Abstract
Research on the impact of diet and memory has garnered considerable attention while exploring the link between obesity and cognitive impairment. High-fat diet (HFD) rodent models recapitulate the obesity phenotype and subsequent cognitive impairments. While it is known that HFD is associated with sensory impairment, little attention has been given to the potential role these sensory deficits may play in recognition memory testing, one of the most commonly used cognitive tests. Because mice utilize their facial whiskers as their primary sensory apparatus, we modified a common recognition test, the novel object recognition task, by replacing objects with sandpaper grits at ground level, herein referred to as the novel tactile recognition task (NTR). First, we tested whisker-manipulated mice in this task to determine its reliance on intact whiskers. Then, we tested the HFD mouse in the NTR. Finally, to ensure that deficits in the NTR are due to cognitive impairment and not HFD-induced sensory deficiencies, we tested the whisker sensitivity of HFD mice via the corner test. Our results indicate that the NTR is a whisker dependent task, and that HFD mice exhibit tactile recognition memory impairment, not accompanied by whisker sensory deficits.
Keywords: Cognitive impairment, obesity, recognition task, vibrissae manipulation, whisker trimming
Recognition memory behavioral tasks are useful tools to assess cognitive deficits in preclinical models [1]. Recognition tasks in humans and monkeys are hippocampal-dependent [2-6]. On the other hand, these findings in rodents have yielded inconsistent reports [1, 7]. Studies suggest that both the hippocampus and the perirhinal cortex play a role in recognition memory in rodents [1, 7, 8]. It is possible that the inconsistencies among the various species may be due to the fact that rodents rely heavily upon their whiskers (vibrissae) for spatial navigation and exploration [9, 10]. Each whisker, which is represented by individual structures within layer 4 of the barrel cortex [11], allows the mouse to gather information about the size, shape, and texture of various stimuli. Hence, normal sensory perception and discrimination is vital for recognition memory.
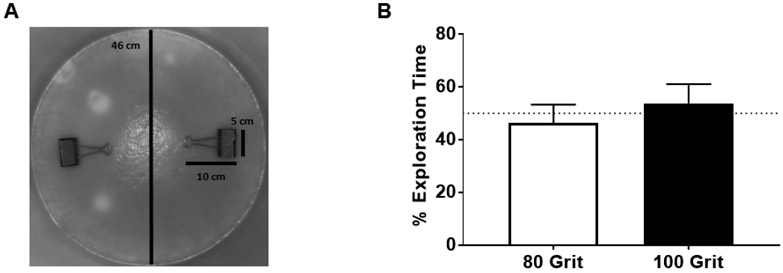
One of the most commonly used recognition memory tasks is the novel object recognition test (NOR), which utilizes the mouse’s innate exploratory behavior and preference for novelty. A previous study modified the NOR by utilizing tactile objects to assess recognition memory [12]. They demonstrated that recognition memory is ablated following whisker removal; however, it is not known whether this impairment is reversible. Hence, we first sought to evaluate the long-term consequences of whisker trimming on tactile discrimination in mice. To create the novel tactile recognition task (NTR), we modified the NOR by replacing objects with 80 and 100 grit sandpaper that we adhered on top and inside of large office paper clips (Fig. 1A). We utilized adult male C57BL6 mice, purchased from the Jackson Laboratory (Bar Harbor, Maine) at 4 weeks of age and placed on a standard diet (STD) consisting of 10% kcal from fat (Research Diets Inc.; #D12450B, New Brunswick, NJ) ad libitum. An opaque-colored circular arena (46 cm diameter x 32 cm height) was used as previously described for NOR [13] (Fig 1A). A camera mounted above the open field was used to record the movements of the mouse for 10 minutes. Following habituation, mice were placed in the arena with an 80 grit paper clip and a 100 grit paper clip. There are no significant differences in the time mice spend exploring the 80 (mean = 46.2%, standard error of the mean = ± 3.1%) vs 100 (53.8% ± 3.1%) grit paper, t(16) = 1.749, p>0.05 (Fig. 1B), analyzed by two-tailed t-test via Prism v7 (GraphPad Software, Inc.). This suggests that the mice do not have an inherent preference to one type of sandpaper grit; hence, the 80 versus 100 sandpaper are valid for use with NTR.
Figure 1. Novel tactile recognition task.
A) Set up and dimensions in centimeters (cm) for the novel tactile recognition task. B) Percent exploration time between the 80 and 100 grit sandpaper in standard diet mice. Data represented as mean and standard error of the mean (SEM); n= 9.
