Abstract
To determine the aetiological role and epidemiological profile of common respiratory viruses in adults with acute respiratory tract infections (ARTIs), a 2‐year study was conducted in Beijing, China, from May 2005 to July 2007. Nose and throat swab samples from 5808 ARTI patients were analysed by PCR methods for common respiratory viruses, including influenza viruses (IFVs) A, B, and C, parainfluenza viruses (PIVs) 1–4, enteroviruses (EVs), human rhinoviruses (HRVs), respiratory syncytial virus (RSV), human metapneumovirus (HMPV), human coronaviruses (HCoVs) OC43, 229E, NL63, and HKU1, and adenoviruses (ADVs). Viral pathogens were detected in 34.6% of patient samples, and 1.6% of the patients tested positive for more than one virus. IFVs (19.3%) were the dominant agents detected, followed by HRVs (6.5%), PIVs (4.3%), EVs (3.2%), and HCoVs (1.1%). ADVs, RSV and HMPV were also detected (<1%). The viral detection rates differed significantly between infections of the lower and upper respiratory tracts in the sample population: PIVs, the second most commonly detected viral agents in lower acute respiratory tract infections (LRTIs), were more prevalent than in upper acute respiratory tract infections, indicating that the pathogenic role of PIVs in LRTIs should be investigated. Currently, this study is the largest‐scale investigation of respiratory virus infections in China with multiple agent detection, providing baseline data for further studies of respiratory virus infections in adults with ARTIs.
Keywords: Acute respiratory tract infections, adult, epidemiology, molecular detection, respiratory virus infection
Introduction
Viruses are among the major causes of acute respiratory tract illnesses (ARTIs) throughout the world. The most common viruses responsible for ARTIs include influenza viruses (IFVs), respiratory syncytial virus (RSV), parainfluenza viruses (PIVs) 1–4, enteroviruses (EVs), human rhinoviruses (HRVs), adenoviruses (ADVs), and human coronaviruses (HCoVs) 229E and OC43 [1, 2, 3, 4]. Improvements in molecular detection techniques have resulted in the recent identification of several new respiratory viruses [5]. Recently discovered viruses such as human metapneumovirus (HMPV), novel strains of coronaviruses (SARS‐CoV, HCoV‐NL63, and HKU1), human bocavirus and novel polyomaviruses (WU and KI) have been detected around the world [5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. The illness and mortality due to respiratory viruses have made viral ARTIs a top priority in the global health challenge. As vaccination is currently unavailable for most of these viruses, it is necessary to monitor epidemic patterns and investigate the spread of respiratory virus infections to efficiently identify, control and prevent future epidemics.
Viral emergence varies from season to season, year to year, and region to region [15]. The risk of respiratory virus infection correlates with age, pre‐existing medical condition, and immune status [16, 17]. Most studies of respiratory viral activity patterns have focused on children, and large‐scale epidemiological investigations of respiratory virus infections in adults have rarely been conducted. In an attempt to characterize the respiratory virus infections and to provide insights into the aetiology and clinical associations of respiratory viruses in adult ARTIs, a 2‐year study was conducted with adults suspected of having ARTIs in Beijing, China.
Materials and Methods
Patients and clinical specimens
Recruitment of patients took place from May 2005 to July 2007 at the Fever Outpatient Clinic Department (FOCD) of the Peking Union Medical College Hospital (PUMCH), Beijing, China. To include the potential viral ARTIs and to exclude typical bacterial infections, patients enrolled in the study were selected by physicians according to the following criteria: ≥14 years of age, with respiratory symptoms such as cough or wheezing, acute fever (body temperature ≥38°C), and normal or low leukocyte count, with or without radiological pulmonary abnormalities. Nose and throat swabs were collected from each patient, and the two swabs were pooled in one tube containing virus transport medium (VTM; Copan, Brescia, Italy). A total of 5808 patient samples (i.e. c. one‐third of the patients who visited the FOCD of PUMCH during the study period) were collected and tested.
Nucleic acid extraction
Total nucleic acids (DNA and RNA) were extracted from 200 μL of each specimen (VTM) using the NucliSens easyMAG apparatus (bioMérieux, Marcy l’Etoile, France), according to the manufacturer’s instructions [18].
