza | Days off school/work: mean (CI) | Yes | No |
| Palomino 2004 [36] | Chile | Inpatient | Prospective observational | 117 | <2 y | Acute lower respiratory infection | NPA | Immunofluorescence | Adv | Duration of hospitalization: median | No | No |
| Resch 2011 [37] | Austria | Inpatient | Retrospective notes review | 425 | <12 m | Lower respiratory infection | NPA | ELISA, immunofluorescence | RSV and influenza | Duration of hospitalization: mean (SD); supplemental oxygen treatment duration | No | No |
| Shaikh 2014 [38] | USA | Outpatient | Prospective observational | 206 | 2–12 y | Acute sinusitis | NPS | Culture | S. pneumoniae, M. catarrhalis, H. influenzae | Days to symptom resolution: median | Yes | No |
| Tsolia 2003 [39] | Greece | Inpatient | Prospective observational and retrospective case review | 636 | <1 y | Bronchiolitis | NPW | Immunofluorescence | RSV | Duration of hospitalization: mean (SD); intensive care admission: yes/no | Yes | No |
| Tsung 2010 [40] | Hong Kong | Inpatient | Prospective observational | 475 | <5 y | Acute respiratory tract infections | NPS and NPA | Immunofluorescence, PCR | Adv, influenza A and B, PIV, RSV, hMPV, M. pneumoniae, RV, enterovirus | Duration of hospitalization: categories: <2 d, 3–4 d, >5 d, median (IQR) | No | No |
d, days; w, weeks; mo, months; y, years; NPA, nasopharyngeal aspirate; PCR, polymerase chain reaction; RT-PCR, reverse-transcriptase PCR; hMPV, human metapneumovirus; HBoV, human bocavirus; SD, standard deviation; IQR, interquartile range; RSV, respiratory syncytial virus; RV, rhinovirus; NPS, nasopharyngeal swab; A&E, accident and emergency department; GP, general practice; NP, nasopharyngeal; ELISA, enzyme-linked immunosorbent assay; HCoV, coronaviruses; Adv, adenovirus; PIV, parainfluenzavirus; NS, nasal swab; TS, throat swab; ED, emergency department; NPW, nasopharyngeal wash; PICU, paediatric intensive care unit.
Most studies used a prospective observational design (17, 81%). Several upper respiratory sampling methods were used: nasopharygeal wash/aspirate (13 studies, 62%); nasopharyngeal swabs (four studies, 19%); combinations of nasal, throat, nasopharyngeal swabs and aspirates (three studies, 14%); and a rhinopharyngeal swab (one study, 5%). Laboratory methods also varied between studies, with 11 (52%) using polymerase chain reaction techniques, three (14%) using immunofluorescence and the remainder using mixed/other methods.
Data were reported for 15 microbes/groups of microbes, including four bacteria, ten viruses and a combined Influenza A/B category. A full list of reported microbes is given later in Table 4. The most data were reported for respiratory syncytial virus (RSV) (15 associations with prognosis investigated), rhinovirus (six) and influenza (six). The majority (13, 62%) reported data only for participants who were positive for the microbe(s) of interest; there is therefore a paucity of ‘control’ data from participants without detected microbes. None of the studies quantified microbial load, and no study reported outcomes stratified by antibiotic consumption or antimicrobial resistance.
Table 4.
Vote count of associations sought between clinical outcomes and microbes reported by studies
Quality assessment is summarized in Table 3 . No study had high risk of bias in the domain assessing attrition. The domain assessing confounding showed high risk of bias, commonly because studies measured limited numbers of microbes such that results could have been confounded by the presence of an untested microbe. High risk of bias was observed in three other domains for at least one study. Three studies had low risk of bias in all domains; ten had high risk of bias in at least one domain.
Table 3.
