Abstract
Respiratory viruses cause acute respiratory diseases with a broad and overlapping spectrum of symptoms. We examined the clinical symptoms and explored the patterns of various respiratory viral infections in children in Hong Kong. Among 2090 specimens collected from outpatient care (2007–2010), 1343 (64.3%) were positive for any virus by the xTAG assay, and 81 (3.9%) were positive for co-infection. The most frequently detected viruses among children aged 6–15 years were enterovirus/rhinovirus and influenza virus A, whereas most non-influenza viruses were more frequently detected in younger children. Higher body temperature was more common for illnesses associated with influenza viruses than for those associated with non-influenza viruses, but other symptoms were largely similar across all infections. The seasonality pattern varied among different viruses, with influenza virus A being the predominant virus detected in winter, and enterovirus/rhinovirus being more commonly detected than influenza virus A in the other three seasons, except for 2009.
Keywords: Acute respiratory illness, burden, outpatient, seasonality, symptom
Introduction
Acute respiratory illness (ARI) represents an important cause of hospitalization and death in all age groups worldwide [1]. Although ARIs can be caused by a wide range of different respiratory viral pathogens, ascertainment of the exact causative agents is rarely clinically indicated, and is thus not routinely performed. Among the small proportion of patients needing hospitalization, significant disease burdens have been attributed to adenovirus (AdV) in children, respiratory syncytial virus (RSV) and influenza virus A (IFVA) in all age groups, and rhinovirus (RhV) and parainfluenza virus 3 (PIV 3) in children and the elderly [2]. However, the full spectrum of disease burden among the majority of patients with ARIs presenting in community outpatient settings has remained largely elusive. Seasonal patterns of ARIs caused by influenza virus [3] and RSV [4] have been better described in some geographical areas, but those of most other respiratory viruses remain poorly understood.
The generally overlapping spectrum of non-specific symptoms makes it very difficult to distinguish between infections with different respiratory viruses [5]. A better understanding of their differential symptom patterns may help to identify cases that are more likely to be influenza virus infections, and thus may benefit clinically from specific antiviral treatment.
The Hong Kong Special Administrative Region is situated in the northern hemisphere, and has a subtropical climate, with an annual variation in temperature from 14.5–18.9°C in January and February to 26.2–31.4°C in June and July, and a mean relative humidity from 69–74% in December and January to 83% in March and April. In this study, we aimed to investigate the burden of ARIs caused by different respiratory viral pathogens among children aged ≤15 years in a community outpatient setting, to describe their seasonal patterns of occurrence, and to characterize their clinical characteristics at presentation.
Materials and methods
Sources of data
As part of a larger study on transmission of influenza viruses in households, we recruited patients from primary-care outpatient clinics in private and public sectors across Hong Kong who met our inclusion criteria, including: (a) being a Hong Kong resident; (b) presenting with at least two symptoms of ARI, including a body temperature of ≥37.8°C, headache, sore throat, cough, runny nose, sputum, and myalgia; (c) onset of symptoms within the preceding 48 h; and (d) living in a household with at least two other people, none of whom had reported ARI in the preceding 14 days. All consenting subjects completed a short data collection form, and had two sets of pooled nasal and throat swab specimens collected by a trained nurse. One specimen was stored immediately in viral transport medium for subsequent virological testing; the other specimen was tested on site with the QuickVue Influenza A + B rapid diagnostic test (Quidel, San Diego, CA, USA). Subjects with a positive rapid test result and their household contacts were further followed up [6], but, in the present analysis, we also analysed laboratory results from the other specimen from all subjects, regardless of their rapid test result. Proxy written informed consent was obtained for all participants from their parents or legal guardians, with additional written consent being obtained from those aged 8–16 years. The study protocol was approved by the Institutional Review Board of Hong Kong University. Weekly meteorological data, such as temperature, humidity, and precipitation, were obtained from the Hong Kong Observatory.
