Abstract
Epidermal growth factor (EGF) promotes gastrointestinal mucosal recovery by stimulating the mitogenic activity of intestinal crypt epithelial cells. The aim of this study was to determine the effects of EGF on atrophic enteritis induced in piglets by experimental infection with porcine epidemic diarrhoea virus (PEDV) strain Dr13. Two groups of 12 conventional, colostrum-deprived, 1-day-old, large White-Duroc cross breed piglets were inoculated orally with PEDV (3 × 105 50% tissue culture infective doses), with or without EGF (10 μg/kg/day, intraperitoneally once daily for 4 days after infection) and compared to 12 uninfected, untreated control piglets.
PEDV + EGF piglets had less severe clinical signs than PEDV only piglets at 48 and 60 h post-infection (hpi). Histologically, the ratio of villous height:crypt depth of PEDV + EGF piglets was significantly higher than PEDV only piglets at 36 and 48 hpi. Immunohistochemistry for Ki67 demonstrated increased proliferation in intestinal crypt epithelial cells of PEDV + EGF piglets compared to PEDV only piglets at 36, 48 and 60 hpi. EGF stimulates proliferation of intestinal crypt epithelial cells and promotes recovery from atrophic enteritis in PEDV-infected piglets.
Keywords: Epidermal growth factor, Porcine epidemic diarrhoea virus, Atrophic enteritis, Mucosal repair
Introduction
Porcine epidemic diarrhoea virus (PEDV), a member of the genus Coronavirus (Family Coronaviridae, Order Nidovirales) causes highly contagious diarrhoea in pigs of all ages and is particularly fatal to piglets less than 2 weeks of age (Pensaert and de Bouck, 1978, Chae et al., 2000). PEDV is an economically important cause of disease in pigs in Korea, resulting in significant morbidity and mortality in neonatal piglets and increased costs due to vaccination and disinfection (Chae et al., 2000, Jung et al., 2006a, Song et al., 2006). Although vaccines can be used to prevent PEDV, there are no effective strategies for treatment of affected pigs during PEDV outbreaks.
PEDV infections are characterised by acute destruction of intestinal villous enterocytes and villous atrophy in the jejunum and ileum, producing severe malabsorptive and maldigestive diarrhoea (Pensaert and de Bouck, 1978, Jung et al., 2006b). Severe intestinal villous atrophy, with 50–60% loss of villi, develops within 3 days in piglets experimentally infected with PEDV (Jung et al., 2006a, Jung et al., 2006b).
The role of growth factors in intestinal mucosal repair has been studied extensively (Lemoine et al., 1992, Stephen Murphy, 1998, Playford, 1995, Playford and Wright, 1996). Five EGF-like peptides, including EGF, transforming growth factor-α, heparin-binding EGF-like growth factor (HB-EGF), amphiregulin and betacellulin, have been identified in the intestinal lumen (Marti et al., 1989, Lemoine et al., 1992, Barnard et al., 1995). Among these, EGF acts primarily as a gastrointestinal surveillance peptide involved in mucosal repair (Playford, 1995, Playford and Wright, 1996).
Epidermal growth factor (EGF) is secreted in the gastrointestinal tract by salivary glands, duodenal Brunner’s glands and ulcer-associated cell lineage (UACL) cells (Barnard et al., 1995). EGF binds to a specific receptor expressed on the basolateral surface of enterocytes during mucosal damage and stimulates mitogenic activity (Playford, 1995, Playford and Wright, 1996). EGF has positive effects on the recovery of damaged intestinal villi by promoting the proliferation of intestinal crypt epithelial cells (Skov Olsen et al., 1986, Chao et al., 2003).
The aim of this study was to determine the effects of EGF on atrophic enteritis induced in piglets by PEDV and to assess whether EGF has therapeutic potential for treatment of viral enteritis in piglets.
Materials and methods
Recombinant human epidermal growth factor
Recombinant human EGF was produced as described previously (Lee et al., 2003) and stored lyophilised at −20 °C. EGF administered to piglets was diluted with sterile saline.
Porcine epidemic diarrhoea virus
Inoculations were performed with fourth passage tissue culture propagated PEDV strain Dr13, a virulent isolate that causes yellowish watery diarrhoea in piglets within 24 h of oral infection (Song et al., 2003). The virus was titrated in Vero cells and viral antigen was detected by immunofluorescence using a monoclonal antibody (Jung and Chae, 2004).
