Abstract
Objective
To compare outcomes of early and delayed treatment with cidofovir for human adenovirus (HAdV) pneumonia.
Methods
A retrospective cohort study in Korean military hospitals was conducted between January 2012 and December 2018. Patients with potentially severe HAdV pneumonia with risk factors for respiratory failure were included and divided into early (within 7 days from symptom onset) and delayed (after 7 days from symptom onset) treatment groups. The primary outcome was respiratory failure development within 21 days after symptom onset.
Results
A total of 89 patients with potentially severe HAdV pneumonia were enrolled in the cohort; they included 62 early and 27 delayed treatment patients. All patients were males in their early 20s. Significantly fewer patients in the early treatment group progressed to respiratory failure (8/62, 12.9%), compared to the delayed group (18/27, 66.7%, p < 0.001). Early treatment was associated with a lower 21-day probability of respiratory failure by the Kaplan–Meier method (p < 0.001). On multivariate analysis, monocyte count, hypoxaemia, confusion, whole lung involvement, and early cidofovir treatment within 7 days from symptom onset were included, and monocyte count (HR 0.995, 95%CI 0.991–1.000, p 0.042), confusion (HR 4.964, 95%CI 1.189–20.721, p = 0.028), and early cidofovir treatment (HR 0.319, 95%CI 0.115–0.883, p = 0.028) were significantly associated with respiratory failure.
Conclusions
Early administration of cidofovir was associated with a lower hazard for respiratory failure development. It is suggested that cidofovir be administered within 7 days from symptom onset to prevent respiratory failure in patients with potentially severe HAdV pneumonia.
Keywords: Adenovirus, Cidofovir, Early, Pneumonia, Respiratory failure
Introduction
Respiratory illnesses caused by the human adenovirus (HAdV) generally occur in infants and children [1], but severe, potentially fatal pneumonia is also reported in the adult population [2]. HAdV pneumonia in adults has been most frequently reported in communal living populations, especially among military trainees [[3], [4], [5], [6], [7], [8]]. HAdV type 55 (HAdV-55), an emerging recombinant strain of types 11 and 14 [9], has spread rapidly throughout China since the first Chinese outbreak in 2006 [[7], [8], [9], [10]]. HAdV-55 has also been noticed in the Korean military from 2012 and caused a large outbreak beginning in the winter of 2014 [6,[11], [12], [13]]. This non-vaccine-type HAdV outbreak is currently ongoing in the Korean military, with a high burden of morbidity and mortality [13]. In managing severe HAdV pneumonia patients in the Korean military, we tried cidofovir based on previous case reports and in vitro studies [[14], [15], [16]], and we experienced several successes with early administration of cidofovir [17]. Since then, we have routinely administered cidofovir for all patients with potentially severe HAdV pneumonia and risk factors for respiratory failure [18], but the administration interval from symptom onset varied between patients. To compare outcomes of early and delayed treatment, we conducted a retrospective cohort study in Korean military hospitals for a 7-year period.
Methods
Study design and patient selection
A retrospective cohort study was performed. We reviewed electronic medical records of cidofovir-treated HAdV pneumonia patients at 14 military hospitals between January 2012 and December 2018. Among the cidofovir-treated HAdV pneumonia patients, we included patients with potentially severe disease who had any of the following risk factors for respiratory failure at initial presentation [18]: (a) hypoxaemia (PaO2 <60 mmHg or SpO2 <90%), (b) monocytopenia (<150/μL), (c) multilobar involvement (three or more lobes on chest computed tomography (CT)), or (d) pleural effusion on chest CT. Included patients were divided into an early treatment group (within 7 days from symptom onset) and a late treatment group (after 7 days from symptom onset), based on the median value of the cohort. The primary outcome was the development of respiratory failure within 21 days after symptom onset. Secondary outcomes included duration of hospital stay, intensive care unit (ICU) care, duration of ICU care, continuous renal replacement therapy (CRRT) support, extracorporeal membrane oxygenation (ECMO) support, and death. Secondary outcomes were assessed during the hospital stay of patients. It was generally recommended to administer cidofovir at a dosage of 5 mg/kg weekly in combination with oral probenecid [17]. Since the second dose of cidofovir was selectively administered among patients who progressed to respiratory failure, we evaluated outcomes associated with the first dose. This study was approved by the Institutional Review Board of the Armed Forces Medical Command (AFMC-19-IRB-099) and informed consent was waived by the board.