Molecular detection of respiratory viruses
The presence of RSV, IFVs A, B, and C, PIVs 1–4, HRVs, EVs, HCoVs (229E, OC43, NL63, and HKU1), HMPV and ADVs was determined by PCR assays, as previously reported [1, 19, 20, 21, 22], and the details of these assays are summarized in Table 1. Briefly, two multiplex nested RT‐PCRs were used for the simultaneous detection of PIVs 1–4, EVs, and HRVs, as well as IFVs A, B, and C, and RSVs A, and B. In addition, two one‐step RT‐PCRs were used to detect HCoVs and HMPV. ADVs were detected by one‐step PCR. The sensitivity of the PCR systems was determined by using cloned, amplified products of each type of virus. Blank VTM was used as a negative control, and 100 copies of invariant β‐actin gene were added to lysis buffer as internal controls to exclude inhibitors for nucleic acid extraction and PCR. Each RT‐PCR amplification was performed using the SuperScript II One‐Step RT‐PCR Platinum Taq kit (Invitrogen), and ExTaq DNA polymerase (Takara) was used for PCR assays. PCR products were analysed by electrophoresis in 2% agarose gel containing ethidium bromide. Of the PCR‐positive samples, half were randomly selected for verification by PCR product sequencing.
Table 1.
PCR reactions used for detection of respiratory viruses
| Viruses detected | Methods | Round | Sequences of primers | Target genes | Thermal profile | Size of PCR products | References |
|---|---|---|---|---|---|---|---|
| IFVs A, B, and C; RSVs A and B | Multiplex nested RT‐PCR | 1st | FluAC1: 5′‐GAACTCRTYCYWWATSWCAAWGRRGAAAT‐3′ | NP | 48°C for 45 min; 94°C for 3 min; 94°C for 30 s, 55°C for 60 s, 72°C for 60 s, 45 cycles. 72°C for 10 min | IFV A: 301 bp IFV B: 226 bp IFV C: 111 bp RSV A: 363 bp RSV B: 611 bp | [1] |
| FluB1: 5′‐ACAGAGATAAAGAAGAGCGTCTACAA‐3′ | |||||||
| FluABC2: 5′‐ATKGCGCWYRAYAMWCTYARRTCTTCAWAIGC‐3′ | |||||||
| RSVAB1: 5′‐ATGGAGYTGCYRATCCWCARRRCAARTGCAAT‐3′ | F | ||||||
| RSVAB2: 5′‐AGGTGTWGTTACACCTGCATTRACACTRAATTC‐3′ | |||||||
| 2nd | FluAB3: 5′‐GATCAAGTGAKMGRRAGYMGRAAYCCAGG‐3′ | NP | 94°C for 3 min; 94°C for 30 s, 55°C for 60 s, 72°C for 45 s, 35 cycles; 72°C for 10 min | ||||
| FluC3: 5′‐AAATTGGAATTTGTTCCTTTCAAGGGACA‐3′ | |||||||
| FluAC4: 5′‐TCTTCAWATGCARSWSMAWKGCATGCCATC‐3′ | |||||||
| FluB4: 5′‐CTTAATATGGAAACAGGTGTTGCCATATT‐3′ | |||||||
| RSVA3: 5′‐TTATACACTCAACAATRCCAAAAAWACC‐3′ | F | ||||||
| RSVA4: 5′‐AAATTCCCTGGTAATCTCTAGTAGTCTGT‐3′ | |||||||
| RSVB3: 5′‐ATCTTCCTAACTCTTGCTRTTAATGCATTG‐3′ | |||||||
| RSVB4: 5′‐GATGCGACAGCTCTGTTGATTTACTATG‐3′ | |||||||
| PIVs 1–4; HRV; EV | Multiplex nested RT‐PCR | 1st | 1PIV13: 5′‐AGGWTGYSMRGATATAGGRAARTCATA‐3′ | HA | 48°C for 45 min; 94°C for 3 min; 94°C for 30 s, 55°C for 60 s, 72°C for 60 s, 45 cycles; 72°C for 10 min | PIV 1: 439 bp PIV 2: 297 bp PIV 3: 390 bp PIV 4: 174 bp EV: 226 bp (200–232 bp) RV: 110 bp (100–120 bp) | [19] |
| 2PIV13: 5′‐CTWGTATATATRTAGATCTTKTTRCCTAGT‐3′ | |||||||
| 1PIV2: 5′‐TAATTCCTCTTAAAATTGACAGTATCGA‐3′ | |||||||
| 1PIV4: 5′‐ATCCAGARRGACGTCACATCAACTCAT‐3′ | 5′NCR‐HA | ||||||
| 2PIV24: 5′‐TRAGRCCMCCATAYAMRGGAAATA‐3′ | HA | ||||||
| 1‐EV/RVF: 5′‐CTCCGGCCCCTGAATRYGGCTAA‐3′ | 5′NCR‐VP4/VP2 | ||||||
| 2‐EV/RVR: 5′‐TCIGGIARYTTCCASYACCAICC‐3′ | |||||||