Experimental design
Five conventional, colostrum-deprived, 1-day-old, large White-Duroc crossbreed piglets were used from a PEDV-seronegative sow from a herd with no history of PEDV to determine the optimal dose and time schedule for treatment with EGF. The intraperitoneal route was selected to minimise the unpredictable toxicological effects of recombinant proteins on piglets. No adverse effects were detected following daily administration of 10 μg/kg/day EGF for 2–6 days.
Thirty-six conventional, colostrum-deprived, 1-day-old, large White-Duroc crossbreed piglets were obtained from 10 PEDV-seronegative sows from a herd with no history of PEDV and randomly divided into three groups. Twenty-four piglets were inoculated orally with 3 mL of 1 × 105 50% tissue culture infective doses/mL of PEDV strain Dr13. Twelve PEDV-infected piglets (PEDV + EGF) were treated with 10 μg/kg/day EGF intraperitoneally once daily from 0–4 days after infection. Twelve control piglets were inoculated with uninfected cell culture medium.
Experimental piglets were held in groups of three in isolators at 28.5 °C and fed a commercial sterile milk substitute. Animals were examined three times daily for clinical signs. Three piglets from each group were sacrificed at 24, 36, 48 and 60 h post-infection (hpi) by electrocution after sedation with IV sodium pentobarbital.
Morphometric analysis
Three segments of jejunum from each piglet were fixed in 10% neutral buffered formalin, dehydrated in graded ethanol solutions and embedded in paraffin wax for histopathology. Ten villi and 10 crypts were measured in well orientated histological sections. The ratio of villous height:crypt depth (VH:CD) was calculated and values were expressed as means ± standard deviation (Jung et al., 2006a, Jung et al., 2006b). Significant differences in VH:CD between groups of piglets were assessed using Wilcoxon matched pairs test. P < 0.05 was considered statistically significant.
Immunohistochemistry for Ki67
Proliferation of intestinal crypt epithelial cells was evaluated by immunohistochemistry for Ki67 (Danilenko et al., 1995). Antigens were retrieved from formalin-fixed paraffin-embedded tissues by pressure cooking, then were incubated with normal goat serum (Sigma) in phosphate-buffered saline (PBS) for 30 min at room temperature to block non-specific reactivity. Sections were incubated with mouse anti-human Ki67 monoclonal antibody (Dako, 1:200) overnight at 4 °C in a humid chamber. After three washes with PBS, sections were incubated for 1 h at 36 °C with goat anti-mouse IgG (Dako, 1:200) conjugated to alkaline phosphatase. After colorimetric detection with red substrate (Roche Applied Science) for 10 min at room temperature, sections were counterstained with Mayer’s haematoxylin.
The Ki67 score (number of positive cells per field under 200× magnification) was evaluated using an image analyser (Image Measurement Standard v4.01, Bersoft). Values were expressed as means ± standard deviation and statistical analysis was performed by analysis of variance (ANOVA) and Student’s t test.
Results
Clinical signs
PEDV only piglets exhibited severe watery diarrhoea and intermittent vomiting from 24 to 60 hpi, some exhibited anorexia from 36 to 60 hpi and most had severe dehydration from 48 to 60 hpi. Most PEDV + EGF piglets had moderate diarrhoea from 24 to 36 hpi and severe diarrhoea from 48 to 60 hpi, although some piglets had severe diarrhoea at 24 hpi. Anorexia and dehydration were observed in PEDV + EGF piglets from 36 to 60 hpi. Subjectively, clinical signs were less severe in PEDV + EGF piglets than PEDV only piglets.
Ratio of villous height to crypt depth
At 24 hpi, the mean VH:CD of PEDV-infected piglets was similar to that of control piglets, but decreased significantly from 36 to 60 hpi (Table 1 ). The mean VH:CD of PEDV + EGF piglets was significantly higher than that of PEDV only piglets from 36 to 48 hpi (P < 0.05).
Table 1.