In order to determine the long-term consequences of whisker trimming on recognition memory, NTR was performed on STD mice first after unilateral, then after bilateral whisker trimming, and finally following 6 weeks of re-growth. Briefly, STD mice were anesthetized prior to whisker trimming, which was performed as previously described [14]. NTR was performed within 2 days of trimming. The NTR was performed in the following sequence at each of these time points: on day 1 (habituation), the mice could explore the empty arena 3 times (10 minutes each). On day 2 (exploration phase), the mice could explore 2 identical sandpaper grits located in opposite halves of the arena (10 minutes) before being returned to their home cage. Following a 30 to 45-minute delay, the mice were returned to the arena with one grit identical to the one used in the exploration phase and a second, novel grit located in the opposite half of the arena. The mice could explore the sandpaper grits for 10 minutes. The arena and sandpaper were both cleaned thoroughly with 70% ethanol after each trial. The time the mice spent exploring each sandpaper was recorded by SMART VIDEO TRACKING Software (Panlab). Normal tactile recognition memory was defined by spending significantly more than 50% (by chance) of time exploring the novel sandpaper grit as determined by a one-sample t-test.
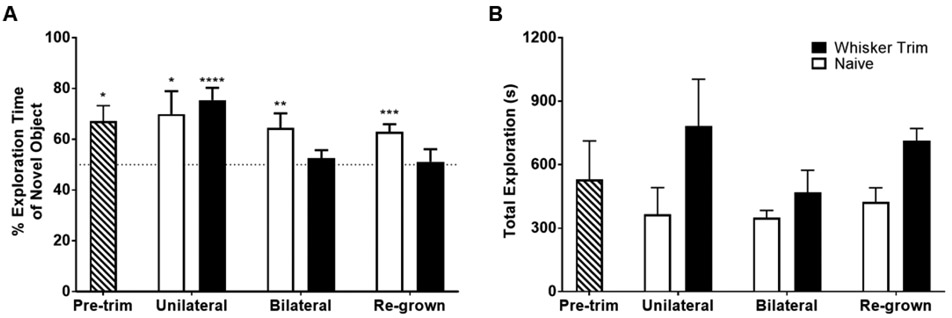
At pre-trim (prior to whisker trim), mice spend significantly more time exploring the novel sandpaper grit (67.1% ± 6.1%, t(22) = 4.000, p<0.001; Fig. 2A). Unilateral whisker trimmed mice (75.4% ± 4.9% t(12) = 7.403, p<0.001; Fig. 2A) maintain the ability to discriminate the novel sandpaper grit similar to mice without any whisker manipulation (naïve; 69.9% ± 9.0%, t(8) = 3.135, p<0.05; Fig. 2A). This suggests that unilateral whisker trimming does not impair tactile recognition memory. We further confirmed this via a paired t-test between unilateral and pre-trim time point, which was not significant (p>0.05). A previous study reported that partial de-whiskered mice can still process sensory information [15]. While naïve mice maintain the ability to discriminate the novel sandpaper grit (64.5% ± 5.7%, t(8) = 3.566, p<0.01; Fig. 2A), bilateral trim mice do not (52.5% ± 3.2%; Fig. 2A). Similar to previous findings [12], this data suggests that bilateral whisker trimming impairs tactile recognition, as bilateral mice perform significantly different compared to the pre-trim timepoint in a paired two-tailed t-test, t(5) = 2.694, p<0.05. This inability to discriminate the novel sandpaper grit remains even after the whiskers have re-grown for 6 weeks in the whisker trimmed mice (51.2% ± 4.9%; Fig. 2A), whereas the naïve mice maintain the ability to discriminate the novel sandpaper grit (62.9% ± 3.0%, t(8) = 6.036, p<0.001; Fig. 2A). Whisker trimming did not impact the ability of the mice to explore the sandpaper as we did not observe significant differences in total exploration time between the naïve and whisker trimmed mice with a two-way ANOVA and Sidak’s multiple comparison test (Fig. 2B). Overall, these results indicate that NTR performance is dependent upon intact whiskers, and that bilateral whisker trimming leads to long-term impairments in whisker sensitivity. This is in line with the literature, which demonstrates that trimming whiskers in neonates is associated with long-term abnormalities in the barrel cortex even after whiskers fully re-grow [16]. Furthermore, trimming whiskers in neonates impairs whisker-dependent discrimination associated behaviors in adulthood [17, 18]. This may be due to synaptic alterations in the hippocampus induced by sensory deprivation via whisker trimming [19]. While this has been reported in whisker trimming experiments conducted during neonatal development [16-18],to our knowledge we are the first to report such a phenomenon in otherwise healthy, adult mice.