Data collection and definitions
Collected data included demographics, presenting symptoms, initial laboratory findings, factors for pneumonia severity measures, initial radiological findings, data on cidofovir and antibiotic treatment, underlying comorbidity, and outcomes. Quantitative variables were used as continuous variables and/or converted into categorical variables using commonly used cut-off values (presented in the results). CURB-65 score was used for the calculation of pneumonia severity [19], and the modified Medical Research Council (mMRC) scale for rating dyspnoea [20]. Initial presentation was assessed on the day of the first hospital visit. All included patients first visited the hospital after pneumonia development and were admitted on the same day. HAdV infections were confirmed by multiplex respiratory virus polymerase chain reaction (PCR) tests using patients' sputum samples, as previously described [13]. Pneumonia was defined as the presence of parenchymal infiltration on chest x-ray or CT with respiratory symptoms. All included patients underwent chest CT unless whole lung involvement was apparent on chest x-ray. Respiratory failure was defined when mechanical ventilation (MV) support was required with a PaO2/FiO2 ratio ≤200 mmHg measured after endotracheal intubation. Renal impairment occurring within 2 weeks of cidofovir treatment was assessed according to the RIFLE (risk, injury, failure, loss and end-stage) criteria [21].
Statistical analysis
To compare clinical factors, either the Student's t-test or the Mann–Whitney U test was used for continuous variables, and either the χ-square or Fisher's exact tests was used for categorical variables. The Kaplan–Meier method was used to calculate the 21-day probability of respiratory failure. The Cox proportional hazard model was used to evaluate potential risk factors for respiratory failure within 21 days. All factors clinically relevant to the outcome were evaluated by univariate analysis, and statistically significant factors were included in the multivariate analysis to adjust early administration of cidofovir within 7 days after symptom onset. If more than two factors that share parameters or are clinically correlated were statistically significant, only one factor with the strongest HR by univariate analysis was included in the multivariate analysis. A subgroup analysis was conducted among patients who received cidofovir before the development of respiratory failure. Patients who received cidofovir on the same day as or after respiratory failure development were excluded, and the primary outcome was evaluated with the same statistical methods. Missing data are presented in footnotes to the Tables. All p-values were two-tailed, and those <0.05 were considered statistically significant. IBM SPSS Statistics version 20.0 (IBM, Armonk, NY, USA) was used for all statistical analyses.
Results
Baseline characteristics and initial presentation
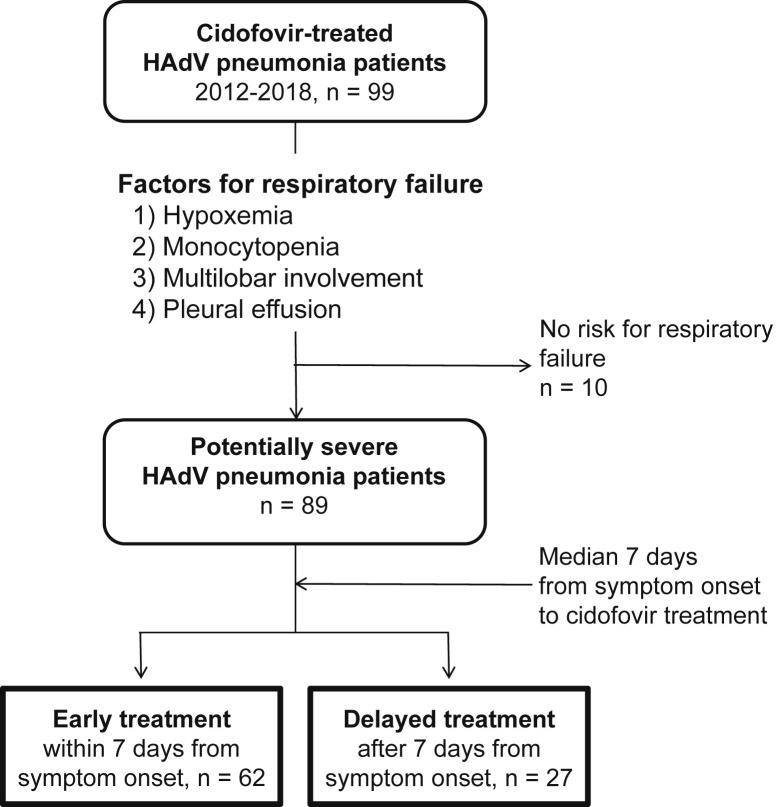
During the study period, a total of 89 patients were included in the cohort and were divided into early (n = 62) and delayed (n = 27) treatment groups (Fig. 1 ). Baseline characteristics and initial presentations of cidofovir-treated HAdV pneumonia patients are presented in Table 1 . All patients were males in their early 20s. Presenting symptoms and laboratory findings did not differ between the two groups. Patients in the early treatment group visited the hospital earlier than those in the delayed group (mean 3.0 versus 