Abstract
Background: The existence of human enteric coronavirus (HEC) has been debated since its first description in stool by electron microscopy (EM) in 1975. Needed to resolve the issue is its cultivation in readily available cell lines.
Objectives: To grow HEC in cell lines. To describe its characteristics and to differentiate it from other human and animal coronaviruses.
Study design: Originally grown in human fetal intestinal organ culture, HEC was passed in J774 cells (a mouse macrophage cell line) and C6/36 cells (a mosquito cell line). Its cytopathic effect (CPE) and pattern of immunofluorescence were described. Its appearance was ascertained by negative staining and transmission EM. Its structural proteins were delineated by polyacrylamide gel electrophoresis (PAGE) and Western blotting (WB). The antigenic character of the virus was determined by immunofluorescence and WB. Agglutination with mouse erythrocytes was performed.
Results: In J774 cells, HEC induced the formation of giant cells and small syncytia. Immunofluorescence in both J774 and C6/36 cells was limited to the cytoplasm. Studies with transmission EM revealed the virus to have the typical appearance of other coronaviruses, to be 80–120 nm in diameter, and to bud into cysternae of the endoplasmic reticulum. By PAGE and WB, its major protein has an average molecular weight (MW) of 41 kilodaltons (kDa). Two other proteins had MWs of 190 and 24 kDa. By immunofluorescence and WB, HEC is antigenically distinct from human coronaviruses 0C43 and 229E and mouse hepatitis virus (A59 strain). Preparations of HEC did not agglutinate mouse erythrocytes.
Conclusion: We conclude that HEC is a human coronavirus that is antigenically unrelated to 0C43 and 229E viruses. Growth of HEC in readily available cell lines should aid in elucidating its role as a pathogen in human diarrheal illnesses.
Keywords: Human enteric coronavirus, J774 cells, C6/36 cells, Adaptation, Growth in cell lines
1. Introduction
Since the first reports in 1975 of its presence in stool as determined by electron microscopy (EM), the existence of human enteric coronavirus (HEC) has been debated (Caul et al., 1975, Mathan et al., 1975). Some investigators have considered these particles as viruses and as potential pathogens in human diarrhea, necrotizing enterocolitis, and malabsorption. Others cite their variable size and appearance, their presence in control stool specimens and failure to be cultivated in tissue culture as evidence that they might represent cell debris or even portions of other microorganisms (Macnaughton and Davies, 1981). Critical to its acceptance as a virus and a potential human pathogen is its growth in (a) readily available cell line(s) and the accessibility of virus seed stocks in a depository like the American Type Culture Collection (ATCC). It will also be important to exclude the possibility that HEC is not a known human respiratory coronavirus strain (229E or OC43) that for some reason has been found in stool or that the virus does not represent a laboratory contaminant of a known animal coronavirus, such as mouse hepatitis virus (MHV).
In 1982–1983, three clusters of cases of necrotizing enterocolitis (NEC) occurred at the Special Care Nursery, Parkland Memorial Hospital, Dallas, TX (Resta et al., 1985). In contrast to endemic NEC, these outbreaks were in older, higher birth weight infants and the case fatality rate was lower. Coronavirus-like particles (CVLPs) were seen in stools by EM and the virus could be passed in human fetal intestinal organ culture. However, embryonic tissues were available in only a few centers and soon we also could not obtain these tissues. This report details the adaptation of HEC to a mouse peritoneal macrophage cell line (J744 cells), and to a mosquito cell line derived from Aedes albopictus mosquitoes (C6/36 cells). J774 cells were used because they support the growth of MHV. The use of C6/36 cells was a chance event. These cells were growing in the laboratory and we included them in the cells that were tested.
2. Materials and methods
Human fetal intestinal (HFI) organ cultures were prepared and used as outlined (Resta et al., 1985). The J774 and C6/36 cells were obtained from the ATCC. The cell lines were fed every 3 days with RPMI medium, 2% fetal calf serum, glutamine, penicillin and amikacin. The C6/36 cells were maintained at 28°C while the other cells were kept at 37°C. Virus seed stock was prepared by passing HEC 15 times in HFI organ cultures and three times in J774 cells. The material from this last passage was frozen and thawed once and 1 ml of the resuspended slurry was placed into nunc vials and stored at −70°C. It has been determined to be bacteriologically sterile and free from mycoplasma contamination and has been sent to the ATCC. For demonstration of the virus and its antigenic characteristics, this virus seed stock, a later passage in J774 cells or low passage virus in C6/36 cells were used. Virus was also grown in medium containing 10 μgm/ml of trypsin (Storz et al., 1981).
