Abstract
Mobile phones (MPs) are potential reservoirs of nosocomial bacteria, but few data are available concerning viruses. We aimed to evaluate the presence of virus RNA from epidemic viruses including metapneumovirus, respiratory syncytial virus, influenza viruses, rotavirus (RV) and norovirus on the MPs used by healthcare workers (HCWs) and to relate it to hygiene measures. An anonymous behavioural questionnaire about MP use at hospital was administered to the HCWs of four adult and paediatric departments of a university hospital. After sampling personal (PMP) and/or professional MPs (digital enhanced cordless telephone, DECT), virus RNAs were extracted and amplified by one-step real-time reverse transcription–quantitative PCR. The molecular results were analysed in a masked manner in relation to the behavioural survey. Questionnaires from 114 HCWs (35 senior physicians, 30 residents, 32 nurses, 27 nurses' assistants) working either in adult (n = 58) or paediatric (n = 56) departments were analysed. Medical personnel used their PMP more frequently than paramedical HCWs (33/65 vs. 10/59, p <0.001). MPs were used during care more frequently in adult wards than in paediatric ones (46/58 vs. 27/56, p <0.001). Virus RNA was detected on 42/109 (38.5%) collected MPs, with RV found on 39, respiratory syncytial virus on three and metapneumovirus on one. The presence of virus RNA was significantly associated with MPs from the paediatric HCWs (p <0.001). MPs routinely used in hospital, even during care, can host virus RNA, especially RV. Promotion of frequent hand hygiene before and after MP use, along with frequent cleaning of MPs, should be encouraged.
Keywords: Epidemic viruses, healthcare workers, hospital-acquired infections, mobile phones
Introduction
Mobile phones (MPs) are becoming commonplace in both community and hospital settings. More than 50% of healthcare workers (HCWs) admit using MPs (either personal or professional devices) in their clinical environment and practice, including during physical contact with patients [1], [2], [3], [4]. The use of MPs can improve the quality, rapidity and efficiency of communication in healthcare settings [1].
Bacterial contamination on these devices has been described [1], with up to 25% of MPs being found to be contaminated [5]. Nosocomial bacteria such as methicillin-resistant Staphylococcus aureus, Acinetobacter species, vancomycin-resistant enterococci, Pseudomonas species and coliforms have been retrieved from MPs [2], [5], [6], [7]. These devices may thus serve as a reservoir of bacteria known to cause nosocomial infections [4], [5] and may play a role in their transmission to patients through the hands of HCWs [8]. Adequate hand hygiene as well as device cleaning and disinfection could decrease this risk. Unfortunately, as reported in reviews about this topic [4], [5], HCWs do not regularly apply hygiene procedures such as regular cleaning of their mobile devices and do not perform hand hygiene before or after their use, even though most physicians are aware that these devices could carry pathogenic microorganisms [9].
In contrast to bacterial contamination, evidence of viral contamination of MPs such as digital enhanced cordless telephones (DECTs) or personal mobile phones (PMPs) are, to our knowledge, lacking. However, epidemic viruses such as influenza viruses, rotavirus (RV) and norovirus (NV) have been shown to be able to adhere and contaminate inert surfaces as well as medical devices close to the patients' environment [10], [11], [12]. NV and RV were shown to be particularly resistant; they can survive for weeks, even months, on surfaces and in the hospital environment [11], [13], [14], [15], [16]. Contamination of hospital surfaces by these viruses may therefore play a role in nosocomial epidemics [11], [13]. Respiratory viruses have been shown to persist on surfaces for a few days [14], [17], with a potential role in nosocomial transmission, as emphasized during the severe acute respiratory syndrome epidemic of 2003 [18]. Epidemic viruses have already been retrieved from electronic-device surfaces such as keyboards, computers and telephone handsets [10], [12], [17], [19], [20], [21]. However, the viral contamination of HCW MPs using up-to-date methods has not been studied.
