Abstract
Mycoplasmas are frequently isolated from many animal species. In domestic cats, mycoplasmas may be isolated from respiratory and ocular mucosae, but other sites are also occasionally colonized by these organisms. No cases of Mycoplasma species-associated neurologic disease have been reported in cats. We describe a case of Mycoplasma felis-associated meningoencephalitis in a 10-month-old domestic shorthair cat.
Mycoplasmas are important pathogenic and commensal organisms isolated from many animal species. 1–6 Mycoplasmas are members of the class Mollicutes, which contains approximately 200 known species. Mycoplasmas are the smallest known prokaryotes. 1–3,5,6 They also represent the majority of the relatively few pathogenic Mollicutes found in animals. 1,5,6 Mycoplasmas have several characteristics that distinguish them from most other bacteria; notably they lack a cell wall, are one of the smallest independent replicating organisms, and the majority require sterols for growth. 1,5–7 Pathogenic mycoplasmas have a propensity for certain sites in animals, namely respiratory, ocular, and genital mucosa. 5,6,8 Some Mycoplasma species may cause persistent superficial infections, disseminate systemically, and invade surrounding tissues. 5,6,8,9 Mycoplasma felis is frequently isolated from the feline conjunctiva, respiratory tract, and urogenital tract. 3,10–14 M felis is strongly suspected as an etiologic agent in feline conjunctivitis, respiratory disease, and polyarthritis. 3,5,9,11–13 However, it has not been reported to cause feline central nervous system disease, nor has any other mycoplasma been implicated in neurologic disease except Mycoplasma pneumoniae in humans. 1,15–18 The purpose of the present study is to report a case of M felis-associated meningoencephalitis in a young cat.
A 10-month-old male castrated domestic shorthair cat presented to the Veterinary Teaching Hospital at The Ohio State University (OSU-VTH) for a 2-week history of a worsening neurologic condition and suspected inner ear disease. A month previously the cat was acutely lethargic and was treated with one 24 mg dose (0.3 ml) of injectable cefovecin (Convenia; Pfizer) and enrofloxacin/silver sulfadiazine otic drops for 10 days (Baytril Otic; Bayer). Two weeks after this treatment, the cat developed a left-sided head tilt and a reported fever ranging from 103–104°F. The cat was then treated with a single 1 mg dose (0.2 ml) of injectable meloxicam (Metacam; BoehringerIngelheim) and sent home on one half tablet of 25 mg marbofloxacin once q 24 h (Zenequin; Pfizer) and 0.3 mg (0.2 ml) of meloxicam (Metacam; BoehringerIngelheim) oral solution. These treatments helped resolve the fever but did not abate the neurologic signs. Other pertinent history obtained included that the cat lived strictly indoors, was current on rabies and feline viral rhinotracheitis, calicivirus, panleukopenia vaccines, and there were no other pets in the household. The cat had been adopted from a shelter 4 months previously and had been reportedly healthy until presentation at the referring veterinarian.
One week after beginning the marbofloxacin (Zenequin; Pfizer) the cat was seen at OSU Veterinary Hospital. Routine physical and neurological examination revealed quiet mentation, marked cervical weakness manifested as ventroflexion, left-sided head tilt and severe tetraparesis with the thoracic limbs worse than the pelvic limbs. Menace response was bilaterally absent, and there was decreased nasal sensation bilaterally, horizontal nystagmus with the fast phase to the right, and left supraspinatus muscle atrophy. Proprioceptive positioning and hopping responses were decreased in all four limbs but worse in the thoracic limbs. Patellar and flexor reflexes were normal in both pelvic limbs but the thoracic limb withdrawal reflex was decreased bilaterally. Spinal pain was consistently elicited upon spinal palpation between the second and fourth lumbar vertebrae (L2–L4). Based on the neurological findings, a lesion was localized to the left rostral medulla, bilateral cortex and cervical spinal cord segments C1–C8, as well as L2–L4 vertebrae.