Laboratory methods
Each pooled nasal and throat swab specimen was stored in viral transport medium (5% bovine serum albumin in Earle's balanced salt solution with antibiotic), kept at 2–8°C immediately after collection, and cryopreserved at −70°C within 36 h. The specimens were tested for eight common respiratory viruses (including types and subtypes), namely IFVA (subtypes H1 and H3), influenza virus B (IFVB), RSV (subtypes A and B), PIV (types 1–4), metapneumovirus (MPV), enterovirus (EnV)/RhV, AdV, bocavirus (BoV), and coronavirus (CoV) (types NL63, HKU1, 229E, and OC43), with the xTAG RVP FAST version 2.0 multiplex assay (Luminex Molecular Diagnostics, Toronto, Ontario, Canada), and this was followed by product detection and identification with a Luminex suspension microarray [7]. Total nucleic acid was extracted from the clinical specimens with the NucliSens easyMAG extraction system (bioMerieux, Zaltbommel, The Netherlands), according to the manufacturer's instructions. The extracted nucleic acid was tested for respiratory viruses.
Statistical analysis
Detection rates (and co-detection rates for co-infection) of each virus, stratified by age group (0–5 years and 6–15 years), were calculated by dividing the number of specimens positive for the corresponding virus by the total of positive specimens in that age group. Symptomatology was examined by comparing clinical symptoms of different respiratory virus infections by the use of Pearson's chi-square test (χ 2) or Fisher's exact test (FE). Logistic regression was used to examine the association of different symptoms with influenza or non-influenza virus infection. To assess the seasonal pattern, percentages of different positive specimens across different seasons were compared by use of the chi-square test or FE. Here, we defined winter as December to February, spring as March to May, summer as June to August, and autumn as September to November. Also, logistic regression was used to assess the association between virus detection and meteorological factors, including temperature, absolute humidity, and precipitation. All statistical analyses were performed with R version 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 2090 swab samples were obtained from children aged ≤15 years from February 2007 to December 2010 (187, 1224, 570 and 109 in 2007, 2008, 2009 and 2010, respectively). The detection rate was significantly different across the 4 years, with the highest rate being in 2009, which might due to the pandemic H1N1 IFVA. There was no difference in sex distribution across the years, whereas there was a significant difference in age group percentage across years, with a higher percentage of children in the older age group (6–15 years) than in the younger age group (<6 years) for the first 3 years (2007–2009) but not for 2010 (Table S1).
There were 1343 (64.3%) specimens positive for at least one of the respiratory viruses, 81 (3.9%) specimens positive for more than one respiratory virus, and two specimens positive for three respiratory viruses. Overall, EnV/RhV (19.6%) and IFVA (23.4%) were the two most frequently detected viruses throughout the 4 years. On comparison of the two age groups, viral aetiology was detected significantly more frequently in the younger age group than in the older age group (Table 1 ). Specifically, IFVA and IFVB were detected significantly more frequently in the older age group, whereas most of the other non-influenza viruses were more frequently detected in the younger age group, with the exception of CoV, which was detected at similar frequencies in the two age groups. We further stratified the analysis by separating 2009 from the other 3 years, to determine whether the occurrence of the pandemic H1N1 IFVA in 2009 had affected the seasonal pattern of other common respiratory viruses. On comparison with the other 3 years with usual seasonal influenza activities, the detection pattern of other respiratory viruses during 2009 was broadly similar, with most non-influenza viruses being more frequently detected in the younger age group. Important exceptions included CoV and IFVA, which were more frequently detected in the older age group, and IFVB and BoV, for which no significant difference was seen between the two age groups for 2009 (Table S2). AdV was more frequently detected in the younger age group in 2009, but not in the other 3 years.
Table 1.