| 2nd | 3PIV13: 5′‐ACGACAAYAGGAARTCATGYTCT‐3′ | HA | 94°C for 3 min; 94°C for 30 s, 55°C for 60 s, 72°C for 30 s, 35 cycles; 72°C for 10 min | ||||
| 4PIV1: 5′‐GACAACAATCTTTGGCCTATCAGATA‐3′ | |||||||
| 4PIV3: 5′‐GAGTTGACCATCCTYCTRTCTGAAAAC‐3′ | |||||||
| 3PIV24: 5′‐CYMAYGGRTGYAYTMGAATWCCATCATT‐3′ | |||||||
| 4PIV2: 5′‐GCTAGATCAGTTGTGGCATAATCT‐3′ | |||||||
| 4PIV4: 5′‐TGACTATRCTCGACYTTRAAATAAGG‐3′ | |||||||
| 3‐EV/RVF: 5′‐ACCRASTACTTTGGGTRWCCGTG‐3′ | 5′NCR‐VP4/VP2 | ||||||
| 4‐EV/RVR: 5′‐CTGTGTTGAWACYTGAGCICCCA‐3′ | |||||||
| HCoV | RT‐PCR | HCoVFc: 5′‐GGTTGGGACTATCCTAAGTGTGA‐3′ | Pol | 48°C for 45 min; 94°C for 3 min; 94°C for 30 s, 48°C for 30 s, 72°C for 45 s, 40 cycles; 72°C for 10 min | 440 bp | [20] | |
| HCoVRc: 5′‐CCATCATCAGATAGAATCATCATA‐3′ | |||||||
| HMPV | RT‐PCR | HMPVR: 5′‐CATGCCCACTATAAAAGGTCAG‐3′ | L | 48°C for 45 min; 94°C for 3 min; 94°C for 30 s, 55°C for 30 s, 72°C for 45 s, 40 cycles; 72°C for 10 min | 171 bp | [21] | |
| HMPVF: 5′‐CACCCCAGTCTTTCTTGAAA‐3′ | |||||||
| ADV | PCR | AdvF: 5′‐GCCSCARTGGKCWTACATGCACATC‐3′ | Hexon | 94°C for 3 min; 94°C for 30 s, 55°C for 30 s, 72°C for 45 s, 35 cycles; 72°C for 10 min | 301 bp | [22] | |
| AdvR: 5′‐CAGCACSCCICGRATGTCAAA‐3′ | |||||||
EV, enterovirus; HCoV, human coronavirus; HMPV, human metapneumovirus; HRV, human rhinovirus; IFV, influenza virus; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
Statistical analysis
Comparison among groups was performed using a chi‐square test with a significance level of p <0.05.
Results
Overall detection of respiratory viruses
The investigation was performed on working days from May 2005 to July 2007, with the exception of July and November 2005, when clinical samples were not collected. Between 11 and 54 (average 33) patients per day were diagnosed with ARTIs at the FOCD, PUMCH during the study period. On average, ten patients with ARTIs were selected each day, according to the criteria described in Materials and Methods, providing 5808 patients in total. Patient ages ranged between 14 and 97 years (median, 30 years; mean, 35.7 years). Specimens were collected from both females (3137, 54.0%) and males (2671, 46.0%). The sensitivity of detection of viruses was as follows: 1–10 molecules for PIVs 2 and 4, EVs, HRVs, IFVs A, B, and C, RSVs A and B, HMPV and ADVs; and 10–100 molecules for PIVs 1 and 3, and HCoVs.
Respiratory samples from 2010 (34.6%) patients were found to be positive for at least one virus, and those from 3798 (65.4%) patients were negative for all respiratory viruses tested. Pathogens in such patients as these need to be further investigated. The monthly detection rates of respiratory viruses ranged from 12.7% to 69.8% of patients tested (Fig. 1). There were no significant differences between viruses infecting men and women (data not shown). All of the common respiratory viruses were detected. As expected, IFV infection was dominant, being detected in 1119 (19.3%) patients. The detection rate of HRVs was 6.5%, and those of other viruses were as follows: PIVs, 4.3%; EVs, 3.2%; and HCoVs, 1.1%. ADVs, RSV and HMPV were rarely detected, displaying positivity rates of <1% (Table 2).
Figure 1.