5.4 days from symptom onset, p 0.001). Patients in the delayed treatment group had lower PaO2/FiO2 ratios and were more likely to have hypoxaemia compared to those in the early treatment group (both p < 0.001), while factors for CURB-65 did not differ. Among radiological findings, significantly more patients in the delayed treatment group presented with whole lung involvement (11/27, 40.7%) than those in the early treatment group (8/62, 12.9%, p 0.003). In addition to HAdV, other respiratory viruses were co-detected in five patients in the early treatment group (5/62, 8.1%) and two patients in the delayed treatment group (2/27, 7.4%).
Fig. 1.
Patient selection for the retrospective cohort. Patients who did not have any risk factors for respiratory failure were excluded. Patients were divided into early (≤7 days from symptom onset) and delayed (>7 days from symptom onset) treatment groups, based on the median value of the cohort. HAdV, human adenovirus.
Table 1.
Baseline characteristics and initial presentation of cidofovir-treated patients with human adenovirus (HAdV) pneumonia
| Variables | Early treatment, within 7 days from symptom onset (n = 62) | Delayed treatment, after 7 days from symptom onset (n = 27) | p value |
|---|---|---|---|
| Demographics: | |||
| Age, years | 20.4 ± 1.4 | 20.4 ± 2.0 | 0.980 |
| Male sex | 62 (100) | 27 (100) | NA |
| Presenting symptoms: | |||
| Fever | 62 (100) | 27 (100) | NA |
| Cough | 62 (100) | 26 (96.3) | 0.303 |
| Sputum | 59 (95.2) | 25 (92.6) | 0.637 |
| Dyspnoea (MMRC > II) | 28 (45.2) | 13 (48.1) | 0.795 |
| Diarrhoea | 21 (33.9) | 7 (25.9) | 0.458 |
| Interval from symptom onset to admission | 3.0 ± 1.5 | 5.4 ± 3.1 | 0.001 |
| Initial laboratory tests: | |||
| WBC count (/μL) | 4186.8 ± 2320.1 | 3857.4 ± 2331.3 | 0.540 |
| Leukopenia (<4000/μL) | 33 (53.2) | 18 (66.7) | 0.239 |
| Lymphocyte count (/μL) | 800.0 ± 346.5 | 706.4 ± 325.2 | 0.242 |
| Lymphopenia (<1500/μL) | 59 (95.2) | 26 (100) | 0.552 |
| Monocyte count (/μL) | 214.1 ± 149.2 | 147.5 ± 125.9 | 0.049 |
| Monocytopenia (<150/μL) | 29 (46.8) | 18 (69.2) | 0.054 |
| Platelet count (x103/μL) | 116.5 ± 52.6 | 112.4 ± 43.9 | 0.722 |
| Thrombocytopenia (<150 × 103/μL) | 49 (79.0) | 23 (85.2) | 0.497 |
| C-reactive protein (mg/dL) | 11.7 ± 5.3 | 10.7 ± 5.7 | 0.457 |
| Pneumonia severity on admission: | |||
| PaO2/FiO2 ratio (mmHg) | 295.4 ± 71.0 | 233.0 ± 80.79 | <0.001 |
| Hypoxaemiaa | 21 (33.9) | 20 (74.1) | <0.001 |
| Confusion | 2 (3.2) | 2 (7.4) | 0.582 |
| Tachypnoea (RR ≥ 30) | 13 (21.0) | 10 (37.0) | 0.111 |
| Shock (SBP <90 or DBP ≤60 mmHg) | 40 (64.5) | 14 (51.9) | 0.261 |
| CURB-65 (median, IQR)b | 1 (0–1) | 1 (1–1) | 0.549 |
| Radiological finding | |||
| Multilobar involvement | 31 (50.0) | 18 (66.7) | 0.146 |
| Whole lung involvement | 8 (12.9) | 11 (40.7) | 0.003 |
| Pleural effusion | 44 (71.0) | 22 (81.5) | 0.298 |
| Microbiological test results: | |||
| Co-detection of other respiratory viruses | 5 (8.1) | 2 (7.4) | 1.000 |
| Rhinovirus | 2 (3.2) | 1 (3.7) | 1.000 |
| Respiratory syncytial virus B | 1 (1.6) | 1 (3.7) | 0.517 |
| Influenza Ac | 1 (1.6) | 0 (0.0) | 1.000 |
| Coronavirus OC43 | 1 (1.6) | 0 (0.0) | 1.000 |
| Detection of bacterial pathogens | 0 (0.0) | 0 (0.0) | NA |
Data are expressed as the number (%) of patients or mean ± SD unless indicated otherwise. Only one patient in the delayed treatment group had underlying asthma, while others had no comorbidities. One patient in the delayed treatment group had no data for differential count.