Detection and co-detection of each common respiratory virus in different age groups
| Detection | All (n = 2090), no. (%) | Age 0–5 years (n = 822), no. (%) | Age 6–15 years (n = 1268), no. (%) | p |
|---|---|---|---|---|
| Any detection | 1343 (64.3) | 591 (71.9) | 752 (59.3) | 0.000 |
| EnV/RhV | 490 (23.4) | 234 (28.5) | 256 (20.2) | 0.000 |
| IFVA | 409 (19.6) | 119 (14.5) | 290 (22.9) | 0.000 |
| IFVB | 132 (6.3) | 31 (3.8) | 101 (8.0) | 0.000 |
| MPV | 111 (5.3) | 69 (8.4) | 42 (3.3) | 0.000 |
| RSV | 103 (4.9) | 90 (10.9) | 13 (1.0) | 0.000 |
| PIV | 63 (3.0) | 42 (5.1) | 21 (1.7) | 0.000 |
| CoV | 59 (2.8) | 19 (2.3) | 40 (3.2) | 0.282 |
| AdV | 53 (2.5) | 29 (3.5) | 24 (1.9) | 0.023 |
| BoV | 6 (0.3) | 6 (0.7) | 0 (0.0) | 0.004 |
| Co-detection | 81 (3.9) | 46 (5.6) | 35 (2.8) | 0.002 |
| EnV/RhV | 62 (3.0) | 36 (4.4) | 26 (2.1) | 0.003 |
| IFVA | 36 (1.7) | 16 (1.9) | 20 (1.6) | 0.606 |
| IFVB | 8 (0.4) | 1 (0.1) | 7 (0.6) | 0.159 |
| MPV | 8 (0.4) | 5 (0.6) | 3 (0.2) | 0.276 |
| RSV | 20 (1.0) | 19 (2.3) | 1 (0.1) | 0.000 |
| PIV | 6 (0.3) | 5 (0.6) | 1 (0.1) | 0.038 |
| CoV | 13 (0.6) | 3 (0.4) | 10 (0.8) | 0.269 |
| AdV | 6 (0.3) | 4 (0.5) | 2 (0.2) | 0.219 |
| BoV | 5 (0.2) | 5 (0.6) | 0 (0.0) | 0.009 |
p-Values were estimated with the chi-square test or Fisher's exact test.
AdV, adenovirus; BoV, bocavirus; CoV, coronavirus; EnV, enterovirus; IFVA, influenza virus A; IFVB, influenza virus B; MPV, metapneumovirus; PIV, parainfluenza virus; RhV, rhinovirus; RSV, respiratory syncytial virus.
Co-detection of more than one virus was, overall, more frequent in the younger age group than in the older age group (5.6% vs. 2.8%) (Table 1). For specific viruses, this mainly involved co-detection of EnV/RhV, RSV, PIV, and BoV. After stratification by pandemic (2009) and other years, the co-detection pattern was generally preserved, with overall co-detection and co-detection of BoV being significant in 2009 but not in the other years, and co-detection of PIV not being significantly different for all years (Table S2).
Among children with positive viral detection, the most frequently reported clinical symptoms at presentation included cough (81.8%), runny nose (83.4%), fever (59.2%), and sputum (55.2%) (data not shown). On comparison of infections with different viruses detected (BoV was excluded here, because there was no detection of BoV in the older age group), fever was reported significantly more often for IFVA (83.2% and 79.7%), IFVB (80.6% and 82.2%) and AdV (82.8% and 75.0%) than for the other viruses, in both age groups (χ 2, p < 0.001) (Table 2 ). Cough was more frequent for MPV (100%), BoV (100%) and RSV (96.7%) in the younger age group, and for MPV (97.6%) and IFVA (85.5%) in the older age group (FE, p < 0.001). The presence of a runny nose was most frequent for EnV/RhV in the younger age group (FE, p 0.003), but was not significant in the older age group. Sputum was most frequently reported for RSV (72.2%) in the younger age group, and for MPV (81.0%) in the older age group (χ 2, p < 0.001). Results from logistic regression suggested that the occurrence of fever was associated with a significantly increased likelihood of influenza virus (IFVA and IFVB) infection vs. infection with other respiratory viruses (OR 4.78, 95% CI 2.96–7.74) in the younger age group. Cough, runny nose and sputum were also associated with an increased likelihood of influenza virus detection in the older age group (Table S2).
Table 2.