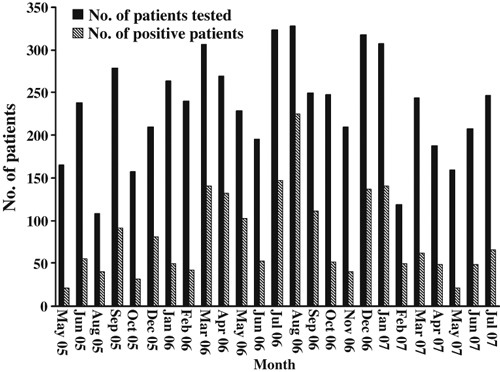
Numbers of patients tested and numbers with positive virus detection for each month of the investigation.
Table 2.
Distribution of respiratory virus infections according to age groups
| Age group (years) | ||||
|---|---|---|---|---|
| 14–25 | 26–65 | ≥66 | Total | |
| Patientstested (no.) | 1918 | 3434 | 456 | 5808 |
| IFVs | 384 (20.0)a | 667 (19.4) | 68 (14.9) | 1119 (19.3) |
| HRVs | 152 (7.9) | 197 (5.7) | 27 (5.9) | 376 (6.5) |
| PIVs | 89 (4.6) | 136 (4.0) | 27 (5.9) | 252 (4.3) |
| EVs | 84 (4.4) | 95 (2.8) | 9 (2.0) | 188 (3.2) |
| HCoVs | 18 (0.9) | 27 (0.8) | 20 (4.4) | 65 (1.1) |
| ADVs | 31 (1.6) | 19 (0.6) | 1 (0.2) | 51 (0.9) |
| RSV | 2 (0.1) | 19 (0.6) | 9 (2.0) | 30 (0.5) |
| HMPV | 4 (0.2) | 10 (0.3) | 5 (1.1) | 19 (0.3) |
ADV, adenovirus; EV, enterovirus; HCoV, human coronavirus; HMPV, human metapneumovirus; HRV, human rhinovirus; IFV, influenza virus; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
aNumbers in parentheses are percentages.
Seasonality of respiratory virus infection
IFV A was detected at the highest frequency and throughout the study period. Two major peaks were observed: in a period from July to September 2006, and in a period from December 2006 to February 2007. Three minor IFV A peaks were observed in a period between August and September 2005, in December 2005, and in March 2006 (Fig. 2a). Unlike IFV A, IFV B is active in spring in Beijing. Apart from two distinct peaks in March and April of 2006 and 2007, rates of IFV B infection were low throughout the study period. The IFV B peaks occurred following IFV A peaks, indicating that epidemic peaks may alternate between IFVs A and B in Beijing (Fig. 2a). IFV C (15 patients, 0.3%) was rarely detected.
Figure 2.
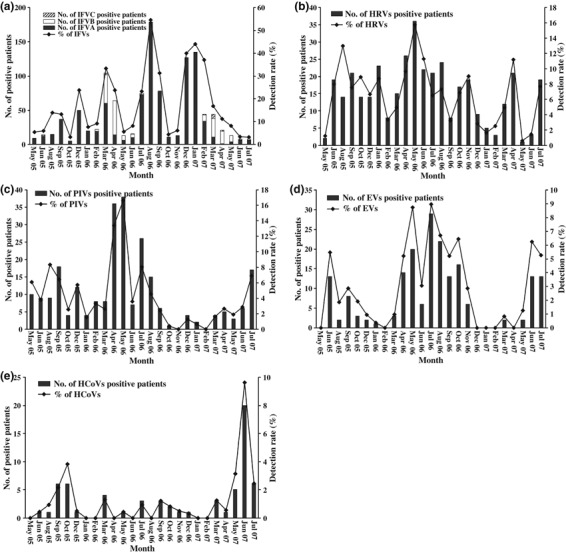
Numbers of patients with positive detection of individual viruses, and detection rate (% of patients) for each month of the investigation. (a) influenza viruses (IFVs); (b) human rhinovirus (HRV); (c) parainfluenza viruses (PIVs); (d) enteroviruses (EVs); (e) human coronaviruses (HCoVs).
HRVs were the second most frequently detected viral agents, and presented during nearly the entire year, lacking regular seasonality. These findings indicate the important role of HRVs in adult ARTIs (Fig. 2b).
PIV infection varied in incidence between the 2 years of the study (Fig. 2c). PIVs were not detected in November 2006 and February 2007, and a distinct PIV peak was observed in the spring (April and May) of 2006. The incidence of EVs was low between December and March in both 2005 and 2006; indeed, EVs were not detected during February of each year. More cases of EV were found in early summer (June of 2005 and 2007, and July of 2006 and 2007) (Fig. 2d).