HAdV, human adenovirus; MMRC, modified Medical Research Council; WBC, white blood cell; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen ratio; SpO2, peripheral capillary oxygen saturation; RR, respiratory rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; IQR, interquartile range; SD, standard deviation; BUN, blood urea nitrogen; NA, not applicable.
PaO2 <60 mmHg or SpO2 <90%.
Calculating CURB-65, no one met the criteria for elevated BUN or old age.
The patient detected with influenza A received oseltamivir treatment.
Treatment and outcomes
Factors associated with treatment and outcomes are presented in Table 2 . All the included patients were followed for at least 21 days from symptom onset for outcome assessment (median 23 days). Compared to the early treatment group, significantly more patients in the delayed treatment group progressed to respiratory failure within 21 days after the onset of symptoms (8/62, 12.9% versus 18/27, 66.7%; p < 0.001). Among patients who progressed to respiratory failure, significantly more patients in the delayed treatment group exhibited late progression to respiratory failure after 7 days from symptom onset compared to those in the early treatment group (16/18, 88.9% versus 3/8, 37.5%; p 0.014). The duration of hospital stay and ICU care were significantly longer in the delayed treatment group than in the early treatment group (both p < 0.05). Significantly more patients in the delayed treatment group received ECMO support than in the early treatment group (6/27, 22.2% versus 3/62, 4.8%; p 0.020). Death occurred in one patient in the delayed treatment group.
Table 2.
Treatment and outcomes of cidofovir-treated patients with human adenovirus (HAdV) pneumonia
| Variables | Early treatment, within 7 days from symptom onset (n = 62) | Delayed treatment, after 7 days from symptom onset (n = 27) | P value |
|---|---|---|---|
| Cidofovir treatment: | |||
| Dosage (mg/kg) | 5.0 ± 0.4 | 5.2 ± 0.5 | 0.095 |
| Under-dosing (<5 mg/kg) | 21 (33.9) | 8 (29.6) | 0.695 |
| Interval from symptom onset to Tx, days | 5.6 ± 1.4 | 10.0 ± 2.2 | <0.001 |
| Interval from admission to Tx, days | 2.6 ± 1.5 | 4.6 ± 2.5 | 0.001 |
| Repeated treatment (≥2 doses, weekly) | 5 (8.1) | 11 (40.7) | 0.001 |
| 2 doses | 4 (6.5) | 10 (37.0) | NA |
| 3 doses | 0 (0.0) | 1 (3.7) | NA |
| 4 doses | 1 (1.6) | 0 (0.0) | NA |
| Initial antibiotics and other modalities: | |||
| β-Lactams | 61 (98.4) | 27 (100) | 1.000 |
| Macrolides | 33 (53.2) | 16 (59.3) | 0.599 |
| Fluoroquinolones | 49 (79.0) | 21 (77.8) | 0.894 |
| Intravenous immunoglobulin | 6 (9.7) | 7 (25.9) | 0.057 |
| Outcome measures: | |||
| Respiratory failure within 21 days after symptom onset | 8 (12.9) | 18 (66.7) | <0.001 |
| Interval from symptom onset (median, range) | 7.0 (6.0–10.0) | 9.5 (5.0–16.0) | 0.030 |
| After 7 days from symptom onset | 3/8 (37.5) | 16/18 (88.9) | 0.014 |
| Interval from admission (median, range) | 3.5 (1.0–5.0) | 4.0 (1.0–10.0) | 0.461 |
| Hospital stay, days | 22.0 ± 22.5 | 34.9 ± 19.5 | 0.012 |
| ICU care | 60 (96.8) | 25 (92.6) | 0.582 |
| ICU stay, days | 5.8 ± 5.9 | 10.3 ± 7.2 | 0.008 |
| CRRT supporta | 1 (1.6) | 1 (3.7) | 0.517 |
| ECMO support | 3 (4.8) | 6 (22.2) | 0.020 |
| Death | 0 (0.0) | 1 (3.7) | 0.303 |