The aims of this study were (a) to evaluate the contamination of MPs by epidemic viruses including RV, NV, influenza A and B viruses, syncytial respiratory virus (RSV) and metapneumovirus (hMPV) in clinical settings, (b) to evaluate the behaviour of HCWs using their MPs in our center by using a blindly recorded questionnaire and (c) to correlate viral contamination of MPs with the behaviour of HCWs.
Materials and Methods
The study took place at the University Hospital of Saint-Étienne, France, from January to March 2013, i.e. the period of circulation of epidemic viruses (influenza viruses, RSV, gastroenteritis-associated viruses) in our setting [22] (personal data). HCWs of the paediatric and adult emergency rooms, as well of those of the general paediatric and the infectious diseases departments, were involved. The term ‘mobile phones’ was used to indicate both PMPs and DECTs. Physicians and residents were considered to be medical staff (n = 55); nurses and nurses' assistants were considered to be paramedical HCWs (n = 59). The design of the study is summarized in Fig. 1 . Medical students were excluded from the survey, but because the sampling of MPs was performed in their unit during the study, we also sampled their PMPs. None of the HCWs declared or presented signs of epidemic viral infection at the time of MP sampling.
Fig. 1.
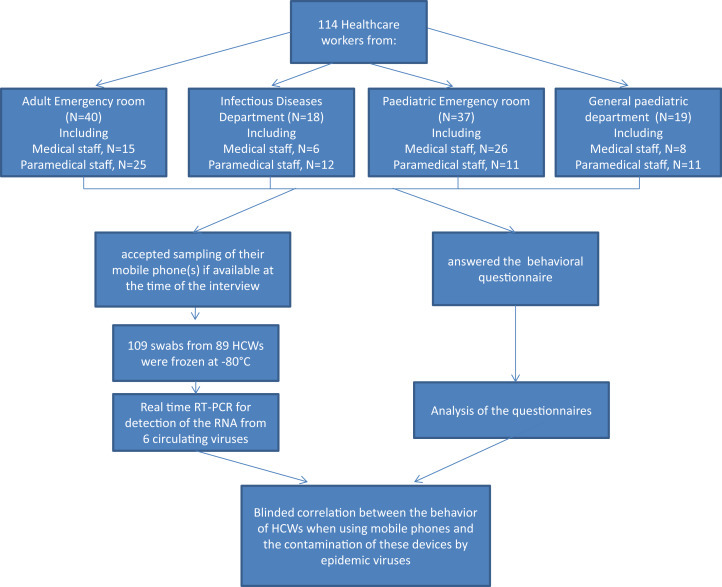
Overall study design.
Behavioural patterns in the use of MPs by HCWs
Each department was visited twice by SP and EBN during the study period. A questionnaire was administered to all HCWs in the visited departments, without previous information about the study being provided. Participants were volunteers and answered anonymously; they all agreed that the MPs they used could be sampled. General data about the use of PMPs or DECTs during work were recorded, such as using the device close to patients, using an alcohol-based hand rub before and after use and cleaning the MPs.
Sampling of MPs
The MPs were wiped with a 480CE e-swab (Copan, Brescia, Italy), and the swabs, placed in transport medium, were frozen at −80°C before virologic analysis. When HCWs used PMPs and DECTs at hospital, both MPs were sampled. In some cases, several HCWs shared one DECT; in this situation, the DECT was sampled once. PMPs were sampled only if used at hospital.
Detection of virus RNA on MPs
A volume of 200 μL of transport medium was extracted by using the Specific B protocol on the NUCLISENS easyMAG instrument (bioMérieux, Marcy l'Etoile, France). The elution volume was 50 μL. The amplification step was performed immediately after extraction, without freezing of nucleic acids. Ten microlitres of extract was mixed with ready-to-use commercial mastermix, and one-step reverse transcription and quantitative PCR (RT-qPCR) reactions were performed on an ABI7500fast real-time cycler (Applied Biosystems, Foster City, CA, USA), according to the manufacturers' recommendations. The enteric viruses (RV and NV) were detected by using KHRV and KHPNOV kits from Ceeram (La Chapelle-sur-Erdre, France); the respiratory viruses (influenza A and B viruses, RSV and hMPV) were detected by using the MWS kits from bioMérieux [22]. The molecular results were analysed in a masked manner to the results of the behavioural survey.