Figure 2. Novel tactile performance in whisker trimmed mice.
A) The percentage (%) of time spent exploring the novel sandpaper grit in mice prior to (pre-trim; striped black and white bar) and following a unilateral and bilateral whisker trim, and after 6 weeks of re-growth. A separate cohort of “naïve” mice were used as a control after whisker trimming. Data represented as mean and standard error of the mean (SEM); n= 5 and 7 for the “Naïve” and “Whisker Trim” cohorts, respectively; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
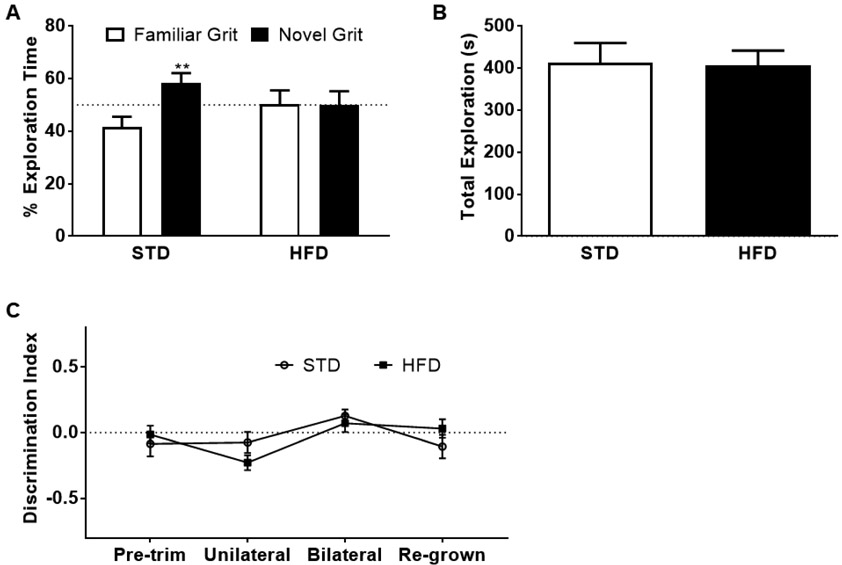
A high-fat diet (HFD), consisting of 54% kcal from fat (Research Diets Inc.; D05090701) ad libitum beginning at 4 weeks of age, can lead to significant weight gain and impaired performance in novel discrimination tasks [13]. We demonstrate that HFD (mean weight in grams = 25.227, ± 0.795) leads to significant weight increase compared to STD mice (21.023, ± 0.638) as early as 1 month of diet and continued throughout the study (p=0.0398, determined through a two-way ANOVA with Sidak’s multiple comparisons. See Supp. Fig. 1). Given that we already demonstrated that STD mice perform on the NTR (see Fig. 2), we next sought to test HFD mice in this task. A cohort of mice was placed on a HFD for 30-33 weeks and subjected to NTR. Based on a one-sample t-test, HFD mice fail to spend more than 50% of time exploring the novel sandpaper grit (49.84% ± 5.312%). We also demonstrate that naïve, age-matched STD mice perform on the NTR (58.3%, ± 3.782%, t(6) = 15.42, p<0.0001; Fig 3A), again validating the use of this task in STD mice. The lack of discrimination in the HFD cohort is not due to a lack of exploration, as there were no significant differences in total exploration time between the dietary groups (Fig. 3B). These results add to previous findings, where we and others demonstrated that a HFD led to impairments in both spatial and novelty detection tasks [13, 20].
Figure 3. Tactile recognition memory impaired by HFD.
A) The percentage of time spent exploring the familiar and novel sandpaper grit and B) total exploration time of mice following 30-33 weeks of diet on either the standard diet (STD) or the high-fat diet (HFD) during novel tactile recognition (NTR). The dashed line represents 50% exploration; n= 7 and 8 for the STD and HFD groups, respectively. C) Discrimination index from corner test to distinguish the direction in which mice turn when approaching a corner following unilateral and bilateral whisker trim, and after 6 months of re-growth. The dashed line represents a discrimination index value of 0; data represented as mean and standard error of the mean (SEM); n= 9 and 12 in the STD and HFD groups, respectively; **p<0.01.
