Abstract
Purpose
Mycoplasma pneumoniae (M. pneumoniae) is a common causative agent of pneumonia in children. The aim of this study is to determine whether there is any difference in selected cytokine or chemokines response in asthmatic children compared to non-asthmatic children during acute M. pneumoniae pneumonia.
Methods
Seventy-five children, 6–12 years of age, admitted with M. pneumoniae pneumonia were enrolled. Two patient groups were defined: the children with known asthma (N = 40) and non-asthmatic children (N = 35). Interleukin (IL)-18 and selected chemokines, IL-8, CXCL9, CXCL10, and regulation upon activation normal T-cell expressed and secreted (RANTES) were measured by means of ELISA in the plasma samples of the patients collected on admission. We investigated the values of these mediators in relation to the asthma status and symptom severity of the patients. Twenty age-matched, non-infected controls were also studied.
Results
Plasma levels of IL-18 and the chemokines increased significantly in the patients with M. pneumoniae pneumonia compared to non-infected, age-matched controls (P < 0.01). However, the asthmatic patients showed significantly reduced IL-18 and CXCL10 responses (P < 0.01, <0.05, respectively) and had more severe pneumonia symptoms (P < 0.01) compared to non-asthmatic patients. IL-18 was significantly lower in severe pneumonia group than in non-severe group (P < 0.05).
Conclusions
Our study suggests that IL-18 and the chemokines are importantly involved in the pathogenesis of M. pneumoniae pneumonia. It also indicates that some asthmatic children have deficient IL-18 response when affected by M. pneumoniae pneumonia, which might be associated with more severe pneumonia observed in this group of patients.
Keywords: Mycoplasma, IL-18, Chemokine, Asthma
1. Introduction
Mycoplasma pneumoniae (M. pneumoniae) is a common causative agent of pneumonia in children. Although the mechanism of M. pneumoniae pathogenesis remains to be elucidated, one of the known important components of it is the induction of various cytokines and chemokines [1], [2]. Host immune factors may influence the outcome of infection, and a recent study has reported that asthmatic children have deficient cellular and humoral responses to M. pneumoniae infection [3] . However, the difference in selected cytokine or chemokine response between the patients with known asthma and non-asthmatic patients has not been well studied yet.
Interleukin (IL)-18 is a proinflammatory cytokine which was initially identified as a co-stimulatory factor for inducing interferon (IFN)-γ [4], [5], [6]. Recent studies have investigated the role of IL-18 in the host response to infection. We have previously reported that IL-18 is significantly increased in the serum of the children with acute M. pneumoniae pneumonia [7]. Interferon-inducible protein (CXCL10/IP-10) and monokine-induced by γ-interferon (CXCL9/MIG) are chemokines known to selectively attract Th1 cells [8], [9]. IL-8 is a potent chemoattractant for neutrophils and regulated upon activation normal T-cell expressed and secreted (RANTES) is known to recruit eosinophils [8], [9]. These chemokines have also been reported to be induced by M. pneumoniae infection in different model systems [1], [10], [11].
In the present study, we evaluated the plasma levels of IL-18 and the chemokines, IL-8, CXCL9, CXCL10, and RANTES to investigate their possible roles in the pathogenesis of acute M. pneumoniae pneumonia in children. We also aimed to examine whether there is any difference in these cytokine or chemokines responses in asthmatic children compared to non-asthmatic children.
2. Materials and methods
2.1. Subjects
Seventy-five children, aged 6–12 years, admitted with M. pneumoniae pneumonia were enrolled. Diagnosis of pneumonia was based on both clinical and radiological findings.
The patients were divided into two groups: asthma group (N = 40) and non-asthma group (N = 35). Asthma was defined with the physician’s diagnosis of bronchial hyperresponsiveness upon methacholine challenge (PC20 ⩽ 16 mg/ml) or at least 12% of reversibility of FEV1 after β2 agonist inhalation. The asthma group was divided again by their atopic status: atopic asthma (N = 28) and non-atopic asthma (N = 12). Atopy was defined as having one of the following: at least one positive skin prick test (SPT) to common allergens (egg, milk, soybean, peanut, cat fur, dog hair, grass mix, tree mix, D. farinae, D. pteronyssinus, Alternaria, Cladosporium; Bencard, Brentford, UK) with >3 mm reaction to histamine. The patients who had taken systemic corticosteroid or any controller medicine within 6 weeks of admission were excluded. The non-asthma group had no previous history of asthma and showed negative results to methacholine challenge and SPT.