A complete blood count (CBC) revealed increased plasma protein (8.0 g/dl; reference interval (RI) 5.6–7.4 g/dl), mild leukocytosis (15.0×109/l; RI 4.0–14.5×109/l) with an increase in segmented neutrophils (12.6×109/l; RI 3.0–9.2×109/l) and monocytosis (0.9×109/l; RI 0–0.5×109/l). Serum biochemistry showed hypercalcemia (11.2 mg/dl; RI 8.4–10.1 mg/dl), hypochloremia (111.1 mEq/l; RI 114–126), and low alkaline phosphatase (9 IU/l; RI 15–65 IU/l). Electrolytes panel the following day, revealed Na+, K+, total serum Ca++, Cl−, and total serum Mg++, to be within normal limits. Feline immunodeficiency virus and feline leukemia virus enzyme-linked immunosorbent assays (Snap; Idexx) were negative. A coronavirus titer was not performed.
Cerebrospinal fluid (CSF) fluid was collected from both cerebellomedullary (CBM) and lumbar cisterns. The CBM cisternal CSF was clear, colorless, with a total protein of 71 mg/dl (RI<25 mg/dl), a white blood cell (WBC) count of 151 cells/μl (RI<5 cells/μl), and a red blood cell (RBC) count of 6 cells/μl (RI<5 cells/μl). The cytological proportions were 70% non-degenerate neutrophils, 9% mononuclear cells, and 21% lymphocytes, which includes plasma cells. The lumbar CSF was xanthochromic and cloudy. Lumbar CSF protein was 2590 mg/dl (RI<40 mg/dl), WBC count was 86,400 μl (RI<5 cells/μl), and RBC count was 3600 μl (RI<5 cells/μl). A hundred percent of the WBC was non-degenerate neutrophils. The findings of the CBM and lumbar cistern CSF were consistent with a marked neutrophilic inflammation. CSF was submitted for culture and sensitivity.
Based on the CSF findings, the primary differential diagnoses were bacterial or fungal meningoencephalomyelitis, and feline infectious peritonitis. Due to the severity of the clinical signs, the guarded prognosis associated with our differential diagnoses and the lack of response to antimicrobial therapy previously instituted by the referring veterinarian, the owner elected to humanely euthanase the cat. A complete necropsy was performed.
A 5 mm (approximate) diameter area of purulent material was observed on the meninges covering the ventral aspect of the right cerebellum and portion of the brainstem. The meninges of the left side of the brain ventral to the cerebrum and lateral to the mamillary bodies contained a 2 mm (approximate) in diameter, pocket of inspissated purulent material (Fig 1). The spinal cord in the area of C2 and C3 appeared light green when the dura was intact. The dura covering the spinal cord was white and very soft on cut surface. The left bulla was filled with green-tinged translucent viscous fluid. Other gross findings included multifocal gingival ulceration, acute diffuse moderate pulmonary congestion and edema (interpreted as terminal changes). Histologic examination of the spinal cord (including brainstem), cerebellum and cerebrum exhibited inflammation of varying severity. The leptomeninges in sections of spinal cord from C3–C6 were markedly expanded and effaced by accumulations of intact and degenerate neutrophils admixed with fibrin. Numerous coalescing aggregates of lymphocytes, plasma cells and histiocytes were also present adjacent to the outer edge of the white matter. Multifocal randomly distributed areas of white matter contained dilated myelin sheaths with occasional dilated axons and axonophagia. Areas of parenchymal rarefaction were present in the white matter, interpreted as pan-necrosis. Similar inflammatory and axonal changes were observed at the levels of T2, L2–L3 and L4–L5, however, all changes were judged as mild to moderate when compared to the cervical changes. Inflammation in the cerebellum and brainstem was similar in composition, predominantly on the ventral aspect of the brainstem (Figs 2 and 3).
Fig 1.