Fluorescence microscopy was performed by preparing a suspension of cells; low speed centrifugation at room temperature, resuspending the cells in phosphate buffered saline (PBS), recentrifugation and final resuspension of the cells. Ten wells of a multi-welled slide were filled with this final suspension, air dried and fixed in acetone at −20°C for 10 min. Slides were stored at −20°C. Infected cells and uninfected control cells were overlaid with immune and non-immune human sera, incubated at 37°C for 30 min, washed with PBS and then stained with flouresceinated anti-human Ig (IgG, IgA, and IgM) or anti-human IgM or anti-animal IgG. J774 cells are known to contain receptors for the Fc portion of IgG. To perform flourescence microscopy on these cells, we used only anti-human IgM conjugates.
Negative stain electron microscopy (EM) was performed by fixing the supernatants of infected cells with 2% glutaraldehyde, low speed centrifugation after 24 h fixation and subsequent centrifugation of the remaining supernatant at 130 000 g for 90 min at 4°C. The precipitate was resuspended in distilled water. Negative staining was accomplished with 2% phosphotungstic acid (PTA) at a pH of 7.2. Transmission EM with J774 cells was performed by standard methods (Bencosme and Tsutsumi, 1970).
Preparations of HEC for polyacrylamide gel electrophoresis (PAGE) and Western blotting (WB) were made by growing the virus in J774 and C6/36 cells. When the infected cell suspension as determined by immunofluorescence approximated 25%, the cells were scraped from the surface of the flask, the contents frozen and thawed once and the supernatant was separated after centrifugation at 10 000 g for 20 min at 4°C. The saline concentration of the supernatant was adjusted to 2.3% and polyethylene glycol (PEG) added to a concentration of 7%. After standing overnight at 4°C, the solution was centrifuged at 10 000 g for 20 min at 4°C. The PEG precipitate was solubilized by adding ethylenediaminotetraacetic acid in normal saline that was buffered at a pH of 7.2. The resulting solution was placed on a 20–60% sucrose gradient and centrifuged at 130 000 g for 18 h at 4°C. The bands obtained at or near mid-gradient were subjected to PAGE and silver stained.
WB was accomplished by taking the bands in the mid or near-mid sucrose gradient, adding a buffer containing sodium dodecylsulfate (SDS) without a reducing substance and heated at 100°C for 5 min. The solubilized proteins were separated by PAGE in a tris-buffered (pH 8.2) solution containing glycine and SDS. The PAGE separated proteins were transferred to a nitrocellulose membrane and blocked with 5% bovine serum albumin and 0.2% NP40 in TBS overnight. After drying, strips were cut for WB. The WB strips were reacted overnight with sera at a 1:50 dilution followed by the addition of biotinylated anti-human or animal antisera (1:250 dilution), streptavidin peroxidase (1:250 dilution) followed by color development after the addition of a chromogenic substrate. Between each step, the strips were washed three times in tris-buffered saline. The biotinylated antisera and streptavidin peroxidase were obtained from Kirkegaard and Perry Laboratories, Gaithersburg, MD. Hemagglutination studies were performed with mouse erythryocytes, 0.05%, suspended in PBS, pH 7.4. OC43 and 229E antigens and antisera were obtained from Dr John Hierholzer of the Centers for Disease Control, Atlanta, GA. For WB, antigens were further purified by equilibrium sucrose density ultracentrifugation. Dr Kathryn Holmes of the University of Colorado Medical Center, Denver, CO supplied rabbit antiserum (α N) against the N protein of mouse hepatitis virus (MHV A59).