The severity of pneumonia was assessed on the scores from 0 to 5 according to the number of following clinical findings observed in the patients during admission (modified criteria based on the previous studies [12], [13]): fever (>38.5 °C), rapid breathing (and/or lower chest wall indrawing), decreased oxygen saturation breathing room air (<92%), more than 7 days of hospital stay, more than 2 affected pulmonary lobes on chest X-rays. The patients with severity score ⩾3 was defined as severe pneumonia group (N = 38) and ⩽2 as non-severe pneumonia group (N = 37).
Twenty age-matched controls who were admitted with minor surgical problems were also studied. The children who had no respiratory symptoms within at least 2 weeks of admission, no previous history of wheezing or asthma, and no evidence of M. pneumoniae infection were enrolled as controls.
Informed consents were obtained from all parents and this study was approved by the Catholic University of Taegu Institutional Review Board.
2.2. Diagnosis of M. pneumoniae infection
Current M. pneumoniae infection was diagnosed serologically if the subjects had both of the following criteria as suggested by a previous study [14]: (1) positive specific IgM antibodies in the first and/or second sample (2) seroconversion or fourfold or greater increase in follow-up specific IgG antibodies, or >1:640 specific IgG titer. Specific IgM antibody was determined by ELISA (Genbio, San Diego, CA), and the values were considered positive when they were above the reference range of non-infected subjects (cut-off) recommended by the manufacturers. IgG antibodies were determined using microparticle agglutination assay (MAG) (Serodia-Myco II, Fujirebio, Tokyo, Japan). The control subjects were defined to have no evidence of M. pneumoniae infection when they showed negative serologic results: neither specific IgM nor IgG response on admission.
The presence of co-infection was studied using multiplex RT-PCR (Seeplex™ RV Detection kit, Seegene, Seoul, Korea) for viral infections; respiratory syncytial virus, rhinovirus, human bocavirus, human metapneumovirus, influenza A & B, adenovirus, corona virus, parainfluenza virus and culture of nasopharyngeal aspirates was done for bacterial infections. Only the subjects who showed negative results were included in this study.
2.3. Evaluation of IL-18 and chemokines
The cytokine/chemokines response during respiratory infection is known to change over time. In the present study, the plasma samples were collected immediately after admission and stored at <−70 °C until analysis. IL-18, IL-8, CXCL9, CXCL10, and RANTES were measured using quantikine colorometric sandwich ELISA kits (R&D, Minneapolis, MN) according to the manufacturer’s instruction. ELISA sensitivity was 12.5 pg/mL for IL-18, 7.5 pg/mL for IL-8, 11.3 pg/mL for CXCL9, 4.46 pg/mL for CXCL10, and 2.0 pg/mL for RANTES, respectively. All assays were performed in duplicate for each sample, and the mean values were reported.
2.4. Statistical analysis
Analysis of data was done using SPSS win ver 13.0. For comparison between two groups, since not all distributions were normal, Mann–Whitney U test was used. Chi-square test was also used to compare the frequencies. A P < .05 was considered statistically significant. Data are presented as medians (interquartile range).
3. Results
3.1. Subject characteristics
There was no difference in age and sex ratio among two patient groups and controls. The clinical characteristics are presented in Table 1 . Pneumonia symptom severity score was significantly higher in the patients with known atopic asthma than in non-atopic, non-asthmatic patients (median, 3 vs 2, P = 0.01). The number of patients showing severe symptoms (⩾3 score) was 23 out of 40 (57%) in asthma group, and 15 out of 35 (43%) in non-asthma group, which was not significantly different (P = 0.09) (Table 1).
Table 1.
Clinical characteristics of two patient groups and controls.
| Asthma group (N = 40) | Non-asthma group (N = 35) | Controls (N = 20) | |
|---|---|---|---|
| Age (years, mean) | 6–12 (7.5) | 6–12 (8.7) | 4–15 (7.1) |
| Sex (M/F) | 21/19 | 14/21 | 9/11 |
| Severe/non-severe | 23/17 | 15/20 | |
| Severity score | 3 (2–4)⁎ | 2 (1.25–3) |
Data are presented medians (interquartile range).