HCoVs were detected in only (1.1%) 65 patients, with HCoV‐OC43 being most frequent. They appeared sporadically during the study years, but an epidemic peak occurred in June 2007(Fig. 2e).
Respiratory virus detection among different age groups
IFVs were the most frequent respiratory viruses affecting all age groups tested, especially those ≤65 years of age. Detection rates for EVs, HRVs and ADVs were higher in the 14–25‐year age group, whereas HCoVs, RSV and HMPV were detected mainly in elderly adults (Table 2).
Co‐detection of respiratory viruses
Among the positive cases, multiple (≥2) respiratory viruses were observed in 94 patients. Dual infections were present in 93 of these patients, with triple infections being found in one patient (Table 3). HRVs, detected in 52 cases, were the most frequently found viral agents in co‐infections. IFV A was co‐detected in 39 cases, PIVs in 29, EVs in 23, IFV B in 23, HCoVs in eight, ADVs in six, HMPV in three, IFV C in five, and RSV in one. Co‐infections were significantly more frequent in younger and older groups than in the 26–65‐year age group (≤25 years, p 0.013; ≥66 years, p 0.008).
Table 3.
Co‐detection of respiratory viruses in adults with acute respiratory tract infections
| Virus co‐detection status | Positive cases among age groups | Total cases, n = 5808 | ||
|---|---|---|---|---|
| 14–25 years, n = 1918 | 26–65 years, n = 3434 | ≥66 years, n = 456 | ||
| IFV A + PIVs | 4 | 5 | 0 | 9 |
| IFV A + EVs | 3 | 3 | 0 | 6 |
| IFV A + HRVs | 4 | 14 | 2 | 20 |
| IFV A + HCoVs | 1 | 0 | 0 | 1 |
| IFV A + HMPV | 1 | 0 | 0 | 1 |
| IFV A + IFV C | 2 | 0 | 0 | 2 |
| IFV B + PIVs | 1 | 1 | 1 | 3 |
| IFV B + EVs | 2 | 2 | 0 | 4 |
| IFV B + HRVs | 6 | 3 | 1 | 10 |
| IFV B + ADVs | 1 | 0 | 0 | 1 |
| IFV B + HMPV | 0 | 1 | 0 | 1 |
| IFV B + IFV C | 2 | 1 | 0 | 3 |
| PIVs + EVs | 2 | 3 | 0 | 5 |
| PIVs + HRVs | 4 | 2 | 0 | 6 |
| PIVs + ADVs | 1 | 0 | 0 | 1 |
| PIVs + HCoVs | 0 | 0 | 2 | 2 |
| PIVs + RSV | 0 | 0 | 1 | 1 |
| PIVs + HMPV | 0 | 0 | 1 | 1 |
| EVs + HRVs | 4 | 2 | 1 | 7 |
| EVs + HCoVs | 0 | 1 | 0 | 1 |
| HRVs + ADVs | 3 | 1 | 0 | 4 |
| HRVs + HCoVs | 3 | 1 | 0 | 4 |
| HRVs + IFV B + PIVs | 1 | 0 | 0 | 1 |
| Total | 45 (2.3)a | 40 (1.2) | 9 (2.0) | 94 (1.6) |
ADV, adenovirus; EV, enterovirus; HCoV, human coronavirus; HMPV, human metapneumovirus; HRV, human rhinovirus; IFV, influenza virus; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
aNumbers in parentheses are percentages.
Association of respiratory viruses with lower and upper ARTIs
Among the 5808 patients affected by ARTIs, 276 (4.8%) had lower ARTIs (LRTIs) (e.g. pneumonia) and 5532 (95.2%) had upper ARTIs (URTIs) (e.g. rhinitis, pharyngitis, or laryngitis) (Table 4). The relative occurrence of LRTIs and URTIs was not significantly different among the age groups. IFVs, PIVs and HRVs were the main pathogens involved in both LRTIs and URTIs. A chi‐square test showed that IFV A had a greater tendency to cause URTIs (p 0.010), whereas PIVs tended to cause LRTIs (p 0.015). This observation indicates that more attention should be paid to the pathogenicity of PIVs in addition to IFVs.
Table 4.