| Subgroup analysis: among patients received cidofovir before respiratory failure:b | |||
| Respiratory failure within 21 days after symptom onset | 4/58 (6.9) | 4/13 (30.8) | 0.033 |
| Interval from symptom onset (median, range) | 9.5 (6.8–10.0) | 10.0 (9.0–11.8) | 0.486 |
| After 7 days from symptom onset | 3/4 (75.0) | 4/4 (100) | 1.000 |
| Interval from admission (median, range) | 3.5 (2.3–4.8) | 5.0 (1.8–9.0) | 0.486 |
| Hospital stay, days | 19.0 ± 16.2 | 32.0 ± 16.3 | 0.011 |
| ICU care | 56/58 (96.6) | 11/13 (84.6) | 0.151 |
| ICU stay, days | 5.2 ± 5.4 | 6.1 ± 4.8 | 0.607 |
| CRRT supportb | 1/58 (1.7) | 0/13 (0.0) | 1.000 |
| ECMO support | 1/58 (1.7) | 0/13 (0.0) | 1.000 |
| Death | 0/58 (0.0) | 0/13 (0.0) | NA |
Data are expressed as the number (%) of patients or mean ± SD unless indicated otherwise.
Tx, treatment; ICU, intensive care unit; MV, mechanical ventilation; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; NA, not applicable.
Although one of each group received CRRT support, one in the delayed treatment group experienced renal impairment before cidofovir administration and the other in the early treatment group experienced after 2 weeks of cidofovir treatment. Both of them recovered from the renal injury and did not require maintenance haemodialysis.
The 21-day probability of respiratory failure was also significantly lower in the early treatment group of the subgroup (p 0.017 by log-rank test). Multivariate analysis was not conducted in the subgroup due to limited number of patients.
Cidofovir was administered at around the recommended dosage of 5 mg/kg. Under-dosing was observed in 32.6% of patients (29/89) and did not differ between the two groups. Time intervals from admission to treatment and from symptom onset to treatment were significantly shorter in the early treatment group (both p < 0.001). Only 8.1% of the early treatment group (5/62) were treated with a second dose of cidofovir, while 40.7% of the delayed treatment group (11/27) were repeatedly treated (p = 0.001). Only one patient in the early treatment group experienced acute kidney injury (AKI) within 2 weeks of cidofovir treatment; the patient recovered thereafter. Two patients received CRRT support that was not associated with cidofovir treatment (Table 2, footnote).
Analyses for 21-day probability of respiratory failure
The clinical courses of the early and delayed treatment groups are depicted in Supplementary material Fig. S1. Early treatment was significantly associated with a lower hazard of respiratory failure progression (Supplementary material Fig. S2, p < 0.001). Univariate analysis for 21-day probability of respiratory failure is presented in Supplementary material Table S1. Among statistically significant factors, monocyte count, hypoxaemia, confusion, and whole lung involvement were included in the multivariate analysis to adjust cidofovir treatment within 7 days after symptom onset (all p < 0.05). Detailed reasons for variable selection are presented in Table 3 (footnote). In the multivariate analyses, monocyte count (HR 0.995, 95%CI 0.991–1.000, p 0.042), confusion (HR 4.964, 95%CI 1.189–20.721, p 0.028), and cidofovir treatment within 7 days from symptom onset (HR 0.319, 95%CI 0.115–0.883, p = 0.028) were statistically significant (Table 3).