Characteristic and clinical symptoms of different viral infections by age group
| All | EnV/RhV | IFVA | IFVB | MPV | RSV | PIV | CoV | AdV | BoV | Negative | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 0–5 years (n) | 591 | 234 | 119 | 31 | 69 | 90 | 42 | 19 | 29 | 6 | 231 | |
| Male (%) | 50.9 | 52.1 | 56.3 | 51.6 | 43.5 | 55.6 | 52.4 | 42.1 | 27.6 | 66.7 | 49.4 | 0.149 |
| Fever (%) | 56.5 | 43.6 | 83.2 | 80.6 | 52.2 | 47.8 | 61.9 | 47.4 | 82.8 | 50.0 | 58.9 | 0.000 |
| Cough (%) | 84.3 | 83.8 | 84.9 | 74.2 | 100.0 | 96.7 | 76.2 | 63.2 | 58.6 | 100.0 | 68.0 | 0.000 |
| Runny nose (%) | 83.6 | 90.2 | 80.7 | 83.9 | 73.9 | 85.6 | 73.8 | 84.2 | 69.0 | 83.3 | 71.0 | 0.003 |
| Sputum (%) | 48.7 | 46.2 | 48.7 | 35.5 | 55.1 | 72.2 | 35.7 | 31.6 | 34.5 | 66.7 | 34.6 | 0.000 |
| Age 6–15 years (n) | 752 | 256 | 290 | 101 | 42 | 13 | 21 | 40 | 24 | 0 | 516 | |
| Male (%) | 49.9 | 57.0 | 47.6 | 47.5 | 42.9 | 61.5 | 47.6 | 40.0 | 54.2 | — | 54.1 | 0.233 |
| Fever (%) | 61.3 | 38.3 | 79.7 | 82.2 | 42.9 | 46.2 | 57.1 | 50.0 | 75.0 | — | 57.9 | 0.000 |
| Cough (%) | 79.9 | 73.8 | 85.5 | 81.2 | 97.6 | 84.6 | 71.4 | 80.0 | 54.2 | — | 59.3 | 0.000 |
| Runny nose (%) | 83.2 | 86.3 | 81.7 | 87.1 | 76.2 | 92.3 | 76.2 | 82.5 | 66.7 | — | 55.0 | 0.139 |
| Sputum (%) | 60.2 | 52.0 | 64.5 | 63.4 | 81.0 | 69.2 | 47.6 | 65.0 | 33.3 | — | 41.7 | 0.000 |
p-Values were estimated with the chi-square or Fisher's exact test, except for BoV, owing to limited numbers.
AdV, adenovirus; BoV, bocavirus; CoV, coronavirus; EnV, enterovirus; IFVA, influenza virus A; IFVB, influenza virus B; MPV, metapneumovirus; PIV, parainfluenza virus; RhV, rhinovirus; RSV, respiratory syncytial virus.
Fig. 1 shows the percentages of all positive specimens attributable to each viral pathogen during different seasons in 2009 and other years. IFVA and EnV/RhV were generally the two most frequently detected pathogens in all seasons. IFVA was most frequently detected in winter, both for 2009 (57.3%, p < 0.001) and for the other years (31.3%, p < 0.005). In fact, in 2009, when the pandemic H1N1 IFVA was predominant, it was responsible for the vast majority of all positive specimens (30.2–57.3%) in different seasons. EnV/RhV was next most frequently detected virus over the whole year, particularly in spring and autumn. RSV was more frequently detected in non-winter seasons (12.7%) during all years, but this was less marked in 2009. For other viral pathogens, the seasonal patterns were similar and not significantly different. For various meteorological factors, logistic regression results showed that influenza virus was negatively associated with temperature, whereas RSV was positively associated with temperature. EnV/RhV was positively associated with precipitation, and none of the other viruses was significantly associated with any of the three meteorological factors (Table S3).
Fig. 1.
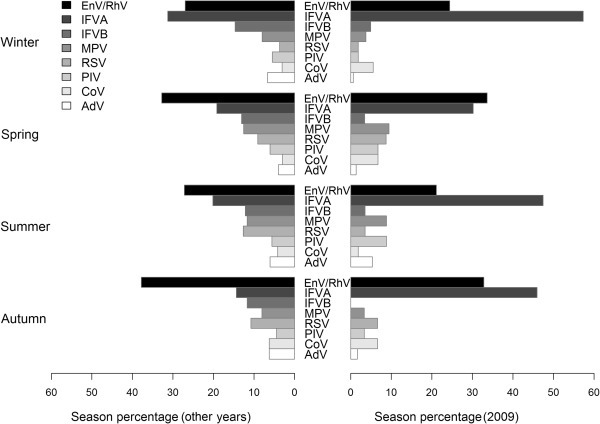
Distribution of the different respiratory viruses detected across four seasons. AdV, adenovirus; CoV, coronavirus; IFVA, influenza virus A; IFVB, influenza virus B; EnV, enterovirus; MPV, metapneumovirus; PIV, parainfluenza virus; RhV, rhinovirus; RSV, respiratory syncytial virus.