Quality assessment of full-text studies using the QUIPS tool
| Author | Study participation | Study attrition | Prognostic factor measurement | Outcome measurement | Study confounding | Statistical analysis and reporting |
|---|---|---|---|---|---|---|
| Lau 2006 | 2 | 1 | 1 | 2 | 3 | 2 |
| Shaikh 2014 | 1 | 1 | 1 | 1 | 3 | 1 |
| Laundy 2003 | 1 | 1 | 2 | 2 | 2 | 3 |
| Lambert 2007 | 1 | 1 | 1 | 2 | 1 | 2 |
| Mansbach 2008 | 1 | 1 | 1 | 1 | 3 | 2 |
| Bennet 2007 | 1 | 1 | 2 | 1 | 2 | 2 |
| Chan 2007 | 1 | 1 | 2 | 1 | 3 | 2 |
| Garcia-Garcia 2017 | 1 | 1 | 1 | 1 | 2 | 1 |
| Marguet 2009 | 1 | 1 | 1 | 1 | 1 | 1 |
| Tsung 2010 | 2 | 1 | 1 | 1 | 2 | 2 |
| Franz 2010 | 1 | 1 | 1 | 1 | 1 | 1 |
| Lambert 2007 | 1 | 1 | 1 | 1 | 1 | 1 |
| Palomino 2004 | 1 | 1 | 3 | 1 | 2 | 1 |
| Tsolia 2003 | 2 | 1 | 2 | 2 | 3 | 1 |
| Chiu 2010 | 1 | 1 | 2 | 1 | 2 | 1 |
| Resch 2011 | 1 | 1 | 2 | 2 | 2 | 1 |
| Foshaug 2015 | 1 | 1 | 1 | 1 | 3 | 1 |
| Mullins 2011 | 1 | 1 | 2 | 1 | 2 | 1 |
| Iwane 2011 | 1 | 1 | 1 | 1 | 3 | 1 |
| Cohen 2015 | 2 | 1 | 1 | 1 | 1 | 1 |
| Gullu 2017 | 1 | 1 | 2 | 1 | 3 | 1 |
| Bamberger 2012 | 1 | 1 | 1 | 1 | 2 | 1 |
Risk of bias: high (3), moderate (2) or low (1).
Outcomes
Prognostic outcomes are listed in Table 4 , which shows a ‘vote count’ of associations examined between reported microbes and outcomes, and whether associations were reported by the primary study authors as statistically significant. In total, 26 differently measured outcomes were reported, the majority of which fell into three categories: (a) hospitalization duration (nine measures); (b) symptom duration (eight measures); and (c) healthcare use (six measures).
The most commonly reported outcome was duration of hospitalization, which was reported by at least one study for all microbes (Table 4). Symptom duration was reported using at least one measure for ten out of 15 microbes, and healthcare use was reported using at least one outcome for nine out of 15 microbes.
Relationship between microbes and prognosis
The 26 outcome and 15 microbe categories reported in Table 4 give a total of 390 possible associations. Of these, 99 (25%) were examined by one or more study, with a total of 134 associations reported by all studies. There were an additional five microbes for which we sought data, but identified no relevant studies.
Statistical tests were used to assess relationships between microbe detection and outcomes for 107 out of 134 outcomes. These were reported by the study authors to be statistically significant (p < 0.05) in 38 out of 107 (36%). Twenty-seven associations were reported in which the authors did not use statistical tests, but reported raw data.
Owing to the diversity of outcome measures reported, opportunities for meta-analyses were limited. We considered use of methods designed for synthesis of diversely reported outcomes including the albatross plot [15], but were unable to proceed due to insufficient primary data.
Meta-analysis was possible for duration of hospitalization. Means and standard deviations were provided by seven studies for RSV and three for adenovirus. Data were also available from three studies, pooling results for influenza A and B. A forest plot for these analyses is given in Fig. 2 . Significant heterogeneity was observed for all three pooled estimates and as such they should be interpreted with caution.
Fig. 2.
Forest plot showing mean duration of hospital stay for patients positive for adenovirus, influenza A and B and RSV.
One additional study (Laundy et al. [16]) provided mean duration of hospitalization for patients with RSV and influenza A, but could not be included in the meta-analysis as no standard deviation was reported. When compared with the results of the meta-analysis, the mean duration of hospitalization for RSV (2.2 days) and influenza A (6.0 days) do not fall within the confidence intervals for the pooled estimates. However, the Laundy study was small, with eight participants identified with influenza A and nine with RSV, which means the contribution of the study, if incorporated into meta-analysis, would be low.
It was not possible to examine whether antibiotic prescribing or antimicrobial resistance status influenced prognosis as the studies did not report results stratified by these factors.