Table 3.
Multivariate analysis for 21-day probability of respiratory failure in cidofovir-treated patients with human adenovirus (HAdV) pneumonia
| Factors for respiratory failure | Multivariate analysis |
|
|---|---|---|
| HR (95%CI) | p value | |
| Monocyte count | 0.995 (0.991–1.000) | 0.042 |
| Hypoxaemia | 1.876 (0.681–5.168) | 0.224 |
| Confusion | 4.964 (1.189–20.721) | 0.028 |
| Whole lung involvement | 1.343 (0.473–3.815) | 0.580 |
| Cidofovir treatment within 7 days after symptom onset | 0.319 (0.115–0.883) | 0.028 |
Among factors significant in the univariate analysis, monocyte count, hypoxaemia, confusion, whole lung involvement, and cidofovir treatment within 7 days after symptom onset were included in the multivariate analysis. Lymphocyte count was not included in the multivariate analysis since it showed colinearity with monocyte count which showed a stronger HR. PaO2/FiO2 ratio (partial pressure of arterial oxygen/fraction of inspired oxygen) and tachypnoea were not included in the multivariate analysis because they shared parameters or associated with hypoxaemia which showed the highest HR among these factors. CURB-65 was not included since confusion, which is a component of the CURB-65, showed a higher HR than CURB-65. Time intervals between symptom onset, admission, and cidofovir treatment were not included because they shared values with cidofovir treatment within 7 days after symptom onset, which is the main exposure of the cohort. Admission time from symptom onset was associated with cidofovir administration time because if the patient admitted to the military hospital early, the patient would have a higher chance of receiving cidofovir early. These two variables showed statistically significant linear association.
Discussion
Fatal HAdV pneumonia in adults has been reported, but an effective treatment modality has not been well evaluated [13,22]. Among currently available antiviral agents, cidofovir—a cytosine nucleotide analogue inhibiting DNA polymerase—is recommended for the treatment of severe HAdV infections based on in vitro and case reports [14,16,22,23]. However, it is not approved for HAdV treatment because of lack of clinical trials. In addition, its high cost and potential adverse effects hinder general use of the drug [22]. Since winter 2014, the Korean military has experienced an explosion of cases of severe HAdV pneumonia due to the ongoing outbreak of HAdV-55 and tried cidofovir for potentially severe HAdV pneumonia patients based on previous experience [6,13,17,18]. Since we treated most potentially severe HAdV cases with cidofovir after the outbreak, a comparison between treated and non-treated patients was not feasible. However, we observed that early administration of cidofovir might prevent progression to respiratory failure and therefore designed the present retrospective cohort study comparing administration time of cidofovir. To our knowledge, the present study is the first comparative study to evaluate the effect of cidofovir for HAdV pneumonia, and could provide supporting data for further clinical studies.
Of note, the early treatment group within 7 days from symptom onset was significantly associated with a lower probability of respiratory failure, and late progression to respiratory failure after 10 days from symptom onset was not observed in this group. The treatment interval of 7 days from symptom onset dividing the early and delayed treatment groups was statistically significant in the multivariate analysis. These findings suggest clinical effectiveness of early cidofovir treatment for HAdV pneumonia. The importance of early treatment of respiratory viruses has been emphasized in studies assessing aerosolized ribavirin for respiratory syncytial virus [24], neuraminidase inhibitors for influenza [25], and convalescent plasma infusion therapy for severe acute respiratory syndrome (SARS) and severe influenza [26]. Likewise, cidofovir would be effective for treating HAdV pneumonia when administered early in the course of the disease, possibly before the progression to respiratory failure. Since cidofovir is expensive and approved only for the treatment of cytomegalovirus infection, it is not readily available in most settings, especially in resource-limited countries. In South Korea, patients need to purchase cidofovir individually through the Korea Orphan and Essential Drug Centre, and the process delays cidofovir administration by at least 1 day. For timely treatment of patients with potentially severe HAdV pneumonia and for further evaluation of clinical efficacy, easy access to cidofovir should be provided.