While HFD-associated recognition memory impairment is in line with the literature [13, 20], it is not known whether this tactile recognition deficiency is due solely to memory impairment or whether deficits are due to underlying whisker-dependent sensory deficits. In fact, in the periphery, a HFD is associated with sensory deficits [21]. Hence, to determine the potential impact of HFD on whisker sensitivity, mice were subjected to the corner test after 7-15 weeks of diet to assess whisker sensitivity, as previously described [22]. Trained observers recorded the direction in which the mouse turned when approaching the 30° corner of the triangle. The scores represent the average of 10 trials performed on each mouse and from at least 3 trained observers. Turning bias was defined by a directional discrimination score, calculated as the ratio of right turns (RT) minus left turns (LT) over total trials (TT) (Discrimination index = (RT-LT) / TT). A score less than 0 represents a turning bias to the left, whereas a score greater than 0 represents a turning bias to the right. Mice were tested after a unilateral and then bilateral trim as well as following 6 months of re-growth as described above. A two-way ANOVA was run to determine if there were difference in turning behavior. Based on a two-way ANOVA, there is a significant interaction with trimming (f(3,263) = 4.566, p=0.0039); however, Sidak’s multiple comparisons test demonstrates that there is no significant differences between the STD and HFD mice (Fig. 3C). The HFD mice do not display differences in turning bias, hence this data suggests that they do not have impaired whisker sensitivity. Similarly, a previous study in rats revealed that a HFD does not impact sensory processing, evaluated by assessing whisker reflex and tactile sensitivity [23]. We both confirm a memory deficit in the HFD mouse model and demonstrate that this deficit is not due to sensory deficits. In summary, we confirm that the NTR is a whisker dependent, valid, and reproducible cognitive task in a mouse model of cognitive impairment. Bilateral, but not unilateral, whisker trimming impairs acute and long-term tactile discrimination in healthy mice performing the NTR, indicating its reliance on whiskers. HFD mice, a proven model of recognition memory impairment, do not exhibit whisker insensitivity.
We demonstrate that the NTR is valid test of recognition memory in mice. Given the task’s relative simplicity, and its ability to assess tactile recognition, the NTR may be useful for testing other mouse models of cognitive impairment. The field of neurodegenerative research relies heavily on episodic memory testing as proxy for cognitive functioning in animal models; this is primarily assessed using visual tasks. This may be a limitation in the field, given that in one study nearly 40% of the transgenic Alzheimer’s disease mice were homozygous for the retinal degeneration gene rd, leading to gene-associated impairments in discrimination tasks [24]. As whisker discrimination is a separate sensory system all together from vision [11], the NTR may be an alternative and powerful task to assess discrimination memory in models with comorbid visual impairment. Furthermore, the NOR tasks utilize a variety of shapes for the explored objects, leading to a potential for variability in animal response. The NTR utilizes sandpaper, which can be held consistent between experiments and throughout laboratories.
Supplementary Material
Highlights.
Novel tactile recognition is a vibrissae dependent activity
High-fat diet impairs tactile recognition memory in mice
High-fat diet does not impair whisker sensitivity in mice
Acknowledgements.
The authors wish to acknowledge Guadalupe Sanchez, Kevin Boyd, Taylor Lowry, Stacey Nguyen, and Madison Patrick for assistance analyzing behavioral videos.
Funding. This work was supported by the National Institute of Health [NHLBI HL007260 and R25 HL092611, NINDS 1R01NS099595 and 1R01NS099595-02S1; NIGMS P20 GM109040]; the Alzheimer’s Association [AARGD-16-440893]. These funding sources were not involved in: study design; collection, analysis, and interpretation of data; in writing of the report; the decision to publish.
Abbreviations
- (ANOVA)
Analysis of variance
- (AAALAC, Intl)
Association for the Assessment and Accreditation of Laboratory Animal Care International
- (HFD)
high-fat diet
- (kcal)
kilocalorie
- (LT)
left turns
- (OLAW)
NIH Office of Laboratory Animal Welfare
- (NOR)
novel object recognition task
- (NTR)
novel tactile recognition task
- (RT)
right turns
- (STD)
standard control diet
- (TT)
total trials
Footnotes
Competing interests. The authors declare no competing interests.