Respiratory viruses detected in acute upper respiratory tract infections (URTIs) and acute lower respiratory tract infections (LTRIs)
| 14–25 years | 26–65 years | ≥66 years | Total number (%) | |||||
|---|---|---|---|---|---|---|---|---|
| URTIs | LRTIs | URTIs | LRTIs | URTIs | LRTIs | URTIs | LRTIs | |
| Patients tested | 1871 | 47 | 3275 | 159 | 386 | 70 | 5532 | 276 |
| Total positives | 710 (37.9)a | 12 (25.5) | 1088 (33.2) | 43 (27.0) | 129 (33.4) | 28 (40.0) | 1927 (34.8) | 83 (30.1) |
| Single detections | 666 (35.6) | 11 (23.4) | 1051 (32.1) | 40 (25.2) | 123 (31.9) | 25 (35.7) | 1840 (33.3) | 76 (27.5) |
| Dual detections | 43 (2.3) | 1 (2.1) | 37 (1.1) | 3 (1.9) | 6 (1.6) | 3 (4.3) | 86 (1.6) | 7 (2.5) |
| IFV A | 294 (15.7) | 3 (6.4) | 561 (17.1) | 15 (9.4) | 48 (12.4) | 11 (15.7) | 903 (16.3)a | 29 (10.5) |
| IFV B | 80 (4.3) | 1 (2.1) | 86 (2.6) | 1 (0.6) | 7 (1.8) | 2 (2.9) | 173 (3.1) | 4 (1.5) |
| IFV C | 10 (0.5) | 0 | 5 (0.2) | 0 | 0 | 0 | 15 (0.3) | 0 |
| HRVs | 148 (7.9) | 4 (8.5) | 188 (5.7) | 9 (5.7) | 24 (6.2) | 3 (4.3) | 360 (6.5) | 16 (5.8) |
| PIVs 1–4 | 87 (4.6) | 2 (4.3) | 125 (3.8) | 11 (6.9) | 20 (5.2) | 7 (10) | 232 (4.2) | 20 (7.3)b |
| EVs | 82 (4.4) | 2 (4.3) | 92 (2.8) | 3 (1.9) | 8 (2.1) | 1 (1.4) | 182 (3.3) | 6 (2.2) |
| HCoVs | 18 (1.0) | 0 | 24 (0.7) | 3 (1.9) | 17 (4.4) | 3 (4.3) | 59 (1.1) | 6 (2.2) |
| ADVs | 31 (1.7) | 0 | 16 (0.5) | 3 (1.9) | 1 (0.3) | 0 | 48 (0.9) | 3 (1.1) |
| RSV | 1 (0.1) | 1 (2.1) | 18 (0.5) | 1 (0.6) | 8 (2.1) | 1 (1.4) | 27 (0.5) | 3 (1.1) |
| HMPV | 4 (0.2) | 0 | 10 (0.3) | 0 | 2 (0.5) | 3 (4.3) | 16 (0.3) | 3 (1.1) |
ADV, adenovirus; EV, enterovirus; HCoV, human coronavirus; HMPV, human metapneumovirus; HRV, human rhinovirus; IFV, influenza virus; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
aNumbers in parentheses are percentages.
bRepresents significant difference between the incidence of virus infection by chi‐square test.
Discussion
The accurate and rapid analysis of a broad range of viral agents is critical for aetiological investigations. In this study, multiple viral agents, including IFVs A, B, and C, RSV, PIVs 1–4, HRVs, EVs, HCoVs (229E, OC43, NL63, and HKU1), HMPV, and ADVs, were analysed in a large population, with the goal of providing comprehensive data for viral infection in adults with ARTIs. To our knowledge, this is the largest‐scale investigation of respiratory virus infections in China in adults with ARTIs. The overall detection rate of respiratory viruses in this study was 34.6%, similar to those obtained by others [2, 3]. However, these results may underestimate the role of virus infection, because viral loads in nose and throat swabs, as used in this study, are usually lower than those in aspirate or lavage [23]. Furthermore, infections from as yet unknown viruses may be responsible for some ARTIs. The prevalence of bacterial ARTI was not investigated in this study.
Some of the tested known viruses, such as HRVs and RSV, were detected at lower rates than those reported by other investigators [24, 25]. There are multiple explanations for these differences. First, detection methods differ from one study to another. It is difficult to compare the results, as the reported data were obtained with different detection methods or PCR primers [2, 24]. Future comparative studies to evaluate the sensitivity and specificity of these detection methods should clarify this issue. Second, the infection rates may vary with geographical location, and with the particular period chosen for testing [15, 24]. Third, the patient population and its environment may influence the results. Previous studies on RSV infections have mostly been conducted in hospitalized elderly adults with medical conditions, such as cardiopulmonary diseases [25], which may predispose them to viral infections that are not so common in the general population. This study involved patients who displayed specific ARTI criteria, in Beijing, China. We tested a larger sample of the population and detected more viral agents in parallel than did previously published studies.