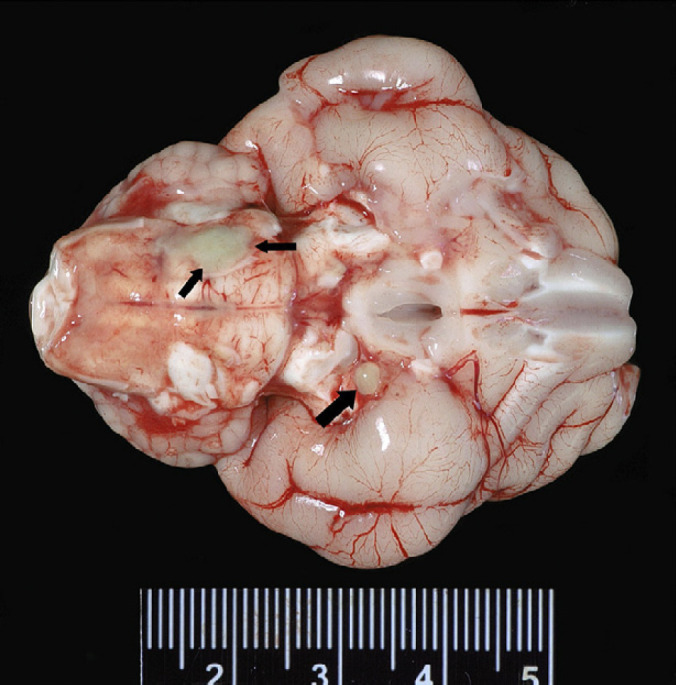
Gross pathology of lesions seen on the ventral aspect of brain and brainstem. One large black arrow points to an approximately 2 mm in diameter hard pocket of green purulent material on the meninges of the left side of the brain, ventral to the cerebrum. Two smaller black arrows highlight an approximately 5 mm in diameter area of green purulent fluid on the meninges ventral to the cerebellum and brainstem.
Fig 2.
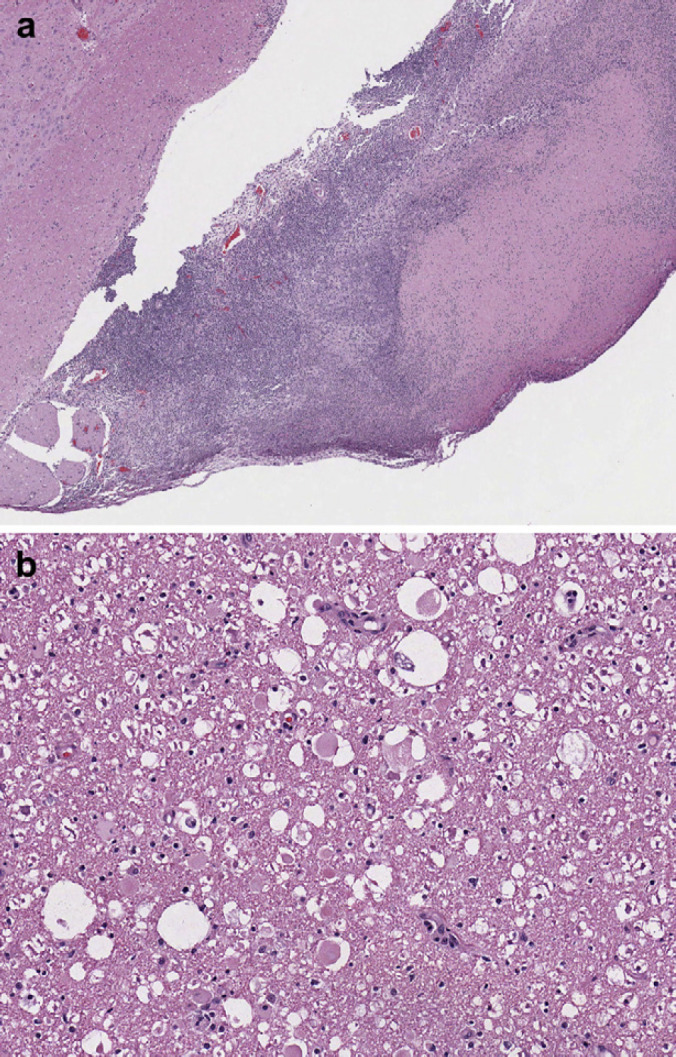
(a) Section of brainstem at 4× showing large scale lymphoplasmacytic infiltration into the superficial parenchyma of the brainstem. (b) Section of brainstem at 40× showing intact and degenerative neutrophils and lymphoplasmacytic infiltration representative of severe inflammation.