3. Results
The addition of 0.2 ml of the virus seed stock caused the appearance of a cytopathic effect (CPE) in J774 cells in 7–14 days. The CPE was accelerated by the addition of 10 μgm/ml of trypsin. The CPE consisted of the formation of giant cells and small syncytia. In C6/36 cells there was no apparent CPE. Immunofluorescent staining of the cytoplasm of both cell types could be demonstrated by the addition of immune but not non-immune sera followed by the appropriate fluoresceinated conjugate. Immunofluorescence appeared first in J774 cells and soon after in C6/36 cells; in an occasional titration in C6/36 cells, immunofluorescence appeared late but was always present 21 days after inoculation. With J774 cells, the addition of fluoresceinated anti-human Ig resulted in fluorescence of all the cells because these cells contain receptors for the Fc fragment of IgG. Specific reactions in J774 cells could only be demonstrated with the use of immune sera and a fluoresceinated anti-Hu IgM conjugate. Initially, the only IgM antibody positive sera we had were from the infants identified in the epidemic.
Patients with human immunodeficiency virus (HIV) infection have been noted to have CVLPs in stool (Kern et al., 1985, Grohmann et al., 1993). We surveyed a group of these patients and found that some of them had high titers of Ig and IgM antibody to HEC. By using these sera, we were able to follow passages and determine whether or not the quantity of virus was increasing. Because of the presence of Fc receptors on J774 cells, we could not determine whether mouse antiserum to OC43 virus or guinea pig antiserum to 229E virus would specifically stain infected cells. However, C6/36 cells could be utilized. Although infected C6/36 cells were brightly stained by human immune sera, they were not stained by a 1:10 dilution of OC43 antiserum and only non-specifically by 229E antiserum. This non-specific immunofluorescence affected all cells, was identical in control cells, was not reduced by prior reaction with immune human sera and completely disappeared at a 1:40 dilution.
Transmission electron microscopy (TEM) of infected J774 cells revealed the presence of CVLPs budding into cysternae of the endoplasmic reticulum (Fig. 1 ). An interior nucleocapsid-like structure could be seen along with elongated peplomers which had clubbed ends. By TEM, the CVLPs had diameters ranging from 80 to 120 nm. Negatively stained EMs of ultracentrifuged glutaraldehyde fixed supernatants from both cell types revealed CVLPs (Fig. 2 ). In negatively stained EMs, the CVLPs were more pleomorphic, ranging from 70 to 125 nm in diameter. A rare particle had a diameter of 220 nm. For 20 particles with typical morphology, the median and mode values were 100 nm each. PEG precipitated virus prepared in J774 cells were subjected to equilibrium sucrose density ultracentrifugation. Two bands at the mid-center of the gradient were collected, subjected to PAGE and silver stained (Fig. 3 ). The band on the right was at mid-qradient, 4.8 cm from the bottom. The band on the left was collected 5.9 cm from the bottom of the gradient.
Fig. 1.
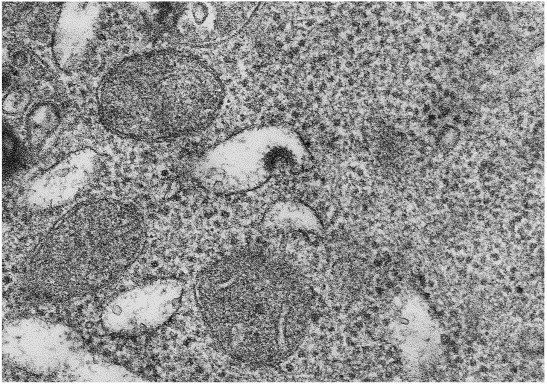
Transmission electron microscopy of infected J774 cells. The picture is at a magnification of 112 500.
Fig. 2.
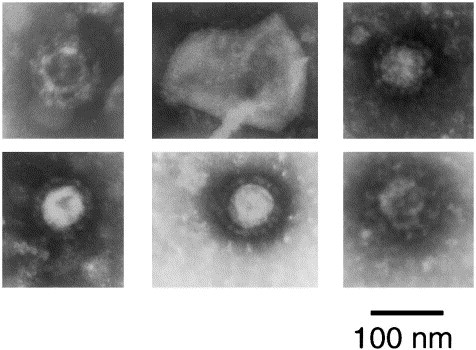
CVLPs from negatively stained resuspended pellets prepared from supernatants of infected cells. Row 1, frames 1 and 2: J774 cells. Row 1, frame 3, row 2, frame 1: C6/36 cells. Row 2, frames 2 and 3: pooled supernatants from J774 and C6/36 cells at 13 and 20 days after inoculation of virus seed stock.