Mean ratio of villous height to crypt depth (VH:CD) in piglets infected with porcine epidemic diarrhoea virus with (PEDV + EGF) or without (PEDV only) epidermal growth factor relative to uninfected piglets (control)
| Group | Hours post-inoculation (hpi) |
|||
|---|---|---|---|---|
| 24 | 36 | 48 | 60 | |
| Control | 6.84 ± 0.53 | 6.92 ± 0.78 | 6.45 ± 0.84 | 6.67 ± 0.66 |
| PEDV only | 6.27 ± 0.91 | 3.24 ± 0.54 | 3.01 ± 0.36 | 2.92 ± 0.30 |
| PEDV + EGF | 6.45 ± 0.74 | 5.54 ± 1.20⁎ | 3.75 ± 0.43⁎ | 3.55 ± 0.55 |
Values are given as mean ± standard deviation.
Significant difference (P < 0.05) in VH:CD between PEDV + EGF and PEDV only piglets.
Ki67 immunohistochemistry
Ki67 scores peaked at 60 hpi in all piglets infected with PEDV (Table 2 ). At 24 hpi, the Ki67 scores of PEDV + EGF piglets were not significantly different from that of PEDV only piglets. However, from 36 to 60 hpi, the Ki67 scores of PEDV + EGF piglets were significantly higher than PEDV only piglets (P < 0.05) (Table 2; Fig. 1 ).
Table 2.
Ki67 score for proliferation of intestinal crypt epithelial cells in piglets infected with porcine epidemic diarrhoea virus with (PEDV + EGF) or without (PEDV only) epidermal growth factor relative to uninfected piglets (control)
| Group | Hours post-inoculation (hpi) |
|||
|---|---|---|---|---|
| 24 | 36 | 48 | 60 | |
| Control | 455 ± 65 | 413 ± 72 | 472 ± 55 | 465 ± 42 |
| PEDV only | 473 ± 52 | 1900 ± 140 | 2380 ± 350 | 2896 ± 280 |
| PEDV + EGF | 482 ± 67 | 2200 ± 127⁎ | 2990 ± 297⁎ | 3450 ± 377⁎ |
Values are given as mean ± standard deviation.
Significant difference (P < 0.05) in Ki67 score between PEDV + EGF and PEDV only piglets.
Fig. 1.
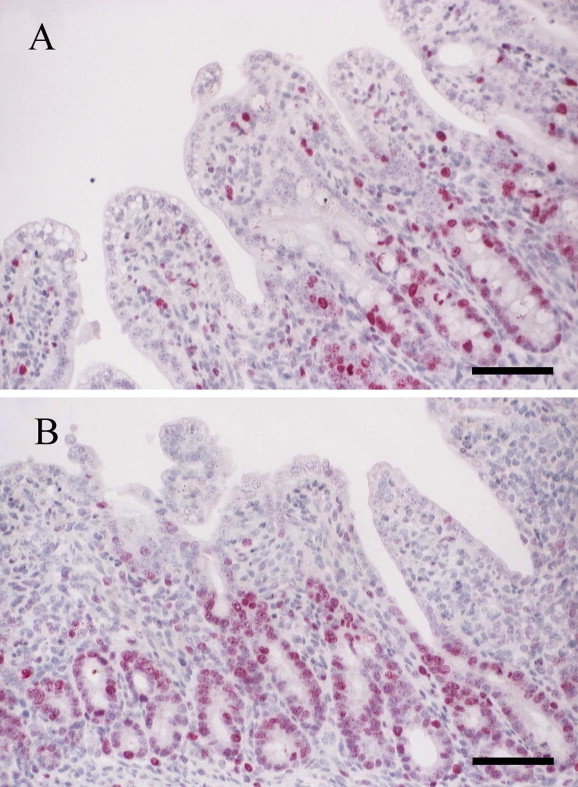
Immunohistochemistry for Ki67. (A) Jejunum of piglet infected with porcine epidemic diarrhoea virus (PEDV) only without epidermal growth factor (EGF) treatment at 60 h post-inoculation (hpi). Positive (red) staining for Ki67 is present in the cytoplasm of crypt epithelial cells. Haematoxylin counterstain. Bar = 50 μm. (B) Jejunum of PEDV + EGF piglet at 60 hpi. Note the higher frequency of Ki67 positive cells in intestinal crypt than the PEDV only piglet. Haematoxylin counterstain. Bar = 50 μm.