Fig 3.
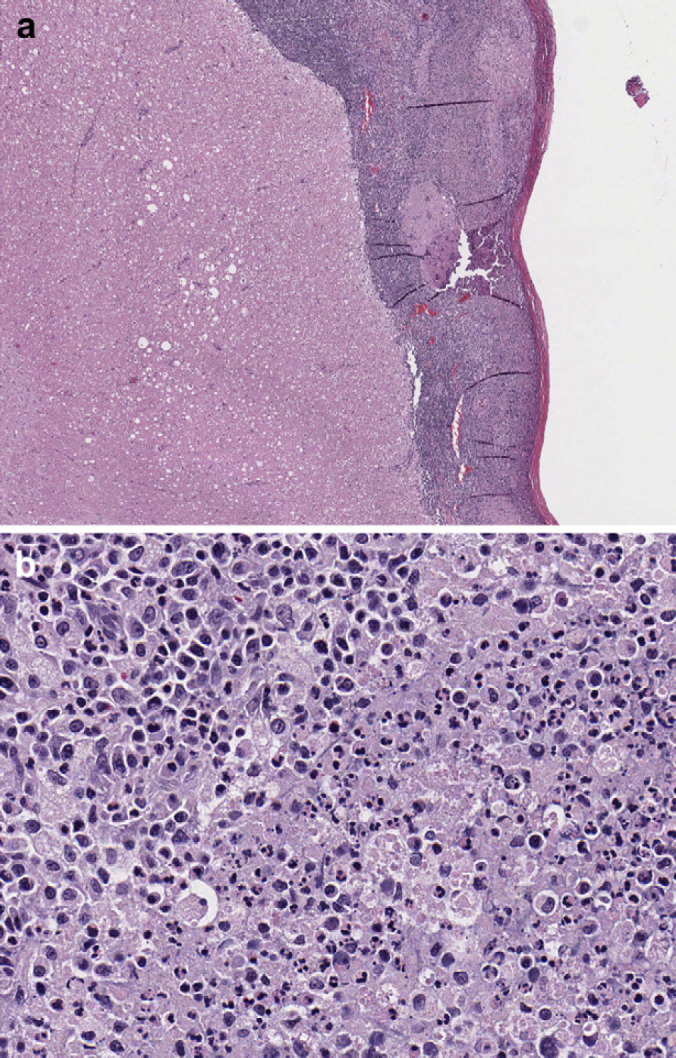
(a) Section of spinal cord at 4× representative of leptomeninges from C3–C6. Multifocal randomly distributed areas of white matter contained dilated myelin sheaths with occasional dilated axons and axonophagia. Areas of parenchymal rarefaction were present in the white matter, interpreted as pan-necrosis. (b) Section of spinal cord at 40× showing leptomeninges that are markedly expanded and effaced by accumulations of intact and degenerate neutrophils admixed with eosinophilic slightly fibrillar material (fibrin). Numerous coalescing aggregates of lymphocytes, plasma cells and histiocytes were also present adjacent to the outer edge of the white matter.
Sections of brain and spinal cord were submitted for aerobic and anaerobic bacterial culture. The cerebrospinal fluid was cultured on trypticase soy agar supplemented with 5% sheep blood and incubated at 35°C in 5% CO2. Pure, heavy growth of pinpoint-sized α-hemolytic colonies was noted at 48 h on all samples. The organism was not visible when using Gram stain reagents, indicating that it might be a mycoplasma, therefore, it was subsequently identified via 16S rDNA sequencing. 19 A search of the GenBank database was carried out using the BLAST algorithm (Basic Local Alignment Search Tool; http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). The 1.4 kb fragment of the 16S rDNA entry yielded a 99% match to M felis.