P = 0.01.
3.2. IL-18 and chemokine responses in relation to the asthma status and severity of pneumonia
The plasma levels of IL-18, IL-8, CXCL9, CXCL10, and RANTES were significantly higher in the patients admitted with M. pneumoniae pneumonia compared to the non-infected controls (Table 2 ). The difference between the M. pneumoniae pneumonia patients and the non-infected controls remained even when the patients were divided into two groups according to their asthma status: asthma group and non-asthma group (data not shown). Between those two patient groups, however, IL-18 and CXCL10 levels in the patients of asthma group were significantly lower than those in non-asthma group (P < 0.01, <0.05, respectively). Meanwhile, other chemokine levels showed no significant difference between asthmatic and non-asthmatic patients (Table 3 ).
Table 2.
Increased IL-18 and chemokines in patients with M. pneumoniae pneumonia.
| Cytokine/chemokines (pg/mL) | Patients (N = 75) | Controls (N = 20) | P value |
|---|---|---|---|
| IL-18 | 491 (376–720) | 17 (12.5–58.9) | <0.01 |
| IL-8 | 13.0 (0–52) | 0 (0–0) | <0.01 |
| CXCL9 | 148 (101.8–237.1) | 83 (71.2–129.8) | <0.01 |
| CXCL10 | 180 (105.9–459.3) | 44 (34.2–73.5) | <0.01 |
| RANTES | 19,817 (18,581–20,802) | 15,399 (12,845–17,724) | <0.01 |
Data are presented as medians (interquartile range).
Table 3.
IL-18 and chemokines in asthma group vs. non-asthma group.
| Cytokine/chemokines (pg/mL) | Asthma group (N = 40) | Non-asthma group (N = 35) | P value |
|---|---|---|---|
| IL-18 | 413.6 (30.8.6–610.4) | 599.2 (456.7–937.8) | <0.01 |
| IL-8 | 13.0 (1.5–29.5) | 17.0 (1.9–53.5) | 0.6 |
| CXCL9 | 126.5 (62.66–193.83) | 161.3 (119.48–267.89) | 0.07 |
| CXCL10 | 157.2 (56.6–320.3) | 223.8 (122.0–717.9) | <0.05 |
| RANTES | 19,604 (18,326–20,756) | 19,747 (18,649–20,802) | 0.6 |
Data are presented as medians (interquartile range).
When the asthma group was divided according to the atopic status of the patients, CXCL10 showed significantly lower values in the atopic patients compared to non-atopic patients (P < 0.05). IL-18 level was also lower in atopic asthma vs. non-atopic asthma, which was not statistically significant (P = 0.1), but other chemokines did not show such difference (Table 4 ).
Table 4.
IL-18 and chemokines in atopic asthma vs. non-atopic asthma.
| Cytokine/chemokines (pg/mL) | Atopic-asthma (N = 28) | Non-atopic asthma (N = 12) | P value |
|---|---|---|---|
| IL-18 | 468.7 (358.5–603.2) | 569.7 (377.5–924.7) | 0.1 |
| IL-8 | 10.8 (1.5–26.0) | 21.9 (3–57) | 0.2 |
| CXCL9 | 158.3 (73.7–209.8) | 146.5 (115.8–256.2) | 0.5 |
| CXCL10 | 151.2 (56.6–320.3) | 200.8 (122.0–717.9) | <0.05 |
| RANTES | 19,604 (18,634–20,620) | 19,643 (18,581–20,825) | 0.7 |
Data are presented as medians (interquartile range).
When the patients were divided according to the severity of pneumonia, significantly lower IL-18 levels were observed in the severe pneumonia group compared to non-severe pneumonia group. However, CXCL10 and other chemokines showed no significant difference in relation to the severity of pneumonia (Table 5 ). Within the asthma group, significantly decreased IL-18 response was still observed in the patients with severe pneumonia compared to the patients with non-severe pneumonia 323.6 (263.0–493.4) vs. 486.1 (362.5–907.6) pg/mL, P < 0.05). Within the non-asthma group, there was a trend toward lower IL-18 levels in the patients with severe pneumonia compared to the patients with non-severe pneumonia, but it did not reach statistical significance 517.3 (453.2–665.8) vs. 765.1 (481.3–962.1) pg/mL, P = 0.09 (Fig. 1 ).