Discussion
Summary of main findings
Our review highlights a paucity of evidence for the prognostic value of upper respiratory tract microbes: of the potential 390 possible associations only 27% have been investigated. That said, of those that have been tested, 36% were reported as significant.
Our meta-analysis suggests hospitalization duration is longer for patients with respiratory syncytial virus than adenovirus and influenza, but we found significant heterogeneity between studies. This is likely to result from the differences in study recruitment setting, country, laboratory methods and participant diagnoses described in Table 2.
Findings in relation to existing literature
Previous work has demonstrated that some specific bacteria and viruses are present more often in the throats of children with acute cough and RTI than in asymptomatic children [12], [13], [17], providing some evidence that acute cough alters the flora of the upper respiratory tract and microbes detected there may be aetiological. However, we have also demonstrated here that there is an absence of evidence as to whether targeting antimicrobial treatment to the results of upper respiratory tract microbial testing would lead to improved outcomes.
Strengths and weaknesses
This review was rigorously conducted and reported according to Cochrane and PRISMA guidelines [14], [18]. The search strategy was designed by subject experts and the quality of included studies assessed using the appropriate QUIPS tool [14]. We used a ‘vote count’ table as the most succinct way to present the overall results of the review, though this does mean that small studies lend as much visual weight to results as their larger counterparts [19].
It is possible that by restricting inclusion, we could have reduced the heterogeneity between studies. However, doing so would have limited our results to a focused population or outcome, limited opportunities for meta-analysis even further and reduced generalizability of any findings to the broader population.
Twenty-six different prognostic measures were identified in the literature. At present, no core outcome set exists for RTI, which leads different studies to measure slightly different outcomes. We aimed to capture all relevant published data in this review, yet had studies (and hence this review) focused on an internationally agreed set of outcomes, it is likely that the percentage of potential associations investigated would have been higher. The large number of associations reported is both a finding in itself, and a limitation of this work.
We were unable to assess the impact of antimicrobial use or antimicrobial resistance on prognosis for patients with/without bacterial detection as studies did not report results stratified by antibiotic use or resistance status.
Clinical and research implications
Our results suggest significant potential for using upper respiratory tract microbes as the target of future POCT studies.
The currently un-investigated microbial-prognosis associations should be urgently subjected to rigorous research, which should include assessments of the impact of microbial load, antibiotic use, resistance status and the value of negative results. Despite a rigorous search, we identified few studies that reported prognostic data for bacterial identification, with the majority of data reporting viral infections. Future studies should also seek and report as large a range of microbes as possible, to minimize confounding by the presence of an untested microbe, and more studies are needed in the primary care setting.
Conclusions
A quarter of potential prognostic associations have been investigated, and of these a third were significant, suggesting considerable potential for POCT. Future research should investigate the prognostic value of both positive and negative tests in both primary and secondary care, and look for interactions between test results, use of antimicrobials and microbial resistance.
Transparency declaration
No conflicts of interest declared. This work was funded by a research grant from the Avon Primary Care Research Collaborative.
Acknowledgement
The authors would like to thank Alison Richards, Information Scientist at CLAHRC West, for her expert help in reviewing, refining and testing the search terms and strategies used in this review.