Discussion
Previous studies have shown the substantial disease burden due to hospitalization or mortality associated with influenza virus in Hong Kong [8], [9]. Our present study further highlighted the important contribution of various non-influenza viruses (EnV/RhV, MPV, RSV, PIV, etc.) to the disease burden in the local community outpatient setting, especially in the younger children aged <6 years, thus giving a more comprehensive picture of the overall disease spectrum caused by these common respiratory viruses.
EnV/RhV and IFVA were the two most frequently identified viruses in both age groups. Generally, most non-influenza viruses were more frequently detected among preschool children (aged 0–5 years) than among older children (aged 6–15 years), except for CoV, and this is consistent with another study [10]. The co-detection rate was also higher in the younger age group, which is consistent with other studies [11]. Co-detection of more than one respiratory virus has been frequently reported; however, the clinical significance of co-detection is unclear, with previous studies giving conflicting results regarding whether co-infection is associated with an increase in the severity of related respiratory disease [12], [13], [14], [15], [16].
Although it is difficult to differentiate between different respiratory viral infections on the basis of clinical symptomatology alone, our results suggested that a number of symptoms were more commonly reported by patients with influenza virus infections than by patients with infections caused by other respiratory viruses. Our study showed that fever was associated with a significantly increased likelihood of influenza virus infection in children aged 0–5 years, whereas fever, cough, runny nose and sputum were also associated with influenza virus infection in children aged 6–16 years. This finding is generally consistent with similar findings of other studies on epidemic influenza [17].
Seasonality patterns always vary with different viruses and regions. Despite the fact that most previous studies have indicated a predominantly winter and autumn seasonality for influenza virus and EnV/RhV [18], [19], we found that both viruses were rather commonly detected over the whole year, with a significant predominance of IFVA in the winter for all of the study years. In 2009, when the H1N1 IFVA was predominant, it was responsible for the majority of positive specimens in all seasons. RSV was more frequently detected in non-winter seasons, and was less marked in 2009. Another study found that RSV activity peaked in July and August, and was positively associated with temperature [20]. The seasonality pattern of an autumn peak for CoV-NL63 infections reported by a previous study, however, could not be demonstrated in this study, as all CoVs were analysed together, without species identification [21]. Although the findings regarding associations with temperature or precipitation may help to shed some light on the observed seasonality patterns of different infections, their underlying mechanism remaines largely controversial [19], [22].
In conclusion, our findings suggest that, although the disease burdens of influenza virus infection in terms of hospitalization and mortality are important, they reflect only the tip of the iceberg, and also understanding the wider spectrum of disease burden in terms of outpatient service attendance and morbidity will always be important. The substantial burden of respiratory infection caused by viruses other than influenza viruses shows the importance of improved surveillance and preventive efforts in the community setting. Further studies are also important to better characterize the burden of these non-influenza viruses from other perspectives, including morbidity and school absence among children. A better understanding of the seasonality patterns of these common respiratory viruses would also help to inform better disease surveillance, to inform proper healthcare action planning and decisions.
Transparency declaration
The authors declare that they have no conflicts of interest.
Editor: D. Raoult
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cmi.2015.05.027
Appendix A. Supplementary data
The following are the supplementary data related to this article:
TABLE S1. Age and gender distribution of the cases across 2007 to 2010.
TABLE S2. Clinical characteristics related to influenza or non-influenza detection.
TABLE S3. Association between virus-specific detection and meteorological factors.