Table 5.
IL-18 and chemokine levels in severe group vs. non-severe group.
| Cytokine/chemokines (pg/mL) | Severity score (⩾3) (N = 38) | Severity score (⩽2) (N = 37) | P value |
|---|---|---|---|
| IL-18 | 457.1 (327.1–623.5) | 608.6 (415.8–956.3) | <0.05 |
| IL-8 | 16.5 (1.5–48.8) | 3.4 (1.5–42.0) | 0.5 |
| CXCL9 | 125.5 (103.1–194.5) | 184.1 (102.8–268.0) | 0.1 |
| CXCL10 | 183.2 (110.1–730.3) | 158.8 (69.1–387.4) | 0.4 |
| RANTES | 19,707 (18,653–21,429) | 19,600 (18,581–20,575) | 0.4 |
Data are presented as medians (interquartile range).
Fig. 1.
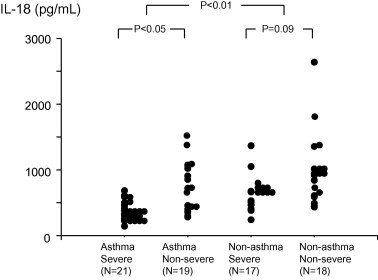
IL-18 levels in asthma group were significantly lower than those in non-asthma group. Within the asthma group, significantly decreased IL-18 response was observed in the patients with severe pneumonia compared to the patients with non-severe pneumonia.
4. Discussion
The present study demonstrated that the plasma levels of IL-18 and selected chemokines, IL-8, CXCL9, CXCL10, and RANTES were significantly increased in the patients with M. pneumoniae pneumonia than non-infected controls. However, it also shows that the patients with known asthma showed significantly reduced IL-18 and CXCL10 responses and had more severe pneumonia symptoms compared to non-asthmatic patients. Moreover, IL-18 level was significantly lower in severe pneumonia group than in non-severe group.
IL-18 has been demonstrated to have many biologic activities, including enhancement of Th1 cell activity and functional development of natural killer (NK) cells. IL-18 was reported to be produced during clinical and various animal models of infection and play a role in host defense against some microbial and fungal pathogens [15], [16], [17], [18]. The role of IL-18 in the pathogenesis of bacterial pneumonia has also been studied. Importantly, IL-18 was shown to improve early host response and have a protective role during Streptococcus pneumoniae pneumonia [19]. More recently, delayed bacterial clearance and higher bacterial loads were observed in the lungs of IL-18 knockout mice during pulmonary infection due to Hemophilus influenzae [20] .
In the present study, plasma levels of IL-18 and CXCL10 were significantly increased in the patients with M. pneumoniae pneumonia compared to non-infected controls. Our results show that these mediators are involved in the pathogenesis of this acute respiratory infection. The values of IL-18 and CXCL10, however, were significantly lower in the group of children with known asthma than those in non-asthma group. When the asthma group was divided according to the atopic status of the patients, CXCL10 was significantly lower and IL-18 showed a tendency toward lower levels in atopic asthma compared to non-atopic asthma. Our finding seems to be consistent with the previous studies that reported significantly decreased serum concentrations of IL-18 [21], [22] and CXCL10 [23] in the patients with asthma or allergic rhinitis. When the patients were divided according to the severity of pneumonia, IL-18 response was significantly decreased in the severe pneumonia group compared to the non-severe group. Decreased IL-18 response in severe pneumonia group vs. non-severe group was observed regardless of asthma status of the patients, which reached statistical significance only within asthma group. A recent study has demonstrated that asthmatic children have deficient cellular and humoral immune responses to respiratory infection with M. pneumoniae and may suffer prolonged or persistent infection [3]. In our study, asthma group presented significantly higher symptom score than non-asthma group, despite the fact that the number of subjects showing severe symptom was not different between the two patient groups. Together with the previous studies, our results suggest that some asthmatic children are more likely to experience severe symptoms when affected by M. pneumoniae pneumonia, which might be associated with deficient early IL-18 response.