Editor: M. Leeflang
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2019.06.024.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Hay A.D., Heron J., Ness A., the ALSPAC study team The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract. 2005;22:367–374. doi: 10.1093/fampra/cmi035. [DOI] [PubMed] [Google Scholar]
- 2.Okkes I.M., Oskam S.K., Lamberts H. The probability of specific diagnoses for patients presenting with common symptoms to Dutch family physicians. J Fam Pract. 2002;51:31–36. [PubMed] [Google Scholar]
- 3.Ashworth M., Cox K., Latinovic R., Charlton J., Gulliford M., Rowlands G. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J Public Health. 2004;26:268–274. doi: 10.1093/pubmed/fdh160. [DOI] [PubMed] [Google Scholar]
- 4.Butler C.C., Hood K., Verheij T., Little P., Melbye H., Nuttall J., et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NICE . 2008. Respiratory tract infections: prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care.https://www.nice.org.uk/https://www.nice.org.uk/guidance/cg69 [PubMed] [Google Scholar]
- 6.Horwood J., Cabral C., Hay A.D., Ingram J. Primary care clinician antibiotic prescribing decisions in consultations for children with RTIs: a qualitative interview study. Br J Gen Pract. 2016;66:e207–e213. doi: 10.3399/bjgp16X683821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S., Little P., Britten N. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ. 2003;326:138. doi: 10.1136/bmj.326.7381.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Neill J. Review on antimicrobial resistance. Wellcome Trust and HM Government; 2016. Tackling drug-resistant infections globally: final report and recommendations.https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf [Google Scholar]
- 9.Hay A.D., Redmond N.M., Turnbull S., Christensen H., Thornton H., Little P., et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med. 2016;4:902–910. doi: 10.1016/S2213-2600(16)30223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Bruel A., Jones C., Thompson M., Mant D. C-reactive protein point-of-care testing in acutely ill children: a mixed methods study in primary care. Arch Dis Child. 2016;101:382–385. doi: 10.1136/archdischild-2015-309228. [DOI] [PubMed] [Google Scholar]
- 11.Kozel T.R., Burnham-Marusich A.R. Point-of-care testing for infectious diseases: past, present, and future. J Clin Microbiol. 2017;55:2313–2320. doi: 10.1128/JCM.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornton H.V., Hay A.D., Redmond N.M., Turnbull S.L., Christensen H., Peters T.J., et al. Throat swabs in children with respiratory tract infection: associations with clinical presentation and potential targets for point-of-care testing. Fam Pract. 2017;34:407–415. doi: 10.1093/fampra/cmw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton H., Blair P., Lovering A., Muir P., Hay A.D. Clinical presentation and microbiological diagnosis in paediatric respiratory tract infection: a systematic review. Br J Gen Pract. 2015;65:e69–e81. doi: 10.3399/bjgp15X683497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden J.A., van der Windt D.A., Cartwright J.L., Cote P., Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 15.Harrison S., Jones H.E., Martin R.M., Lewis S.J., Higgins J.P.T. The albatross plot: a novel graphical tool for presenting results of diversely reported studies in a systematic review. Res Synth Methods. 2017;8:281–289. doi: 10.1002/jrsm.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laundy M., Ajayi-Obe E., Hawrami K., Aitken C., Breuer J., Booy R. Influenza A community-acquired pneumonia in East London infants and young children. Pediatr Infect Dis J. 2003;22:S223–S227. doi: 10.1097/01.inf.0000092192.59459.8b. [DOI] [PubMed] [Google Scholar]
- 17.Rhedin S., Lindstrand A., Rotzen-Ostlund M., Tolfvenstam T., Ohrmalm L., Rinder M.R., et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133:e538–e545. doi: 10.1542/peds.2013-3042. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 19.Grimshaw J., McAuley L.M., Bero L.A., Grilli R., Oxman A.D., Ramsay C., et al. Systematic reviews of the effectiveness of quality improvement strategies and programmes. Qual Saf Health Care. 2003;12:298–303. doi: 10.1136/qhc.12.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Bank Country and Lending Groups [https://datahelpdesk.worldbank.org/knowledgebase/articles/906519].