Fig. 3.
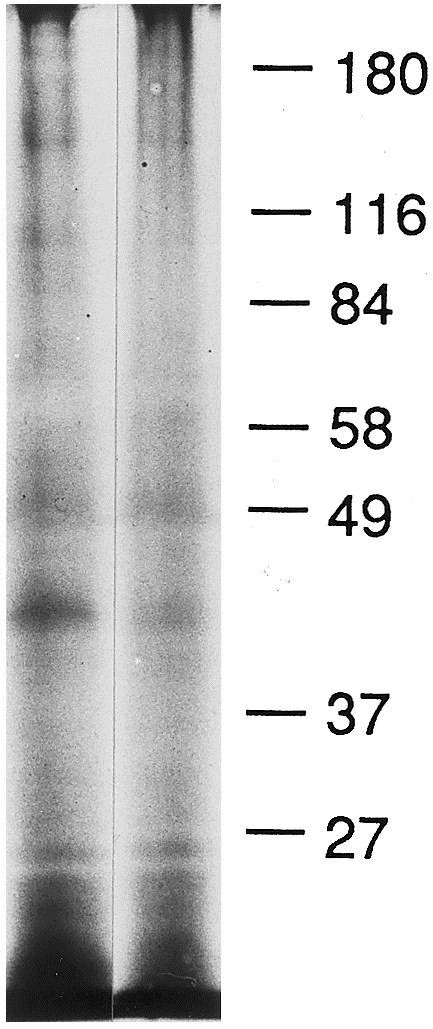
PAGE of PEG precipitate centrifuged on a 20–60% sucrose gradient at 130 000 g for 18 h at 4°C and silver stained. The column on the right was from a band at mid-gradient; the column to the left was from a band just above mid-gradient.
Western blots were prepared using sucrose gradient purified OC43, 229E, and HEC viruses ( Fig. 4, Fig. 5, Fig. 6 ). With the OC43 WB, staining was seen with OC43 antiserum and the α N antiserum to the N protein of MHV (strain A59). There was no staining with 229E antiserum or with Patient 1, IgM. Patients 1, 3, 5 and 6 had AIDS. Patient 2 was from a developing country and Patient 4 was an infant involved in the original nursery outbreak. With the 229E WB, staining is seen with 229E antiserum but not OC43 nor α N nor Patient 1 (Ig). The HEC WB shows three preparations from different samples of infected J774 cells. Note in panel 1 the reaction of Patient 1, IgM to two protein bands located below 49 kDa and the absence of any reactions to these bands with 229E, OC43, and α N antisera. The second and third panels demonstrate IgM antibody reactions to the protein band below 49 kDa as well as to other proteins. A WB was also prepared from infected C6/36 cells and similar and additional bands were observed (Fig. 7 ). Since we used prestained molecular weight markers, we can only estimate the molecular weights of HEC. In average, the proteins range in molecular weight from 24 to 190 kDa; the major protein (N) centers at about 41 kDa. Two other protein bands are seen, one at 124 kDa and the other at 64 kDa, particularly in the C6/36 cell preparation. These bands are similar to and different from the OC43 and 229E WBs in which the same pre-stained molecular weight markers were used. Preparations of HEC did not agglutinate mouse erythrocytes. We had no success in passaging this virus in other cell lines (MRC-5, A549) nor intracerebrally in suckling mice. Human colonic tumor (HCT-8) cells did not consistently replicate the virus.
Fig. 4.

Western blot of OC43 antigen and various antisera. Note that OC43 and A N antisera stain the major protein N at 49 kDa whereas Patient 1 (Ig and IgM) does not nor does antiserum to 229E.
Fig. 5.