It is notable that specific Mycoplasma species culture media was not employed upon primary isolation, as Mycoplasma species were not part of the differential diagnosis. However, many Mycoplasma species are cultivable on simple media. 4,20,21
M felis has never been reported in any animal species as a causative agent of neurologic disease nor to infect nervous tissue. M felis is one of several species of mycoplasma recovered from cats; other mycoplasmas associated with cats are M gatae, M feliminutum, and M arginini. 5,6,9,11–13 An acholeplasma (A laidlawii) and a Ureaplasma species have also been isolated from cats. 5,6,12 M felis has been implicated in few disease processes, most notably conjunctivitis. Respiratory disease and arthritis have also been associated with M felis infection as the etiologic agent. 2,4–6,9,11,12,22 However, the specific pathogenesis of M felis in these disease processes remains unclear.
Literature research on the broader category of Mycoplasma species pathogenesis offers some clues to potential mechanisms but no concrete answers specifically for M felis. Generally, respiratory infections caused by mycoplasmas involve adherence of the organisms to ciliated epithelium, which consequently can cause cell death. 6,23 There are several other proposed mechanisms of Mycoplasma species pathogenesis such as hematologic dissemination and immunomodulatory effects on host cells. In particular, M pneumoniae has been extensively researched and is the only Mycoplasma species known to cause neurologic disease. 1,15–18
M pneumoniae has a limited ability to damage respiratory epithelial cells, but still remains a major pathogen of atypical pneumonia in humans. Moreover, M pneumoniae has been implicated in numerous extrapulmonary diseases, including variable neurologic manifestations. 15–18 The neurologic manifestations seen in people are classified anatomically as brain, spinal cord, and peripheral nerve disorders. Specifically, these are seen as encephalitis, transverse myelitis, and neuropathies of the cranial nerves and nerve roots. 15–18 The underlying pathophysiology has been attributed to three possible mechanisms; a direct type, an indirect type, and a vascular occlusion type. 17 The direct type of extrapulmonary disease by M pneumoniae involves locally induced cytokines that subsequently cause inflammation. The indirect type is immunomodulatory in nature, and extrapulmonary manifestations are the result of humoral immunity, autoimmune reaction, allergy, or production of immune complexes. The vascular occlusion type of neurologic manifestation encompasses both direct and indirect mechanisms following hematogenous spread of M pneumoniae. 17
All the above pathogeneses of M pneumoniae extrapulmonary neurologic disease are neither mutually exclusive nor exhaustive. In the cases of encephalitis and myelitis a combination of direct and indirect pathogenesis has been observed. There are also differences between early-onset and late-onset neurologic disease, as regards pathogenesis and findings. Early-onset encephalitis and early-onset myelitis are typically seen more in children than adults, and detection of M pneumoniae in the CSF is also seen more frequently. 15–18 Hematogenous dissemination and detection of M pneumoniae from CSF are also relevant to our present case report as M felis was cultured from the CSF. The histopathologic diagnosis of the cat's brain and spinal cord (lymphoplasmacytic, suppurative meningoencephalomyelitis) was similar to findings of central nervous system (CNS) disease caused by M pneumoniae. Failure of the cat to improve clinically on appropriate antimicrobial therapy (fluoroquinolones have activity against Mycoplasma species and cross the blood–brain barrier) in this cat supports the indirect type host immune response as a pathomechanism, as seen in M pneumoniae. In fact, immunosuppressive steroid therapy has been recognized as beneficial in cases of human M pneumoniae neurologic disease. 15,16,18
Mycoplasmas are known to cause abscessation, and it is conceivable that extrapulmonary manifestation from a concurrent M felis infection is possible. However, an underlying clinical respiratory infection was not supported by this cat's history and no pulmonary lesions were found at necropsy. Therefore, the pathogenesis of this animal's encephalitis may not parallel the M pneumoniae encephalitides in humans as sequelae to respiratory disease. In this case, it is also unknown whether M felis acted as a sole pathogen, or was a sequela to another bacterial or viral infection. The otitis media-interna seen on necropsy suggested a concurrent infection and could have contributed to the head tilt but not to the multitude of neurologic signs secondary to brain and spinal cord involvement. The veterinary literature reports of middle–inner ear infections in cats are associated with Staphylococcus species, Escherichia coli, Pseudomonas aeruginosa, and Proteus mirabilis. 24 This cat did not have the appropriate dose or length of antibiotic regimen appropriate for otitis media, but none of these organisms were seen on culture of the CSF or CNS tissue. 24 Thus, even if the concurrent otitis media played a role in contributing to vestibular signs, it is doubtful that CNS involvement from a subsequent otitis interna was the cause for this particular cat's severe non-antibiotic responsive neurologic disease. Isolation of only one organism as a pure culture, ie, M felis, from the CSF and CNS lesions supports the conclusion that this cat's multifocal neurological signs were significantly contributed by this organism, even if a comorbid disease was present.