This analysis of the age distribution of viral infections shows that younger and elderly adults were more frequently infected. These data conform previously published data on respiratory infections in an Australian population, where a similar age distribution was reported [2].
As expected, IFVs were the most common agents detected throughout the year. An epidemic of IFVA usually occurs in the winter, and rarely in the summer, in temperate zones [2, 3]. However, during the study period, an epidemic peak of IFV A infection was observed in the summer of 2006. More patients with influenza‐like illness were observed during this period, reflected by a two‐fold increase in visits to the FOCD. The reason for this epidemic is unclear. We speculate that it may be due to antigen drift of IFVA, but this hypothesis needs to be confirmed by haemagglutinin sequencing in future studies. Currently influenza surveillance in north China is mainly conducted between late autumn and early spring (from 1 October to 31 March of the following year), and our findings indicate the necessity to extend the surveillance period of IFV to the entire year.
HRVs were the second most frequently detected respiratory viruses, and were found throughout the year in all age groups, consistent with previous reports [24]. HRVs were the most active viruses during most months, even during periods of peak IFV activity. Moreover, HRVs represented 55.3% (n = 52) of the co‐detection cases. Previous studies have shown that HRVs are implicated in exacerbation of diseases, including chronic bronchitis, acute bronchiolitis, and asthma [26, 27, 28]. Further studies are needed to assess the association between severity of clinical symptoms and multiple infections involving HRVs. Recently, a new group of rhinoviruses has been associated with severe LRTIs [29, 30], confirming the important role of HRVs in respiratory infections.
In this investigation, PIVs were detected at a higher rate in LRTIs than in URTIs, and were the second most frequently detected viruses in LRTIs. PIV4 was the most frequently detected PIV (data not shown). Several recent outbreaks caused by PIVs have been reported, and the incidence of PIV4 infection is increasing [31], indicating the need for further epidemiological investigation, surveillance and control of PIV infections.
HCoVs have recently received special attention because of the discovery and threat of SARS‐CoV [8]. In this study, positive cases were found for all known HCoV types except SARS‐CoV, including HCoV OC43, 229E, NL63, and HKU1. HCoVs were more frequently detected in elderly adults, consistent with the findings of Falsey et al. [32].
The prevalence of respiratory virus co‐infection in adults was lower than that in children [33, 34, 35, 36]. The clinical significance of such co‐detection is unclear. It is possible that respiratory viruses may persist in the nasopharyngeal tract for a long period without causing symptoms [37]. In addition, molecular assays allow the detection of viral nucleic acid materials even if the virus has no replication ability. The amplification of residual genetic material from previous infections or from persistent viruses also remains a complicating factor in the interpretation of multiple virus detection [15].
In summary, the spectrum, seasonality, age distribution and clinical associations of respiratory virus infections in adults with ARTIs were analysed in this study. The findings provide baseline data for evaluating the burden of respiratory virus infection in adults. As the number of patients with LRTIs was limited in this analysis, further studies should be undertaken and longer surveillance periods should provide more information concerning the epidemiology of respiratory virus infections, and their relationships with clinical outcomes.
Transparency Declaration
The authors do not have any conflicting interests with regard to this article.
Acknowledgements
This paper is dedicated to the memory of C. Mérieux, who established the Joint Lab for New Emerging Pathogen Identification through collaborations between IPB, CAMS and bioMérieux. We thank the clinicians of PUMCH for their assistance in sample collection, and F. Komurian‐Pradel for constructive advice and discussions. This study was supported by grants from the IPB, CAMS (2007IPBII), and Fondation Mérieux.