Our findings differ somewhat from those of a prior study that showed increased serum IL-18 in adult patients with M. pneumoniae pneumonia but higher levels in severe cases than in mild cases [24]. The inconsistency between those results and ours may be partially explained by different age distribution and clinical characteristics of the enrolled subjects and different criteria for severe group between the two studies. Different functions of proinflammatory cytokines in experimental pneumonia caused by different respiratory pathogens have been demonstrated. Contrary to the studies showing a protective role of IL-18 during S. pneumoniae or H. influenzae pneumonia stated above [19], [20], IL-18 was reported to impair the pulmonary host defense against Pseudomonas aeruginosa [25] . It was suggested that role of an individual cytokine such as IL-18 may be determined by the underlying state of host and the character of infecting agent [20]. Taken together with those previous studies, our findings suggest that further studies seem to be needed to find out whether the asthma- or severity-related difference in IL-18 response is specific for M. pneumoniae pneumonia in children.
We also studied other chemokines, IL-8, CXCL9, and RANTES that were reported to be induced by M. pneumoniae infection in different model systems. They were significantly increased in the patient group compared to non-infected controls. Our results, in part, support the previous studies that showed increased IL-8 in pleural fluid of the patients with M. pneumoniae pneumonia [10] and increased induction of RANTES in airway epithelial cells infected by M. pneumoniae [11]. However, none of them showed any asthma- or severity-related difference.
Our study has some limitations. One of them is that we did not evaluate IL-18 and CXCL10 in relation to either bacterial clearance or induction of other inflammatory cytokines, which would be necessary to clarify their regulative role in M. pneumoniae infection. Additionally, although all the samples were collected on admission with the aim to observe the role of the mediators in the early host response to M. pneumoniae infection, we are not certain if they were collected at the same stage of infection.
In summary, our study showed that IL-18 and selected chemokines increased significantly in the children with M. pneumoniae pneumonia, which suggests that these mediators are importantly involved in the pathogenesis of this acute respiratory infection. However, the patients with known asthma showed significantly decreased IL-18 and CXCL10 responses and experienced more severe pneumonia compared to non-asthmatic patients. IL-18 level was significantly lower in severe pneumonia group than in non-severe group. Our results suggest that some asthmatic children have deficient IL-18 response when affected by M. pneumoniae pneumonia, which might be associated with more severe pneumonia observed in this group of patients.
References
- 1.Yang J., Hooper W.C., Phillips D.J., Talkington D.F. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004;15:157–168. doi: 10.1016/j.cytogfr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Salvatore C.M., Techasaensiri C., Tagliabue C., Katz K., Leos N., Gomez A.M. Tigecycline therapy significantly reduces the concentrations of inflammatory pulmonary cytokines and chemokines in a murine model of Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2009;53:1546–1551. doi: 10.1128/AAC.00979-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson T.P., Duffy L.B., Pendley D., Dai Y., Cassell G.H. Deficient immune response to Mycoplasma pneumoniae in childhood asthma. Allergy Asthma Proc. 2009;30:158–165. doi: 10.2500/aap.2009.30.3207. [DOI] [PubMed] [Google Scholar]