Ethics
The ethics committee of the University Hospital of Saint-Étienne approved the study.
Statistical analysis
The software used for the collection of data was Excel (Microsoft, Redmond, WA, USA). Statistical analyses were performed by SPSS 20.0 software (IBM, Armonk, NY, USA). For the univariate and bivariate analyses, Fisher's exact test and t tests were used (p <0.05 was considered significant). To adjust for confounding factors, variables with p <0.2 in univariate analysis were entered into a multiple logistic regression model.
Results
During the study period, 114 HCWs (all of those interviewed) responded to the questionnaire. The partition of HCWs by category and department is presented in Fig. 1. The majority of participants were women (74.6%); the mean age was 33.5 ± 10.0 years.
Behaviour of HCWs in use of MPs during work at hospital
All HCWs owned a PMP, and 99 of them (86.8%) used a DECT daily at work. All the HCWs declared that they knew that MPs could host infectious agents. The participants received more than ten calls per workday in 65.6% of cases (75/114 HCWs); no statistical difference was found among categories. Table 1 lists the results of the questionnaire analysis. HCWs used their PMPs in hospital in 37.7% (43/114) of cases, with medical HCWs using their PMP more frequently than paramedical HCWs (respectively, 33/65 vs. 10/53, p <0.001). Seventy-three HCWs (64%) used MPs during care. Among them, 28.8% (21/73) never performed hand hygiene before using their MP, whereas 37.0% (27/73) of HCWs never performed it after using their MP. Overall, 15 HCWs (20.6%) never performed hand hygiene both before and after using a MP. As shown in Table 1, paediatric staff used MPs during care significantly less frequently than adult staff (27/56 vs. 46/58, p <0.001). Regarding hand hygiene before or after use of MPs during care, there was no difference among categories. Paediatric staff disinfected their DECTs less frequently than adult staff (13/52 vs. 25/47, p <0.005). Seventy-eight HCWs (68.4%) halted patient care to answer their MP (their own MP or a shared MP). This proportion was significantly lower in paediatric staff than in adult staff (27/56 vs. 46/58, p <0.001). Among HCWs who stopped care to hang up a MP, 18 (23.1%) never performed hand hygiene after using the MP and before continuing care, with no significant difference among categories.
Table 1.
Declared practices of hygiene and analysis of viral contamination of MPs used by healthcare workers at the University Hospital of Saint-Étienne, France
| Practice | Paediatric ward |
Adult ward |
p | ||
|---|---|---|---|---|---|
| Paramedical staff | Medical staff | Paramedical staff | Medical staff | ||
| Use of MP (personal or professional) at hospital (%) | 20/22 (90.9%) | 33/34 (97.1%) | 33/37 (89.2%) | 21/21 (100%) | NS |
| PMP | 4/20 (20.0%) | 17/33 (51.5%) | 6/33 (18.2%) | 16/21 (76.2%) | <0.0001a |
| DECT | 18/20 (90.0%) | 29/33 (87.9%) | 32/33 (97.0%) | 20/21 (95.2%) | NS |
| Use of MP (personal or professional) during care (%) | 9/22 (40.9%) | 18/34 (52.9%) | 28/37 (75.7%) | 18/21 (85.7%) | <0.001b |
| Hand hygiene never performed before its use | 3/9 (33.3%) | 5/18 (27.8%) | 8/28 (28.6%) | 5/18 (27.8%) | NS |
| Hand hygiene never performed after its use | 3/9 (33.3%) | 6/18 (33.3%) | 11/28 (39.3%) | 7/18 (38.9%) | NS |
| Never disinfect their MP (personal or professional) | |||||
| PMP | 1/4 (25.0%) | 4/17 (23.5%) | 2/6 (33.3%) | 6/16 (37.5%) | NS |
| DECT | 10/18 (55.6%) | 15/29 (51.7%) | 5/32 (15.6%) | 8/20 (40.0%) | <0.005b |
| Halt care provision to answer a call on MP (own or shared) (%) | 9/22 (40.9%) | 25/34 (73.5%) | 25/37 (67.6%) | 19/21 (90.5%) | <0.005a |
| Hand hygiene never performed after its use in this context | 1/9 (11.1%) | 4/25 (20.0%) | 8/25 (32.0%) | 5/19 (26.3%) | NS |
| Detection of virus on at least one MPc | 10/15 (66.7%) | 19/34 (55.9%) | 1/19 (3.4%) | 7/21 (33.3%) | <0.0005b |
| PMP | 3/3 (100%) | 5/12 (41.7%) | 0/3 (0%) | 5/11 (45.5%) | NS |
| DECT | 7/12 (58.3%) | 14/22 (63.6%) | 1/16 (6.3%) | 2/10 (20%) | <0.05b |
DECT, digital enhanced cordless telephone; MP, mobile phone; PMP, personal mobile phone.