Discussion
EGF exerts positive effects on the recovery of damaged intestinal villi by accelerating the proliferation of crypt epithelial cells (Skov Olsen et al., 1986, Chao et al., 2003). In our study, immunohistochemistry for Ki67 revealed that mucosal proliferation in the small intestine of PEDV + EGF piglets was significantly enhanced in comparison with PEDV only piglets from 36 to 60 hpi. Similarly, VH:CD of PEDV + EGF piglets were higher than those of PEDV only piglets from 36 to 48 hpi. These findings suggest that EGF stimulates the proliferation of intestinal crypt cells and promotes the recovery of atrophied villi in the small intestine of PEDV-infected piglets.
A variety of growth factors have been used in clinical therapeutics in humans. EGF and fibroblast growth factor are used for skin wound healing, such as in diabetic foot ulcer (Ito et al., 2005, Hong et al., 2006). Keratinocyte growth factor (KGF) has been used to assist recovery of damaged mucosa in the alimentary tract following radiation and chemotherapy for cancer (Farrell et al., 1998, Farrell et al., 2002). KGF increases the thickness and cellularity of the intestinal mucosa by accelerating mitotic activity in crypt epithelial cells (Housley et al., 1994, Farrell et al., 1998, Playford et al., 1998). EGF may have a similar mechanism of action to KGF in the small intestine.
Severe intestinal villous atrophy develops within 3 days after experimental infection of piglets with PEDV (Jung et al., 2006a, Jung et al., 2006b), whereas intestinal mucosal damage following radiation or chemotherapy is manifested after 5–10 days (Dorr et al., 2005). In our study, EGF was effective in promoting recovery from acute atrophic enteritis within three days after treatment.
Conclusions
This study has shown that EGF enhances proliferation of intestinal crypt epithelial cells and promotes the recovery of damaged villi in piglets infected with PEDV. While treatment with recombinant EGF is currently expensive and requires further toxicological and field studies, we propose EGF as a potential novel therapy to promote intestinal villous recovery in piglets with PEDV infection and possibly other species with viral atrophic enteritis.
References
- Barnard J.A., Beauchamp R.D., Russell W.E., Dubois R.N., Coffey R.J. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108:564–580. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Chae C., Kim O., Choi C., Min K., Cho W.-S., Kim J., Tai J.H. Prevalence of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus infection in Korean pigs. Veterinary Record. 2000;147:606–608. doi: 10.1136/vr.147.21.606. [DOI] [PubMed] [Google Scholar]
- Chao J.C., Liu K.Y., Chen S.H., Fang C.L., Tsao C.W. Effect of oral epidermal growth factor on mucosal healing in rats with duodenal ulcer. World Journal of Gastroenterology. 2003;89:710–716. doi: 10.3748/wjg.v9.i10.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko D.M., Ring B.D., Tarpley J.E., Morris B., Van G.Y., Morawiecki A., Callahan W., Goldenberg M., Hershenson S., Pierce G.F. Differing targets and effects of keratinocyte growth factor, platelet-derived growth factor-BB, epidermal growth factor, and neu differentiation factor. American Journal of Pathology. 1995;147:1261–1277. [PMC free article] [PubMed] [Google Scholar]
- Dorr W., Bassler S., Reichel S., Spekl K. Reduction of radiochemotherapy-induced early oral mucositis by recombinant human keratinocyte growth factor (palifermin): experimental studies in mice. International Journal of Radiation Oncology, Biology and Physics. 2005;62:881–887. doi: 10.1016/j.ijrobp.2005.03.050. [DOI] [PubMed] [Google Scholar]
- Farrell C.L., Bready J.V., Rex K.L., Chen J.N., Dipalma C.R., Whitcomb K.L., Yin S., Hill D.C., Wiemann B., Starnes C.O., Havill A.M., Lu Z.N., Aukerman S.L., Pierce G.F., Thomason A., Potten C.S., Ulich T.R., Lacey D.L. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Research. 1998;58:933–939. [PubMed] [Google Scholar]
- Farrell C.L., Rex K.L., Chen J.N., Bready J.V., Dipalma C.R., Kaufman S.A., Rattan A., Scully S., Lacey D.L. The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Proliferation. 2002;35:78–85. doi: 10.1046/j.1365-2184.35.s1.8.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.P., Jung H.D., Kim Y.W. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Annals of Plastic Surgery. 2006;56:394–398. doi: 10.1097/01.sap.0000198731.12407.0c. [DOI] [PubMed] [Google Scholar]