Although we could not assess patients with severe HAdV pneumonia who were not treated, outcome data can be compared with the previous report of the pre-cidofovir period of the Korean military (2011–2012) [11]. In that report, 12.2% of patients (6/49) with mild and severe HAdV infections progressed to respiratory failure and 6.1% of them (3/49) died. These outcomes would be worse than those of the early treatment group of the present study, with 12.9% (8/62) experiencing respiratory failure and no deaths among patients with potentially severe disease. Although direct comparison with the same severity criteria was not feasible, case fatality rate might have decreased after the introduction of cidofovir in the Korean military.
The present study findings should be interpreted carefully. First, our study does not imply effectiveness of cidofovir administered after progression to respiratory failure. Although this was not included in the scope of present study, we tested several statistical analyses among patients progressed to respiratory failure but could not find any meaningful association between the outcome and timing of cidofovir administration. Patients progressing to respiratory failure should be evaluated in another larger cohort containing both cidofovir-treated and non-treated patients. Second, the study population included young military personnel with minimal comorbidities. This cohort may demonstrate better outcomes and fewer complications than the general population. On the other hand, the study cohort was very homogeneous in terms of age and underlying conditions. Third, previously identified risk factors for respiratory failure—including hypoxaemia, multilobar involvement and pleural effusion—were not statistically significant in the multivariate analysis in the present study. This would be because these factors were used as inclusion criteria to select potentially severe patients. The purpose of the study was to evaluate the effect of cidofovir in the population at risk, not among all patients with HAdV pneumonia. Risk factors for respiratory failure were included in the multivariate analysis to adjust the effect of cidofovir treatment, and statistical non-significance does not necessarily mean that these factors were not associated with pneumonia progression. Fourth, as a retrospective study, there were significant differences between the early and delayed treatment group in terms of severity factors including hypoxaemia and whole lung involvement. Although these factors were adjusted in the multivariate analysis, interpretation of the study results should be cautious. Lastly, HAdV typing was not performed in all included patients. Although the major HAdV type during the study period was type 55 [6,13,17], other types of HAdV might have been included in the study cohort. Since in vitro data on cidofovir activity against various HAdV types are limited [14,27,28], careful interpretation of the study result is required, and further studies evaluating the effect of cidofovir on various HAdV types need to be conducted.
In conclusion, in this retrospective cohort study evaluating 89 cidofovir-treated HAdV pneumonia patients at risk for respiratory failure, early administration of cidofovir was associated with a lower hazard of progression to respiratory failure. It is suggested that cidofovir be administered within 7 days after symptom onset to prevent respiratory failure in patients with potentially severe HAdV pneumonia.
Transparency declarations
All the authors do not have conflict of interests to declare. No funding was received for this work.
Author contributions
J.-H.K. and J.U.L. contributed equally to this paper.
Editor: M. Paul
Footnotes
Preliminary results from this study were presented as poster at the joint meeting of ICIC (4th International Interscience Conference on Infection and Chemotherapy) & ISAAR (12th International Symposium on Antimicrobial Agents and Resistance), September 26–28, 2019, Gyeonju, Korea.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2019.10.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kim J.M., Jung H.D., Cheong H.M., Lee A., Lee N.J., Chu H. Nationwide surveillance of human acute respiratory virus infections between 2013 and 2015 in Korea. J Med Virol. 2018;90:1177–1183. doi: 10.1002/jmv.25069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim F.A., Tleyjeh I.M. Severe adenovirus pneumonia in immunocompetent adults: a case report and review of the literature. Eur J Clin Microbiol Infect Dis. 2008;27:153–158. doi: 10.1007/s10096-007-0416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan M.A., Gray G.C., Smith B., McKeehan J.A., Hawksworth A.W., Malasig M.D. Large epidemic of respiratory illness due to adenovirus types 7 and 3 in healthy young adults. Clin Infect Dis. 2002;34:577–582. doi: 10.1086/338471. [DOI] [PubMed] [Google Scholar]