Data Availability Statement. The datasets generated during the current study are available from the corresponding author upon request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Winters BD, Saksida LM, Bussey TJ, Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval, Neurosci Biobehav Rev 32(5) (2008) 1055–70. [DOI] [PubMed] [Google Scholar]
- [2].Pascalis O, Hunkin NM, Holdstock JS, Isaac CL, Mayes AR, Visual paired comparison performance is impaired in a patient with selective hippocampal lesions and relatively intact item recognition, Neuropsychologia 42(10) (2004) 1293–300. [DOI] [PubMed] [Google Scholar]
- [3].Pascalis O, Bachevalier J, Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task, Hippocampus 9(6) (1999) 609–16. [DOI] [PubMed] [Google Scholar]
- [4].Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE, Impaired recognition memory in monkeys after damage limited to the hippocampal region, J Neurosci 20(1) (2000) 451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nemanic S, Alvarado MC, Bachevalier J, The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys, J Neurosci 24(8) (2004) 2013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McKee RD, Squire LR, On the development of declarative memory, J Exp Psychol Learn Mem Cogn 19(2) (1993) 397–404. [DOI] [PubMed] [Google Scholar]
- [7].Squire LR, Wixted JT, Clark RE, Recognition memory and the medial temporal lobe: a new perspective, Nat Rev Neurosci 8(11) (2007) 872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Broadbent NJ, Squire LR, Clark RE, Spatial memory, recognition memory, and the hippocampus, Proc Natl Acad Sci U S A 101(40) (2004) 14515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mehta SB, Whitmer D, Figueroa R, Williams BA, Kleinfeld D, Active spatial perception in the vibrissa scanning sensorimotor system, PLoS Biol 5(2) (2007) e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pammer L, O'Connor DH, Hires SA, Clack NG, Huber D, Myers EW, Svoboda K, The mechanical variables underlying object localization along the axis of the whisker, J Neurosci 33(16) (2013) 6726–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Petersen CC, The functional organization of the barrel cortex, Neuron 56(2) (2007) 339–55. [DOI] [PubMed] [Google Scholar]
- [12].Wu HP, Ioffe JC, Iverson MM, Boon JM, Dyck RH, Novel, whisker-dependent texture discrimination task for mice, Behav Brain Res 237 (2013) 238–42. [DOI] [PubMed] [Google Scholar]
- [13].Sims-Robinson C, Bakeman A, Bruno E, Jackson S, Glasser R, Murphy GG, Feldman EL, Dietary Reversal Ameliorates Short- and Long-Term Memory Deficits Induced by High-fat Diet Early in Life, PLoS One 11(9) (2016) e0163883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Briner A, De Roo M, Dayer A, Muller D, Kiss JZ, Vutskits L, Bilateral whisker trimming during early postnatal life impairs dendritic spine development in the mouse somatosensory barrel cortex, J Comp Neurol 518(10) (2010) 1711–23. [DOI] [PubMed] [Google Scholar]
- [15].Haridas S, Ganapathi R, Kumar M, Manda K, Whisker dependent responsiveness of C57BL/6J mice to different behavioral test paradigms, Behavioural brain research 336 (2018) 51–58. [DOI] [PubMed] [Google Scholar]
- [16].Simons DJ, Land PW, Early experience of tactile stimulation influences organization of somatic sensory cortex, Nature 326(6114) (1987) 694–7. [DOI] [PubMed] [Google Scholar]
- [17].Lee LJ, Chen WJ, Chuang YW, Wang YC, Neonatal whisker trimming causes long-lasting changes in structure and function of the somatosensory system, Exp. Neurol, 219 (2009) 524–532. [DOI] [PubMed] [Google Scholar]
- [18].Carvell GE, Simons DJ, Abnormal tactile experience early in life disrupts active touch, J Neurosci 16(8) (1996) 2750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Milshtein-Parush H, Frere S, Regev L, Lahav C, Benbenishty A, Ben-Eliyahu S, Goshen I, Slutsky I, Sensory deprivation triggers synaptic and intrinsic plasticity in the hippocampus, Cereb Cortex, 27 (2017) 3457–3470. [DOI] [PubMed] [Google Scholar]
- [20].Cordner ZA, Tamashiro KL, Effects of high-fat diet exposure on learning & memory, Physiol Behav 152(Pt B) (2015) 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL, Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1, Diabetes 58(10) (2009) 2376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M, A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia, J Neurosci Methods 117(2) (2002) 207–14. [DOI] [PubMed] [Google Scholar]
- [23].Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, McEwen BS, Gould E, Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function, Proc Natl Acad Sci U S A 112(51) (2015) 15731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garcia MF, Gordon MN, Hutton M, Lewis J, McGowan E, Dickey CA, Morgan D, Arendash GW, The retinal degeneration (rd) gene seriously impairs spatial cognitive performance in normal and Alzheimer's transgenic mice, Neuroreport 15(1) (2004) 73–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.