References
- 1.WHO . World Health Organization; Geneva, Switzerland: 2008. The global burden of disease: 2004 update. [Google Scholar]
- 2.Gaunt E.R., Harvala H., McIntyre C., Templeton K.E., Simmonds P. Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. J Clin Virol. 2011;52(3):215–221. doi: 10.1016/j.jcv.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipsitch M., Viboud C. Influenza seasonality: lifting the fog. Proc Natl Acad Sci U S A. 2009;106(10):3645–3646. doi: 10.1073/pnas.0900933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borchers A.T., Chang C., Gershwin M.E., Gershwin L.J. Respiratory syncytial virus—a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–379. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox N.J., Subbarao K. Influenza. Lancet. 1999;354(9186):1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 6.Cowling B.J., Chan K.H., Fang V.J., Lau L.L., So H.C., Fung R.O. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362(23):2175–2184. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H., McCormac M.A., Estes R.W., Sefers S.E., Dare R.K., Chappell J.D. Simultaneous detection and high-throughput identification of a panel of RNA viruses causing respiratory tract infections. J Clin Microbiol. 2007;45(7):2105–2109. doi: 10.1128/JCM.00210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P., Goldstein E., Ho L.M., Yang L., Nishiura H., Wu J.T. Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis. 2012;206(12):1862–1871. doi: 10.1093/infdis/jis628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu S.S., Chan K.H., Chen H., Young B.W., Lim W., Wong W.H. Virologically confirmed population-based burden of hospitalization caused by respiratory syncytial virus, adenovirus, and parainfluenza viruses in children in Hong Kong. Pediatr Infect Dis J. 2010;29(12):1088–1092. doi: 10.1097/INF.0b013e3181e9de24. [DOI] [PubMed] [Google Scholar]
- 10.Pretorius M.A., Madhi S.A., Cohen C., Naidoo D., Groome M., Moyes J. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness—South Africa, 2009–2010. J Infect Dis. 2012;206(Suppl 1):S159–S165. doi: 10.1093/infdis/jis538. [DOI] [PubMed] [Google Scholar]
- 11.Drews A.L., Atmar R.L., Glezen W.P., Baxter B.D., Piedra P.A., Greenberg S.B. Dual respiratory virus infections. Clin Infect Dis. 1997;25(6):1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard N., Komurian-Pradel F., Javouhey E., Perret M., Rajoharison A., Bagnaud A. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J. 2008;27(3):213–217. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 13.Calvo C., Garcia-Garcia M.L., Blanco C., Vazquez M.C., Frias M.E., Perez-Brena P. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol. 2008;42(3):268–272. doi: 10.1016/j.jcv.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand H.K., de Groot R., Galama J.M., Brouwer M.L., Teuwen K., Hermans P.W. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012;47(4):393–400. doi: 10.1002/ppul.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papenburg J., Hamelin M.E., Ouhoummane N., Carbonneau J., Ouakki M., Raymond F. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206(2):178–189. doi: 10.1093/infdis/jis333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esper F.P., Spahlinger T., Zhou L. Rate and influence of respiratory virus co-infection on pandemic (H1N1) influenza disease. J Infect. 2011;63(4):260–266. doi: 10.1016/j.jinf.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boivin G., Hardy I., Tellier G., Maziade J. Predicting influenza infections during epidemics with use of a clinical case definition. Clin Infect Dis. 2000;31(5):1166–1169. doi: 10.1086/317425. [DOI] [PubMed] [Google Scholar]
- 18.Cheuk D.K., Tang I.W., Chan K.H., Woo P.C., Peiris M.J., Chiu S.S. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr Infect Dis J. 2007;26(11):995–1000. doi: 10.1097/INF.0b013e3181586b63. [DOI] [PubMed] [Google Scholar]
- 19.Chan P.K., Mok H.Y., Lee T.C., Chu I.M., Lam W.Y., Sung J.J. Seasonal influenza activity in Hong Kong and its association with meteorological variations. J Med Virol. 2009;81(10):1797–1806. doi: 10.1002/jmv.21551. [DOI] [PubMed] [Google Scholar]
- 20.Chan P.K., Sung R.Y., Fung K.S., Hui M., Chik K.W., Adeyemi-Doro F.A. Epidemiology of respiratory syncytial virus infection among paediatric patients in Hong Kong: seasonality and disease impact. Epidemiol Infect. 1999;123(2):257–262. doi: 10.1017/s0950268899002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu S.S., Chan K.H., Chu K.W., Kwan S.W., Guan Y., Poon L.L. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40(12):1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.du Prel J.B., Puppe W., Grondahl B., Knuf M., Weigl J.A., Schaaff F. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49(6):861–868. doi: 10.1086/605435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