- 4.Akira S. The role of IL-18 in innate immunity. Curr Opin Immunol. 2000;12:59–63. doi: 10.1016/s0952-7915(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello C.A. Interleukin-18. Methods. 1999;19:121–132. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K., Tsutsui H., Yoshimoto T., Adachi O., Yoshida N., Kishimoto T. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 7.Chung H.L., Kim S.G., Shin I.H. The relationship between serum endothelin (ET)-1 and wheezing status in the children with Mycoplasma pneumoniae pneumonia. Pediatr Allergy Immunol. 2006;17:285–290. doi: 10.1111/j.1399-3038.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonecchi R., Galliera E., Borroni E.M., Corsi M.M., Locati M., Mantovani A. Chemokines and chemokine receptors: an overview. Front Biosci. 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F., Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9:949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- 10.Narita M., Tanaka H., Yamada S., Abe S., Ariga T., Sakiyama Y. Significant role of interleukin-8 in pathogenesis of pulmonary disease due to Mycoplasma pneumoniae infection. Clin Diagn Lab Immunol. 2001;8:1028–1030. doi: 10.1128/CDLI.8.5.1028-1030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dakhama A., Kraft M., Martin R.J., Gelfand E.W. Induction of regulated upon activation, normal T cells expressed and secreted (RANTES) and transforming growth factor-beta 1 in airway epithelial cells by Mycoplasma pneumoniae. Am J Respir Cell Mol Biol. 2003;29:344–351. doi: 10.1165/rcmb.2002-0291OC. [DOI] [PubMed] [Google Scholar]
- 12.Mathisen M., Strand T.A., Sharma B.N., Chandyo R.K., Valentiner-Branth P., Basnet S. Clinical presentation and severity of viral community-acquired pneumonia in young Nepalese children. Pediatr Infect Dis J. 2010;29:e1–e6. doi: 10.1097/INF.0b013e3181c2a1b9. [DOI] [PubMed] [Google Scholar]
- 13.Kin Key N., Araújo-Neto C.A., Nascimento, Carvalho C.M. Severity of childhood community-acquired pneumonia and chest radiographic findings. Pediatr Pulmonol. 2009;44:249–252. doi: 10.1002/ppul.20988. [DOI] [PubMed] [Google Scholar]
- 14.Souliou E., Almasri M., Papa A., Theodoridou A., Diza E. Laboratory diagnosis of Mycoplasma pneumoniae respiratory tract infections in children. Eur J Clin Microbiol Infect Dis. 2007;26:513–515. doi: 10.1007/s10096-007-0326-0. [DOI] [PubMed] [Google Scholar]
- 15.Netea M.G., Fantuzzi G., Kullberg B.J., Stuyt R.J., Pulido E.J., McIntyre R.C., Jr Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol. 2000;164:2644–2649. doi: 10.4049/jimmunol.164.5.2644. [DOI] [PubMed] [Google Scholar]
- 16.Mastroeni P., Clare S., Khan S., Harrison J.A., Hormaeche C.E., Okamura H. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–483. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohn E., Sing A., Zumbihl R., Bielfeldt C., Okamura H., Kurimoto M. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 18.Stuyt R.J., Netea M.G., Verschueren I., Fantuzzi G., Dinarello C.A., Van Der Meer J.W. Role of interleukin-18 in host defense against disseminated Candida albicans infection. Infect Immun. 2002;70:3284–3286. doi: 10.1128/IAI.70.6.3284-3286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauw F.N., Branger J., Florquin S., Speelman P., van Deventer S.J., Akira S. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J Immunol. 2002;168:372–378. doi: 10.4049/jimmunol.168.1.372. [DOI] [PubMed] [Google Scholar]
- 20.Wieland C.W., Florquin S., van der Poll T. Interleukin 18 participates in the early inflammatory response and bacterial clearance during pneumonia caused by nontypeable Haemophilus influenzae. Infect Immun. 2007;75:5068–5072. doi: 10.1128/IAI.00287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ariano R., Merendino R.A., Minciullo P.L., Salpietro C.D., Gangemi S. Specific immunotherapy effect on interleukin-18 and CD30 serum levels in monosensitized patients with rhinitis. Allergy Asthma Proc. 2003;24:179–183. [PubMed] [Google Scholar]
- 22.Cebeci A.N., Nuhoglu Y., Arslanoglu I., Erguven M., Agachan N. The role of IL-18 in Th1/Th2 balance in children. Allergy Asthma Proc. 2006;27:365–370. doi: 10.2500/aap.2006.27.2894. [DOI] [PubMed] [Google Scholar]
- 23.Lun S.W., Wong C.K., Ko F.W., Ip W.K., Hui D.S., Lam C.W. Aberrant expression of CC and CXC chemokines and their receptors in patients with asthma. J Clin Immunol. 2006;26:145–152. doi: 10.1007/s10875-006-9003-9. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka H., Narita M., Teramoto S., Saikai T., Oashi K., Igarashi T. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121:1493–1497. doi: 10.1378/chest.121.5.1493. [DOI] [PubMed] [Google Scholar]
- 25.Schultz M.J., Knapp S., Florquin S., Pater J., Takeda K., Akira S. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun. 2003;71:1630–1634. doi: 10.1128/IAI.71.4.1630-1634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