Feline infectious peritonitis (FIP) is one of the most common causes of inflammatory neurologic disease in the cat. Although no antemortem coronavirus titers were performed, nor reverse-transcriptase polymerase chain reaction (PCR), it would be possible to utilize immunohistochemistry or reverse-transcriptase PCR on brain tissue directed toward feline coronavirus to rule-out this infection more definitively. However, the gross and histopathologic lesions do not suggest FIP. Typically FIP lesions are pyogranulomatous and perivascular, with associated hydrocephalus. 25,26 Other potential diseases that could cause meningitis, encephalitis, and neurologic lesions in the cat are feline leukemia virus, feline immunodeficiency virus, rabies, Toxoplasma gondii, fungal infections, and feline spongiform encephalopathy. 25,26 These diseases were either ruled out with history, vaccine status, diagnostic testing, or histopathology in this particular cat.
The fact that this is the first reported case of M felis-associated neurologic disease warrants additional examination of the potential role of M felis in CNS disease. Whether this infectious agent is merely a rare opportunist in a uniquely susceptible host or a more common etiologic agent in feline CNS inflammatory diseases remains to be determined.
Addendum
Since acceptance of our manuscript, a case of a dog with meningoencephalitis caused by Mycoplasma edwardii was published (J Vet Diagn Invest 22: 805–818, 2010). We had mentioned in our discussion that there was no report of Mycoplasma species causing CNS disease in animals, which is no longer true. Our report still presents the only case of CNS disease in a cat caused by Mycoplasma.
References
- 1.Baseman J.B., Tully J.G. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety, Emerg Infect Dis 3, 1997, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crisp M.S., Birchard S.J., Lawrence A.E., Fingeroth J. Pulmonary abscess caused by a Mycoplasma sp in a cat, J Am Vet Med Assoc 191, 1987, 340–342. [PubMed] [Google Scholar]
- 3.Foster S.F., Martin P., Braddock J.A., Malik R. A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995–2000), J Feline Med Surg 6, 2004, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnsrude J.D., Christopher M.M., Lung N.P., Brown M.B. Isolation of Mycoplasma felis from a serval (Felis serval) with severe respiratory disease, J Wildl Dis 32, 1996, 691–694. [DOI] [PubMed] [Google Scholar]
- 5.Whitford H.W., Rosenbusch R.F., Lauerman L.H., Committee AAOVLDM. Mycoplasmosis in animals, 1994, Wiley–Blackwell: Oxford. [Google Scholar]
- 6.Razin S., Herrmann R. Molecular biology and pathogenicity of mycoplasmas, 2002, Springer: New York. [Google Scholar]
- 7.Gray L.D., Ketring K.L., Tang Y. Clinical use of 16S rRNA gene sequencing to identify Mycoplasma felis and M gateae associated with feline ulcerative keratitis, J Clin Microbiol 43, 2005, 3431–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker R.D., Walshaw R., Riggs C.M., Mosser T. Recovery of two mycoplasma species from abscesses in a cat following bite wounds from a dog, J Vet Diagn Invest 7, 1995, 154–156. [DOI] [PubMed] [Google Scholar]
- 9.Hooper P.T., Ireland L.A., Carter A. Mycoplasma polyarthritis in a cat with probable severe immune deficiency, Aust Vet J 62, 1985, 352. [DOI] [PubMed] [Google Scholar]