References
- 1. Coiras MT, Pérez‐Breña P, García ML, Casas I. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested‐PCR assay. J Med Virol 2003; 69: 132–144. [DOI] [PubMed] [Google Scholar]
- 2. Druce J, Tran T, Kelly H et al. Laboratory diagnosis and surveillance of human respiratory viruses by PCR in Victoria, Australia, 2002–2003. J Med Virol 2005; 75: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pierangeli A, Gentile M, Di Marco P et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol 2007; 79: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gröndahl B, Puppe W, Hoppe A, Kühne I, Weigl JA, Schmitt HJ. Rapid identification of nine microorganisms causing acute respiratory tract infections by single‐tube multiplex reverse transcription‐PCR: feasibility study. J Clin Microbiol 1999; 37: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fouchier RA, Rimmelzwaan GF, Kuiken T, Osterhaus AD. Newer respiratory virus infections: human metapneumovirus, avian influenza virus, and human coronaviruses. Curr Opin Infect Dis 2005; 18: 141–146. [DOI] [PubMed] [Google Scholar]
- 6. Crowe JE Jr. Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J 2004; 23: S215–S221. [DOI] [PubMed] [Google Scholar]
- 7. Koetz A, Nilsson P, Lindén M, Van Der Hoek L, Ripa T. Detection of human coronavirus NL63, human metapneumovirus and respiratory syncytial virus in children with respiratory tract infections in south‐west Sweden. Clin Microbiol Infect 2006; 12: 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drosten C, Gunther S, Preiser W et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1967–1978. [DOI] [PubMed] [Google Scholar]
- 9. Vabret A, Mourez T, Dina J et al. Human coronavirus NL63, France. Emerg Infect Dis 2005; 11: 1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lau SK, Woo PC, Yip CC et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol 2006; 44: 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 2005; 102: 12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaynor AM, Nissen MD, Whiley DM et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PloS Pathog 2007; 3: 0595–0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allander T, Andreasson K, Gupta S et al. Identification of a third human polyomavirus. J Virol 2007; 81: 4130–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren L, Gonzalez R, Xie Z, et al. WU and KI polyomavirus present in the respiratory tract of children, but not in immunocompetent adults. J Clin Virol 2008; 43: 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larcher C, Jeller V, Fischer H, Huemer HP. Prevalence of respiratory viruses, including newly identified viruses, in hospitalized children in Austria. Eur J Clin Microbiol Infect Dis 2006; 25: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection associated hospitalizations among older adults. J Infect Dis 2002; 185: 1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infection in patients with chronic chest disease. JAMA 2000; 283: 499–505. [DOI] [PubMed] [Google Scholar]
- 18. Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim‐van Dillen PM, Van Der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 1990; 28: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coiras MT, Aguilar JC, García ML, Casas I, Pérez‐Breña P. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested‐PCR assays. J Med Virol 2004; 72: 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woo PC, Lau SK, Chu CM et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 2005; 79: 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peiris JS, Tang WH, Chan KH et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis 2003; 9: 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allard AK, Girones R, Juto P, Wadell G. Polymerase chain reaction for detection of adenoviruses in stools. J Clin Microbiol 1990; 28: 2659–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambert SB, Whiley DM, O’Neill NT et al. Comparing nose–throat swabs and nasopharyngeal aspirates collected from children with symptoms for respiratory virus identification using real‐time polymerase chain reaction. Pediatrics 2008; 122: e615–e620. [DOI] [PubMed] [Google Scholar]
- 24. Bellei N, Carraro E, Perosa A, Watanabe A, Arruda E, Granato C. Acute respiratory infection and influenza‐like illness viral etiologies in Brazilian adults. J Med Virol 2008; 80: 1824–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murata Y. Respiratory syncytial virus infection in adults. Curr Opin Pulm Med 2008; 14: 235–240. [DOI] [PubMed] [Google Scholar]
- 26. Grissell TV, Powell H, Shafren DR et al. Interleukin‐10 gene expression in acute virus‐induced asthma. Am J Respir Crit Care Med 2005; 172: 433–439. [DOI] [PubMed] [Google Scholar]
- 27. Xatzipsalti M, Kyrana S, Tsolia M et al. Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med 2005; 172: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 28. Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol 2004; 14: 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Renwick N, Schweiger B, Kapoor V et al. A recently identified rhinovirus genotype is associated with respiratory‐tract infection in children in Germany. J Infect Dis 2007; 196: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiang Z, Gonzalez R, Xie Z et al. Human rhinovirus group C infection in children with lower respiratory tract infection, China. Emerg Infect Dis 2008; 14: 1665–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lau SK, To WK, Tse PW et al. Human parainfluenza virus 4 outbreak and the role of diagnostic tests. J Clin Microbiol 2005; 43: 4515–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection‐associated hospitalizations among older adults. J Infect Dis 2002; 185: 1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaye M, Skidmore S, Osman H, Weinbren M, Warren R. Surveillance of respiratory virus infections in adult hospital admissions using rapid methods. Epidemiol Infect 2006; 134: 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow‐Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon‐gamma response. Pediatr Infect Dis J 2005; 24: 605–610. [DOI] [PubMed] [Google Scholar]
- 35. Wolf DG, Greenberg D, Kalkstein D et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Infect Dis J 2006; 25: 320–324. [DOI] [PubMed] [Google Scholar]
- 36. Richard N, Komurian‐Pradel F, Javouhey E et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J 2008; 27: 213–217. [DOI] [PubMed] [Google Scholar]
- 37. Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol 2004; 72: 695–699. [DOI] [PubMed] [Google Scholar]