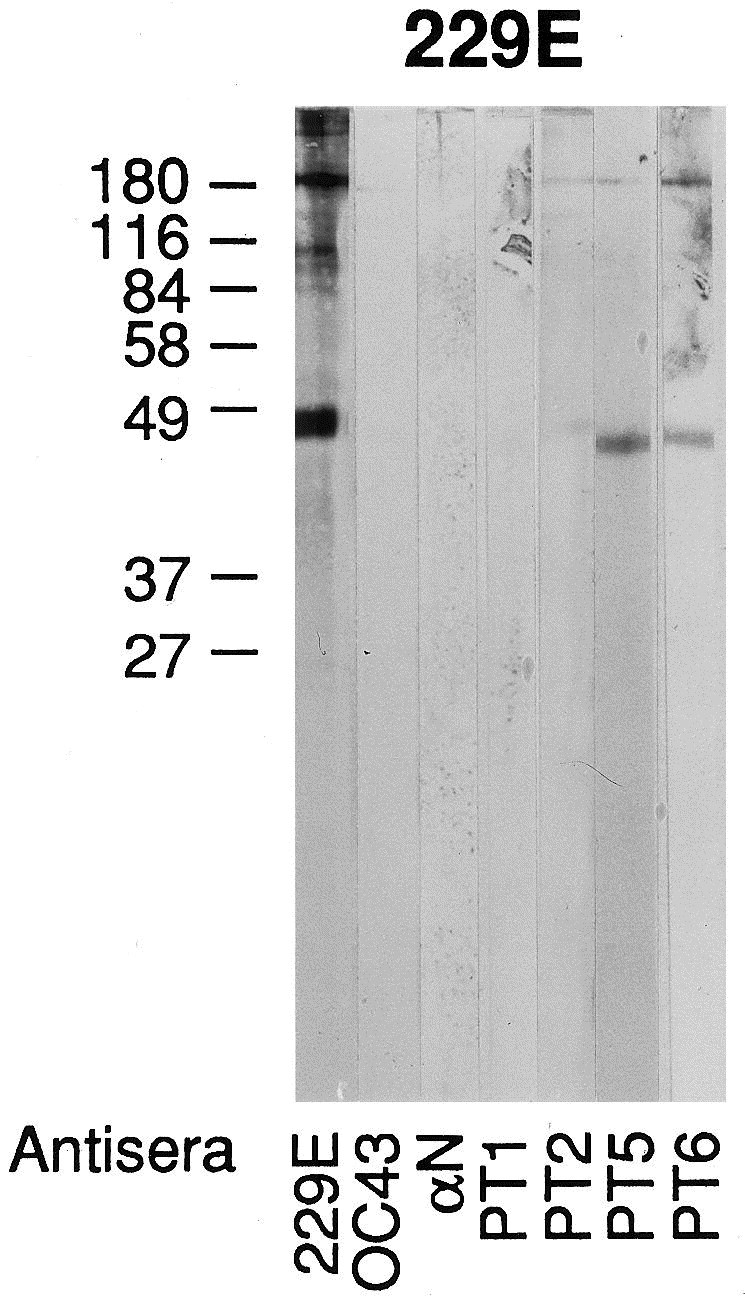
Western blot of 229E antigen and various antisera. Note that 229E antiserum stains the major protein N at or around 49 kDa but this protein is not stained by antisera to OC43, A N or from Patient 1 (Ig).
Fig. 6.
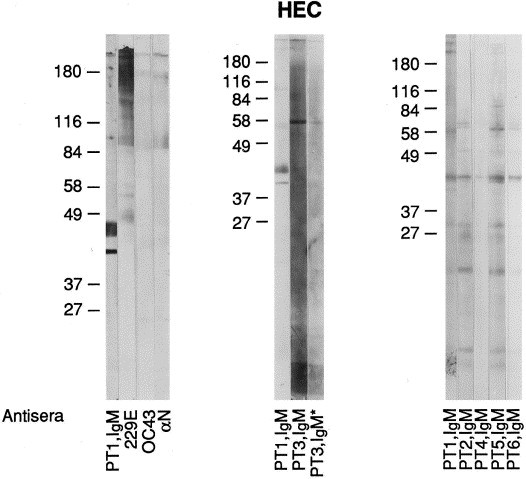
Western blot of HEC preparations from J774 cells. Note patient reactions to the major protein centering at 41 kDa where antisera to 229E, OC43, and α N do not stain this protein. PT3, IgM* represents a serum specimen obtained from patient 3 at a later date.