- 10.Blackmore D.K., Hill A., Jackson O.F. The incidence of mycoplasma in pet and colony maintained cats, J Small Anim Pract 12, 1971, 207–217. [DOI] [PubMed] [Google Scholar]
- 11.Campbell L.H., Snyder S.B., Reed C., Fox J.G. Mycoplasma felis-associated conjunctivitis in cats, J Am Vet Med Assoc 163, 1973, 991–995. [PubMed] [Google Scholar]
- 12.Haesebrouck F., Devriese L.A., van Rijssen B., Cox E. Incidence and significance of isolation of Mycoplasma felis from conjunctival swabs of cats, Vet Microbiol 26, 1991, 95–101. [DOI] [PubMed] [Google Scholar]
- 13.Tan R.J. Susceptibility of kittens to Mycoplasma felis infection, Jpn J Exp Med 44, 1974, 235–240. [PubMed] [Google Scholar]
- 14.Tan R.J., Lim E.W., Ishak B. Ecology of mycoplasmas in clinically healthy cats, Aust Vet J 53, 1977, 515–518. [DOI] [PubMed] [Google Scholar]
- 15.Daxboeck F. Mycoplasma pneumoniae central nervous system infections, Curr Opin Neurol 19, 2006, 374–378. [DOI] [PubMed] [Google Scholar]
- 16.Daxboeck F., Blacky A., Seidl R., Krause R., Assadian O. Diagnosis, treatment, and prognosis of Mycoplasma pneumoniae childhood encephalitis: systematic review of 58 cases, J Child Neurol 19, 2004, 865–871. [DOI] [PubMed] [Google Scholar]
- 17.Narita M. Pathogenesis of neurologic manifestations of Mycoplasma pneumoniae infection, Pediatr Neurol 41, 2009, 159–166. [DOI] [PubMed] [Google Scholar]
- 18.Christie L.J., Honarmand S., Talkington D.F., et al. Pediatric encephalitis: What is the role of Mycoplasma pneumoniae?, Pediatrics 120, 2007, 305–313. [DOI] [PubMed] [Google Scholar]
- 19.Ramos C.P., Foster G., Collins M.D. Phylogenetic analysis of the genus Actinomyces based on 16S rRNA gene sequences: description of Arcanobacterium phocae sp nov, Arcanobacterium bernardiae comb nov, and Arcanobacterium pyogenes comb nov, Int J Syst Bacteriol 47, 1997, 46–53. [DOI] [PubMed] [Google Scholar]
- 20.Thigpen J.E., Cottew G.S., Yeats F., McGhee C.E., Rose D.L. Growth characteristics of large- and small-colony types of Mycoplasma mycoides subsp mycoides on 5% sheep blood agar, J Clin Microbiol 18, 1983, 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ülgen M., Cetin C., Eentürk S., Özel A.E., Özdemir Ü. Urinary tract infections due to Mycoplasma canis in dogs, J Vet Med Series A 53, 2006, 379–382. [DOI] [PubMed] [Google Scholar]
- 22.Liehmann L., Degasperi B., Spergser J., Niebauer G.W. Mycoplasma felis arthritis in two cats, J Small Anim Pract 47, 2006, 476–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood J.L.N., Chanter N., Newton J.R., et al. An outbreak of respiratory disease in horses associated with Mycoplasma felis infection, Vet Rec 140, 1997, 388–391. [DOI] [PubMed] [Google Scholar]
- 24.Sturges B.K., Dickinson P.J., Kortz G.D., et al. Clinical signs, magnetic resonance imaging features, and outcome after surgical and medical treatment of otogenic intracranial infection in 11 cats and 4 dogs, J Vet Intern Med 20, 2006, 648–656. [DOI] [PubMed] [Google Scholar]
- 25.Bradshaw J.M., Pearson G.R., Gruffydd-Jones T.J. A retrospective study of 286 cases of neurological disorders of the cat, J Comp Pathol 131, 1995, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley J.E., Lapointe J.M., Koblik P., Poland A., Pedersen N.C. Diagnostic features of clinical neurologic feline infectious peritonitis, J Vet Intern Med 12, 1998, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]