- 4.Top F.H., Jr., Dudding B.A., Russell P.K., Buescher E.L. Control of respiratory disease in recruits with types 4 and 7 adenovirus vaccines. Am J Epidemiol. 1971;94:142–146. doi: 10.1093/oxfordjournals.aje.a121306. [DOI] [PubMed] [Google Scholar]
- 5.Hoke C.H., Jr., Snyder C.E., Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine. 2013;31:1623–1632. doi: 10.1016/j.vaccine.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Yoo H., Gu S.H., Jung J., Song D.H., Yoon C., Hong D.J. Febrile respiratory illness associated with human adenovirus type 55 in South Korea military, 2014–2016. Emerg Infect Dis. 2017;23:1016–1020. doi: 10.3201/eid2306.161848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B., Huang G.H., Pu Z.H., Qu J.X., Yu X.M., Zhu Z. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest. 2014;145:79–86. doi: 10.1378/chest.13-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Q.B., Tong Y.G., Wo Y., Wang H.Y., Liu E.M., Gray G.C. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009–2012. Influenza Other Respir Virus. 2014;8:302–308. doi: 10.1111/irv.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh M.P., Seto J., Jones M.S., Chodosh J., Xu W., Seto D. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol. 2010;48:991–993. doi: 10.1128/JCM.01694-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Z., Zhang Y., Xu S., Yu P., Tian X., Wang L. Outbreak of acute respiratory disease in China caused by B2 species of adenovirus type 11. J Clin Microbiol. 2009;47:697–703. doi: 10.1128/JCM.01769-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo J.Y., Lee J.E., Kim H.K., Choe K.W. Acute lower respiratory tract infections in soldiers, South Korea, April 2011–March 2012. Emerg Infect Dis. 2014;20:875–877. doi: 10.3201/eid2005.131692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heo J.Y., Noh J.Y., Jeong H.W., Choe K.W., Song J.Y., Kim W.J. Molecular epidemiology of human adenovirus-associated febrile respiratory illness in soldiers, South Korea (1) Emerg Infect Dis. 2018;24:1221–1227. doi: 10.3201/eid2407.171222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko J.H., Woo H.T., Oh H.S., Moon S.M., Choi J.Y., Lim J.U. Ongoing outbreak of human adenovirus-associated acute respiratory illness in the Republic of Korea military, 2013 to 2018. Korean J Intern Med. 2019 doi: 10.3904/kjim.2019.092. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemaly R.F., Hill J.A., Voigt S., Peggs K.S. In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: a systematic literature review. Antivir Res. 2019;163:50–58. doi: 10.1016/j.antiviral.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Doan M.L., Mallory G.B., Kaplan S.L., Dishop M.K., Schecter M.G., McKenzie E.D. Treatment of adenovirus pneumonia with cidofovir in pediatric lung transplant recipients. J Heart Lung Transplant. 2007;26:883–889. doi: 10.1016/j.healun.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Bordigoni P., Carret A.S., Venard V., Witz F., Le Faou A. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2001;32:1290–1297. doi: 10.1086/319984. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.J., Kim K., Park S.B., Hong D.J., Jhun B.W. Outcomes of early administration of cidofovir in non-immunocompromised patients with severe adenovirus pneumonia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon H., Jhun B.W., Kim S.J., Kim K. Clinical characteristics and factors predicting respiratory failure in adenovirus pneumonia. Respirology. 2016;21:1243–1250. doi: 10.1111/resp.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahler D.A., Wells C.K. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 21.Bellomo R., Ronco C., Kellum J.A., Mehta R.L., Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch J.P., 3rd, Kajon A.E. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med. 2016;37:586–602. doi: 10.1055/s-0036-1584923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naesens L., Lenaerts L., Andrei G., Snoeck R., Van Beers D., Holy A. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob Agents Chemother. 2005;49:1010–1016. doi: 10.1128/AAC.49.3.1010-1016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah D.P., Ghantoji S.S., Shah J.N., El Taoum K.K., Jiang Y., Popat U. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68:1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boikos C., Caya C., Doll M.K., Kraicer-Melamed H., Dolph M., Delisle G. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009—15. J Antimicrob Chemother. 2017;72:1556–1573. doi: 10.1093/jac/dkx013. [DOI] [PubMed] [Google Scholar]
- 26.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon Y.J., Araullo-Cruz T.P., Johnson Y.F., Romanowski E.G., Kinchington P.R. Isolation of human adenovirus type 5 variants resistant to the antiviral cidofovir. Invest Ophthalmol Vis Sci. 1996;37:2774–2778. [PubMed] [Google Scholar]
- 28.Hartline C.B., Gustin K.M., Wan W.B., Ciesla S.L., Beadle J.R., Hostetler K.Y. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J Infect Dis. 2005;191:396–399. doi: 10.1086/426831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.