- Housley R.M., Morris C.M., Boyle W., Ring B., Biltz R., Tarpley J.E., Aukerman S.L., Devine P.L., Whitehead R.H., Pierce G.F. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. Journal of Clinical Investigation. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Ito S., Sekine M., Abe M. Reconstruction of the soft tissue of a deep diabetic foot wound with artificial dermis and recombinant basic fibroblast growth factor. Plastic and Reconstructive Surgery. 2005;115:567–572. doi: 10.1097/01.prs.0000149485.60638.30. [DOI] [PubMed] [Google Scholar]
- Jung K., Chae C. Effect of temperature on the detection of porcine epidemic diarrhea virus and transmissible gastroenteritis virus in fecal samples by reverse transcription-polymerase chain reaction. Journal of Veterinary Diagnostic Investigation. 2004;12:237–239. doi: 10.1177/104063870401600312. [DOI] [PubMed] [Google Scholar]
- Jung K., Kim J., Ha Y., Choi C., Chae C. The effects of transplacental porcine circovirus type 2 infection on porcine epidemic diarrhea virus-induced enteritis in preweaning piglets. The Veterinary Journal. 2006;171:445–450. doi: 10.1016/j.tvjl.2005.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Ahn K., Chae C. Decreased activity of brush border membrane-bound digestive enzymes in small intestines from pigs experimentally infected with porcine epidemic diarrhea virus. Research in Veterinary Science. 2006;81:310–315. doi: 10.1016/j.rvsc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Lee Y.S., Suh C.W., Park S.K., Lee E.K. Purification of soluble human epidermal growth factor (hEGF) from recombinant Escherichia coli culture broth by using expanded-bed adsorption chromatography. Biotechnology and Applied Biochemistry. 2003;38:9–13. doi: 10.1042/BA20020113. [DOI] [PubMed] [Google Scholar]
- Marti U., Burwen S.J., Jones A.L. Biological effects of epidermal growth factor, with emphasis on the gastrointestinal tract and liver: an update. Hepatology. 1989;9:126–138. doi: 10.1002/hep.1840090122. [DOI] [PubMed] [Google Scholar]
- Lemoine N.R., Leung H.Y., Gullick W.J. Growth factors in the gastrointestinal tract. Gut. 1992;33:1297–1300. doi: 10.1136/gut.33.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B., de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Archives of Virology. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R.J. Peptides and gastrointestinal mucosal integrity. Gut. 1995;37:595–597. doi: 10.1136/gut.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R.J., Wright N.A. Why is epidermal growth factor present in the gut lumen? Gut. 1996;38:303–305. doi: 10.1136/gut.38.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R.J., Marchbank T., Mandir N., Higham A., Meeran K., Ghatei M.A., Bloom S.R., Goodlad R.A. Effects of keratinocyte growth factor (KGF) on gut growth and repair. Journal of Pathology. 1998;184:316–322. doi: 10.1002/(SICI)1096-9896(199803)184:3<316::AID-PATH3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Skov Olsen P., Poulsen S.S., Therkelsen K., Nexo E. Oral administration of synthetic human urogastrone promotes healing of chronic duodenal ulcers in rats. Gastroenterology. 1986;90:911–917. doi: 10.1016/0016-5085(86)90867-x. [DOI] [PubMed] [Google Scholar]
- Song D.S., Yang J.S., Oh J.S., Han J.H., Park B.K. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF3. Vaccine. 2003;21:1833–1842. doi: 10.1016/S0264-410X(03)00027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D.S., Kang B.K., Oh J.S., Ha G.W., Yang J.S., Moon H.J., Jang Y.S., Park B.K. Multiplex reverse transcription-PCR for rapid differential detection of porcine epidemic diarrhea virus, transmissible gastroenteritis virus, and porcine group A rotavirus. Journal of Veterinary Diagnostic Investigation. 2006;18:278–281. doi: 10.1177/104063870601800309. [DOI] [PubMed] [Google Scholar]
- Stephen Murphy M. Growth factors and the gastrointestinal tract. Nutrition. 1998;14:771–774. doi: 10.1016/s0899-9007(98)00081-1. [DOI] [PubMed] [Google Scholar]