- 21.Bamberger E., Srugo I., Abu Raya B., Segal E., Chaim B., Kassis I., et al. What is the clinical relevance of respiratory syncytial virus bronchiolitis?: findings from a multi-center, prospective study. Eur J Clin Microbiol Infect Dis. 2012;31:3323–3330. doi: 10.1007/s10096-012-1699-2. [DOI] [PubMed] [Google Scholar]
- 22.Bennett B.L., Garofalo R.P., Cron S.G., Hosakote Y.M., Atmar R.L., Macias C.G., et al. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007;195:1532–1540. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- 23.Chan D., Chiu W., Ip P. Respiratory syncytial virus and influenza infections among children ≤3 years of age with acute respiratory infections in a regional hospital in Hong Kong. Hong Kong J Paediatr. 2007;12 15–21+61–62. [Google Scholar]
- 24.Chiu S.S., Chan K.H., Chen H., Young B.W., Lim W., Wong W.H., et al. Virologically confirmed population-based burden of hospitalization caused by respiratory syncytial virus, adenovirus, and parainfluenza viruses in children in Hong Kong. Pediatr Infect Dis J. 2010;29:1088–1092. doi: 10.1097/INF.0b013e3181e9de24. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J.M., Silva M.L., Caini S., Ciblak M., Mosnier A., Daviaud I., et al. Striking similarities in the presentation and duration of illness of influenza A and B in the community: a study based on sentinel surveillance networks in France and Turkey, 2010–2012. PLoS One. 2015;10:e0139431. doi: 10.1371/journal.pone.0139431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foshaug M., Vandbakk-Ruther M., Skaare D., Grude N., Lindbaek M. Mycoplasma pneumoniae detection causes excess antibiotic use in Norwegian general practice: a retrospective case-control study. Br J Gen Pract. 2015;65:e82–e88. doi: 10.3399/bjgp15X683509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franz A., Adams O., Willems R., Bonzel L., Neuhausen N., Schweizer-Krantz S., et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48:239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Garcia M.L., Calvo C., Rey C., Diaz B., Molinero M.D., Pozo F., et al. Human metapnuemovirus infections in hospitalized children and comparison with other respiratory viruses. 2005–2014 prospective study. PLoS One. 2017;12:e0173504. doi: 10.1371/journal.pone.0173504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gullu E., Akin Y., Karaaslan A., Vayvada E., Atabek A., Narter F. RSV infection in Istanbul: risk factors and frequency. J Infect Dev Ctries. 2017;11:691–696. doi: 10.3855/jidc.8871. [DOI] [PubMed] [Google Scholar]
- 30.Iwane M.K., Prill M.M., Lu X., Miller E.K., Edwards K.M., Hall C.B., et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 31.Lambert S.B., Allen K.M., Druce J.D., Birch C.J., Mackay I.M., Carlin J.B., et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens. Pediatrics. 2007;120:e929–e937. doi: 10.1542/peds.2006-3703. [DOI] [PubMed] [Google Scholar]
- 32.Lau S.K., Woo P.C., Yip C.C., Tse H., Tsoi H.W., Cheng V.C., et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansbach J.M., McAdam A.J., Clark S., Hain P.D., Flood R.G., Acholonu U., et al. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad Emerg Med. 2008;15:111–118. doi: 10.1111/j.1553-2712.2007.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marguet C., Lubrano M., Gueudin M., Le Roux P., Deschildre A., Forget C., et al. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One. 2009;4:e4596. doi: 10.1371/journal.pone.0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullins J., Cook R., Rinaldo C., Yablonsky E., Hess R., Piazza P. Influenza-like illness among university students: symptom severity and duration due to influenza virus infection compared to other etiologies. J Am Coll Health. 2011;59:246–251. doi: 10.1080/07448481.2010.502197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palomino M.A., Larranaga C., Villagra E., Camacho J., Avendano L.F. Adenovirus and respiratory syncytial virus-adenovirus mixed acute lower respiratory infections in Chilean infants. Pediatr Infect Dis J. 2004;23:337–341. doi: 10.1097/00006454-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Resch B., Eibisberger M., Morris N., Muller W. Respiratory syncytial virus- and influenza virus-associated hospitalizations in infants less than 12 months of age. Pediatr Infect Dis J. 2011;30:797–799. doi: 10.1097/INF.0b013e318215cf3e. [DOI] [PubMed] [Google Scholar]
- 38.Shaikh N., Wald E.R., Jeong J.H., Kurs-Lasky M., Bowen A., Flom L.L., et al. Predicting response to antimicrobial therapy in children with acute sinusitis. J Pediatr. 2014;164:536–541. doi: 10.1016/j.jpeds.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsolia M.N., Kafetzis D., Danelatou K., Astral H., Kallergi K., Spyridis P., et al. Epidemiology of respiratory syncytial virus bronchiolitis in hospitalized infants in Greece. Eur J Epidemiol. 2003;18:55–61. doi: 10.1023/a:1022556215190. [DOI] [PubMed] [Google Scholar]
- 40.Tsung L.Y., Choi K.C., Nelson E.A., Chan P.K., Sung R.Y. Factors associated with length of hospital stay in children with respiratory disease. Hong Kong Med J. 2010;16:440–446. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.