Medical vs. paramedical staff, whatever the department.
Paediatric vs. adult ward.
In the 89 healthcare workers who provided sampling of at least one MP at the time of interview.
Virus RNA detection on MPs
Virus RNA was detected on 42/109 (38.5%) sampled MPs: RV in 39 cases (92.8%), RSV in three cases and hMPV in one case. NV RNA and influenza virus A and B RNA were not detected on MP samples of interviewed HCWs. One DECT sampled from a paediatric emergency room senior physician was found to be positive for both RSV and RV.
We also sampled 22 PMPs from medical students who were present in the wards during the study; 11 (50%) were found to be positive for virus RNA (RV n = 7, RSV n = 2, influenza virus A and influenza virus B n = 1 each). Because they did not respond to the questionnaire, they were not included in the multivariate analysis.
Correlation of viral contamination of MPs to the behaviour of HCWs
Table 1 shows the detection of viruses on at least one MP by HCW category. By multivariate analysis, the presence of virus RNA was significantly associated with MPs from paediatric HCWs compared to adult HCWs (p < 0.001; 32/59 vs. 10/50; odds ratio increased by 2.76). Other recorded behaviours in using MPs in hospital were not associated with viral contamination. Notably, there were no differences in viral contamination regarding staff categories or hygiene habits related to MP use.
Discussion
We report here for the first time contamination of MPs used by HCWs with epidemic viruses including RV, RSV and hMPV. This finding raises the possible role of MPs in cross-transmission of epidemic viruses in hospitals, with the transfer from nonporous fomites to fingers as described recently [23] and from fingers to fomites including MPs. PMPs may also play a role in the spread of pathogens from community to hospital as well as from hospital to community.
More than one third of sampled MPs were found to be contaminated with virus RNA in the clinical settings we studied. RV RNA was largely recovered from MPs, notably in those from the paediatric staff. This finding was concordant with epidemiologic data showing that RV is the prevalent virus during winter epidemics in the paediatric population, including during the time of this study (data not shown), because RV vaccination coverage is low in France [24]. RV has been frequently found on hospital surfaces for several months after the epidemic period and after the surfaces had been cleaned [11], [14], [15]. The high prevalence of RV in patients on the paediatric ward during the study, together with the capacity of RV to persist in the environment, are probably the main factors explaining the high frequency of RV RNA detection on MPs. It would be interesting to look at the presence of RV RNA on MPs outside the epidemic winter period. Despite NV RNA screening in our samples, it was not detected on MPs in this study. NVs are, however, largely known to be able to survive on several hospital surfaces [10], [13], [19]. The absence of NV RNA was probably due to the reduced circulation of NVs usually associated with benign diarrhoea in adults, and few patients infected with this pathogen may have been hospitalized in adult wards in the course of the study. Failure to detect NVs seems to be less probable, as we used internal controls in both RV and NV RT-qPCR assays in order to avoid false-negative results due to inhibitors [15].