Fig. 7.
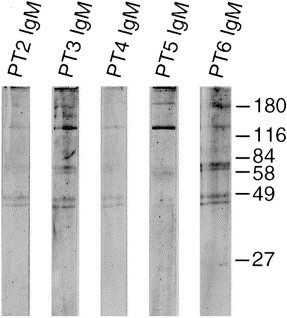
Western blot of HEC purified from infected C6/36 cells and human immune IgM positive antisera. Sera from these patients (Ig) stained the cytoplasm of infected C6/36 cells but antisera to OC43 and 229E viruses did not.
4. Discussion
We have shown the appearance of CPE in J774 cells after the addition of HEC. Viral immunofluorescence appears along with the presence of CPE in these cells and is localized to the cytoplasm. In C6/36 cells, there was no CPE but viral immunofluorescence appeared at about the same time as it did in J774 cells; occasionally immunofluorescence appeared later in C6/36 cells but was always present at 21 days after inoculation. We have shown that CVLPs are present in cysternae of the endoplasmic reticulum of infected J774 cells and have diameters ranging from 80 to 120 nm. After fixation with glutaraldehyde, the supernatants were subjected to ultracentrifugation, the precipitates resuspended and then negatively stained with PTA, pH 7.2. The preparations showed typical CVLPs that were slightly more pleomorphic than seen with TEM. Human immune sera stained the cytoplasm of both infected cell types. Mouse anti-OC43 and guinea pig anti-229E failed to stain infected C6/36 cells. We could not stain J774 cells with these reagents because these cells have Fc receptors for IgG and fluoresce non-specifically when these latter reagents were used.
PAGE of a sucrose equilibrium density gradient revealed silver stained protein bands consistent with these particles being coronaviruses. Western blots of preparations of infected J774 cells and C6/36 cells showed bands that were consistent with those of other coronaviruses yet had some properties that were distinctive from OC43 and 229E viruses. With WBs prepared from infected J774 cells, we obtained bands with IgM positive human antisera that corresponded to those seen by PAGE and silver staining. Staining the HEC WBs with anti-OC43, 229E and α N antisera prepared against the A59 stain of MHV did reveal some non-specific staining but the antisera did not stain the N protein of HEC while IgM antibody of patients stained this protein. Non-specific IgG staining was observed most probably because we were not able to purify the preparations from the Fc receptors for IgG that were on the J774 cells. Antiserum from Patient 1 (Ig and IgM) did not stain the N protein of OC43 and 229E viruses. In WBs prepared from infected C6/36 cells, the IgM of several patients stained appropriate bands. The immunofluorescent stains of infected C6/36 cells revealed specific fluorescence with these antisera but not with antisera to OC43 and 229E viruses.
We conclude from these studies that a coronavirus has been isolated form human feces and that now can be passaged in cell lines. It has the EM appearance and size of a coronavirus and proteins consistent with that of other coronaviruses but distinctive in several regards. These proteins react with immune human sera. Antisera to OC43, 229E viruses, and α N antiserum against MHV (A59) do not stain the N protein of HEC. By averaging different preparations, the proteins of HEC range in molecular weight from 24 kDa, presumably the M protein to 190 kDa, the S protein. Inconstant bands are present at 124 and 64 kDa and could represent breakdown products of the S protein. The major protein, N, appears to center around 41 kDa, a size different from OC43 and 229E viruses. Antibodies to HEC may be prevalent in certain populations as determined by our capacity to obtain sera with IgM reactivity. The patients with AIDS had sera collected in the 1980s, a relatively early part of the HIV epidemic.
As determined by our transmission EMs, the nucleocapsid of HEC is typical of coronaviruses and different from that of toroviruses, a member of the family Coronaviridae (Beards et al., 1984, Koopmans and Horzinek, 1994). Toroviruses can be found in stools of animals and humans and have elongated peplomers. They have, however, a morphologically unique nucleocapsid that is coiled into a hollow tube with a diameter of 23 nm and an average length of 104 nm. The molecular weights of torovirus proteins are different from that of coronaviruses.