The second most frequent virus RNA detected on samples was RSV, which may also be found on environmental surfaces and can lead to inoculation after touching contaminated surfaces [25]. Finally, other respiratory viruses transmitted by droplets, including hMPV and influenza viruses, were recovered from the PMPs of one HCW and two medical students, respectively. These respiratory viruses could also survive on hands and on environmental surfaces [12], [17], [26], leading to the risk of cross-transmission in hospital settings. The yield of contamination could be high because the same MP could be contaminated by several pathogens, as described in our study, and DECTs could be used by several HCWs.
Although most HCWs are aware that MPs may carry pathogenic bacteria [4], most of them do not perform hand hygiene before or after using MPs, and they do not regularly clean their MPs [8]. In the present study, all participating HCWs said they knew that MPs could be contaminated by viruses. However, most of them said they used PMPs and DECTs during their work, notably when they were in contact with patients. This was particularly true among adult staff. A large proportion also did not perform hand hygiene when using a MP, even during physical contact with patients, without difference among categories or departments. Hand hygiene should be performed just before patient contact, as highly recommended by World Health Organization guidelines (http://apps.who.int/iris/bitstream/10665/44102/1/9789241597906_eng.pdf). Beyond possible cross-transmission of pathogenic bacteria [2], [3], [4], [5], [8], we can hypothesize that cross-transmission may also occur with epidemic viruses, as shown in our study. Alcohol-based hand rubs and antiseptic wipes are largely available for all HCWs in our hospital; the lack of hand hygiene before and after using MPs and the lack of cleaning MPs is mostly related to poor adherence to hygiene recommendations by HCWs. Actually, hand washing with soap and the use of alcohol-based disinfectants have been proven to be efficient at removing viruses, especially respiratory ones, from artificially contaminated hands [27]. Gastroenteritis-associated nonenveloped viruses are, however, known to be more resistant to disinfection procedures [28]. Hydroalcoholic hand rub that passes the EN 14476 viral norm or specific tests is needed to kill NVs. However, RVs are sensitive to alcohol disinfection, meaning that the contamination of MPs by these viruses is certainly related to the fact that HCWs did not use alcohol-based hand rubs before using their MPs, rather than more intensive use. Indeed, paediatric MPs were found to be more frequently contaminated; paediatric HCWs used their MPs less frequently than adult HCWs. These results suggest that promotion of the cleaning of MPs should be performed more actively. Indeed, the use of isopropyl alcohol has been shown to be efficient in reducing contamination of fomites [29]; disinfectant wipe intervention on fomites has also been demonstrated to reduce the load of infectious agents as well as the risk of fomite-to-finger microbial transfer [30]. These recommendations should be promoted in paediatric wards, where RVs circulate intensively during epidemics.
Our study has several limitations. Firstly, only virus RNA was detected on MPs, without presumption of the possible infectious potential of the different viruses. The samples were not inoculated onto cell cultures for isolation of respiratory viruses, RV and NV being not cultivable [15]. However, this first demonstration of contamination of MPs by virus RNA should be considered as an effective way for the nosocomial transmission of these viruses, and notably of RVs in paediatric wards. Another limit concerns the absence of virus load determination on MPs by RT-qPCR, as has been done for food contamination [31]. However, to our knowledge, the correlation between virus load on inanimate surfaces and the risk of nosocomial transmission has not been clearly demonstrated. Finally, we were not able to show a correlation between the contamination of the MPs and the frequency of hand hygiene; the sample size of 30 workers, chosen to ensure the validity of the estimation per HCW category, was probably too low to permit the evaluation of this interaction.
After the demonstration by others of contamination by bacteria [4], [5], we show here that contamination by virus RNA also exists on the MPs used by different categories of HCWs from several hospital wards. Our study indicates that more attention must be paid to disinfecting MPs that are largely used in clinical settings and that constitute a reservoir for viral agents. In addition, hand hygiene before and/or after their use must be recommended more strongly, especially in paediatric wards, where viruses may circulate intensively. Because restriction on the use of these devices is not feasible [5], raising awareness in HCWs about the risk of pathogen transmission is urgent. Regular cleaning of MPs should be promoted. Beyond use of MPs, hand hygiene should be performed just before contact with patients.
Acknowledgements
We thank all the HCWs who accepted to participate to this study and E. Delorme for technical assistance. C. Zintilini, C. Janis and P. Bourgeois from bioMérieux and F. Chatigny and F. Loisy from Ceeram are acknowledged for providing the virologic reagents used in this study.
Editor: D. Raoult
Footnotes
Presented in part at the 17th annual meeting of the European Society of Clinical Virology, September 2014, Prague, Czech Republic
Transparency Declaration
bioMérieux and Ceeram provided reagents for RT-qPCR but did not influence the study design or evaluation of the study's results. All authors report no conflicts of interest relevant to this article.
References
- 1.Visvanathan A., Gibb A.P., Brady R.R. Increasing clinical presence of mobile communication technology: avoiding the pitfalls. Telemed J E Health. 2011;17:656–661. doi: 10.1089/tmj.2011.0018. [DOI] [PubMed] [Google Scholar]
- 2.Goldblatt J.G., Krief I., Klonsky T., Haller D., Milloul V., Sixsmith D.M. Use of cellular telephones and transmission of pathogens by medical staff in New York and Israel. Infect Control Hosp Epidemiol. 2007;28:500–503. doi: 10.1086/513446. [DOI] [PubMed] [Google Scholar]
- 3.Ramesh J., Carter A.O., Campbell M.H., Gibbons N., Powlett C., Moseley H., Sr. Use of mobile phones by medical staff at Queen Elizabeth Hospital, Barbados: evidence for both benefit and harm. J Hosp Infect. 2008;70:160–165. doi: 10.1016/j.jhin.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Manning M.L., Davis J., Sparnon E., Ballard R.M. iPads, droids, and bugs: infection prevention for mobile handheld devices at the point of care. Am J Infect Control. 2013;41:1073–1076. doi: 10.1016/j.ajic.2013.03.304. [DOI] [PubMed] [Google Scholar]
- 5.Brady R.R., Verran J., Damani N.N., Gibb A.P. Review of mobile communication devices as potential reservoirs of nosocomial pathogens. J Hosp Infect. 2009;71:295–300. doi: 10.1016/j.jhin.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Ustun C., Cihangiroglu M. Health care workers' mobile phones: a potential cause of microbial cross-contamination between hospitals and community. J Occup Environ Hyg. 2012;9:538–542. doi: 10.1080/15459624.2012.697419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foong Y.C., Green M., Ogden K. Mobile phones as a potential vector of infection in a paediatric ward. J Paediatr Child Health. 2013;49:1083–1084. doi: 10.1111/jpc.12438. [DOI] [PubMed] [Google Scholar]
- 8.Jeske H.C., Tiefenthaler W., Hohlrieder M., Hinterberger G., Benzer A. Bacterial contamination of anaesthetists' hands by personal mobile phone and fixed phone use in the operating theatre. Anaesthesia. 2007;62:904–906. doi: 10.1111/j.1365-2044.2007.05172.x. [DOI] [PubMed] [Google Scholar]
- 9.Brady R.R., Fraser S.F., Dunlop M.G., Paterson-Brown S., Gibb A.P. Bacterial contamination of mobile communication devices in the operative environment. J Hosp Infect. 2007;66:397–398. doi: 10.1016/j.jhin.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Morter S., Bennet G., Fish J., Richards J., Allen D.J., Nawaz S. Norovirus in the hospital setting: virus introduction and spread within the hospital environment. J Hosp Infect. 2011;77:106–112. doi: 10.1016/j.jhin.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 11.Ganime A.C., Carvalho-Costa F.A., Mendonca M.C., Vieira C.B., Santos M., Costa F.R. Group A rotavirus detection on environmental surfaces in a hospital intensive care unit. Am J Infect Control. 2012;40:544–547. doi: 10.1016/j.ajic.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee D.V., Cohen B., Bovino M.E., Desai S., Whittier S., Larson E.L. Survival of influenza virus on hands and fomites in community and laboratory settings. Am J Infect Control. 2012;40:590–594. doi: 10.1016/j.ajic.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Weber D.J., Rutala W.A., Miller M.B., Huslage K., Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care–associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 14.Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Lazaro D., Cook N., Ruggeri F.M., Sellwood J., Nasser A., Nascimento M.S. Virus hazards from food, water and other contaminated environments. FEMS Microbiol Rev. 2012;36:786–814. doi: 10.1111/j.1574-6976.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nenonen N.P., Hannoun C., Svensson L., Toren K., Andersson L.M., Westin J. Norovirus GII.4 detection in environmental samples from patient rooms during nosocomial outbreaks. J Clin Microbiol. 2014;52:2352–2358. doi: 10.1128/JCM.00266-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greatorex J.S., Digard P., Curran M.D., Moynihan R., Wensley H., Wreghitt T. Survival of influenza A(H1N1) on materials found in households: implications for infection control. PLoS One. 2011;6:e27932. doi: 10.1371/journal.pone.0027932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;15(348):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 19.Barker J., Vipond I.B., Bloomfield S.F. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J Hosp Infect. 2004;58:42–49. doi: 10.1016/j.jhin.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73:1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganime A.C., Carvalho-Costa F.A., Santos M., Costa F.R., Leite J.P., Miagostovich M.P. Viability of human adenovirus from hospital fomites. J Med Virol. 2014;86:2065–2069. doi: 10.1002/jmv.23907. [DOI] [PubMed] [Google Scholar]
- 22.Cantais A., Mory O., Pillet S., Verhoeven P.O., Bonneau J., Patural H. Epidemiology and microbiological investigations of community-acquired pneumonia in children admitted at the emergency department of a university hospital. J Clin Virol. 2014;60:402–407. doi: 10.1016/j.jcv.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez G.U., Gerba C.P., Tamimi A.H., Kitajima M., Maxwell S.L., Rose J.B. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl Environ Microbiol. 2013;79:5728–5734. doi: 10.1128/AEM.01030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parez N., Giaquinto C., Du Roure C., Martinon-Torres F., Spoulou V., Van Damme P. Rotavirus vaccination in Europe: drivers and barriers. Lancet Infect Dis. 2014;14:416–425. doi: 10.1016/S1473-3099(14)70035-0. [DOI] [PubMed] [Google Scholar]
- 25.Hall C.B. Respiratory syncytial virus: its transmission in the hospital environment. Yale J Biol Med. 1982;55:219–223. [PMC free article] [PubMed] [Google Scholar]
- 26.Oxford J., Berezin E.N., Courvalin P., Dwyer D.E., Exner M., Jana L.A. The survival of influenza A(H1N1)pdm09 virus on 4 household surfaces. Am J Infect Control. 2014;42:423–425. doi: 10.1016/j.ajic.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Grayson M.L., Melvani S., Druce J., Barr I.G., Ballard S.A., Johnson P.D. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis. 2009;48:285–291. doi: 10.1086/595845. [DOI] [PubMed] [Google Scholar]
- 28.Tuladhar E., Hazeleger W.C., Koopmans M., Zwietering M.H., Duizer E., Beumer R.R. Reducing viral contamination from finger pads: handwashing is more effective than alcohol-based hand disinfectants. J Hosp Infect. 2015;90:226–234. doi: 10.1016/j.jhin.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Brady R.R., Chitnis S., Stewart R.W., Graham C., Yalamarthi S., Morris K. NHS connecting for health: healthcare professionals, mobile technology, and infection control. Telemed J E Health. 2012;18:289–291. doi: 10.1089/tmj.2011.0147. [DOI] [PubMed] [Google Scholar]
- 30.Lopez G.U., Kitajima M., Havas A., Gerba C.P., Reynolds K.A. Evaluation of a disinfectant wipe intervention on fomite-to-finger microbial transfer. Appl Environ Microbiol. 2014;80:3113–3118. doi: 10.1128/AEM.04235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stals A., Van C.E., Uyttendaele M. Viral genes everywhere: public health implications of PCR-based testing of foods. Curr Opin Virol. 2013;3:69–73. doi: 10.1016/j.coviro.2012.11.003. [DOI] [PubMed] [Google Scholar]