Bovine coronavirus (BCV) antibody has been found in human sera and there is a report of diarrhea occurring in a 6-year-old boy having contact with calves in whom a virus most probably identical with BCV was isolated (Storz and Rott, 1981, Zhang et al., 1994). That virus differs form the HEC described in this report in that it has an N protein that approximates 50 kDa and also has a hemagglutinin/esterase glycoprotein. The N protein of BCV has an antigenic relation to the N protein of OC43 whereas the N protein of HEC is apparently distinct from OC43. As far as can be determined, HEC has no hemagglutinating activity toward mouse erythrocytes although BCV does have that capacity.
The first reports of CVLPs in stools of patients with diarrhea, were documented by EM. Caul and associates reported cultivation of these particles in human embryo intestinal organ culture and human embryo kidney monolayers (Caul and Clarke, 1975). They were not able to prepare virus seed stocks that could be distributed to other investigators. In the early 1980s, four investigative groups found evidence of these particles in stool and tried to relate them to disease. In Paris, CVLPs were associated with outbreaks of NEC in 1979 and 1980 in separate hospitals within the city (Chaney et al., 1982, Rousset et al., 1984). In Italy, they were associated with childhood diarrhea and Gerna and colleagues presented evidence that linked them antigenically to OC43 virus (Gerna et al., 1981, Gerna et al., 1984, Gerna et al., 1985). In Arizona, Ray and associates related their presence to diarrheal disease in the nursery and then to diarrhea in the community (Vaucher et al., 1982, Mortensen et al., 1985). In Dallas, Texas in 1982–1983, several outbreaks of NEC and diarrhea occurred in a Special Care Nursery at Parkland Memorial Hospital (Resta et al., 1985). The disease outbreaks suggested a transmissible agent since they affected older children, had a lower case fatality rate, tended to occur as clusters of cases, and disease incidence could be related to breaks in enteric isolation techniques. Using stool samples from these infants, a coronavirus was isolated in human fetal intestinal organ cultures.
Since these tissues are no longer available, we attempted to pass these viruses to cell lines. In this report, we detail their passages in J774 and C6/36 cells and further describe the characteristics of this agent. We have sent this passage virus to the ATCC with aliquots of human immune serum (Ig and IgM positivity) to help identify its presence in tissue culture cells and it now is in the process of authentication. Articles have consistently cited the difficulties in studying these particles and assigning them pathogenic significance (Schnagl et al., 1978, Schnagl et al., 1979, Rettig and Altshuler, 1985). The availability of HEC to interested investigators now should enable a more complete definition of the virus and aid in its identification as a potential pathogen in human diarrheal illnesses.
Acknowledgments
This project was supported in part by a grant from the Thrasher Research Fund, Salt Lake City, UT 84150. We are grateful to the technical expertise of Linda Boney and the secretarial excellence of Lucy Dodd.
References
- Beards G.M, Green J, Hall C, Flewett T.H, Lamouliatte F, Du Pasquier P. An enveloped virus in stools of children and adults with gastroenteritis that resembles the Breda virus of calves. Lancet. 1984;1(8385):1050–1052. doi: 10.1016/S0140-6736(84)91454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencosme S.A, Tsutsumi V. A fast method for processing biologic material for electron microscopy. Lab Invest. 1970;23:447–450. [PubMed] [Google Scholar]
- Caul E.O, Clarke S.K.R. Coronavirus propagated from patient with non-bacterial gastroenteritis. Lancet. 1975;2:953–954. doi: 10.1016/S0140-6736(75)90363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caul E.O, Paver W.K, Clarke S.K.R. Coronavirus particles in faeces from patients with gastroenteritis. Lancet. 1975;1:1192. doi: 10.1016/S0140-6736(75)93176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney C, Moscovici O, Lebon P, Rousset S. Association of coronavirus infection with neonatal necrotizing enterocolitis. Pediatrics. 1982;69:209–214. [PubMed] [Google Scholar]
- Gerna G, Cereda P.M, Revello M.G, Cattaneo E, Battaglia M, Gerna M.T. Antigenic and biological relationships between human coronavirus OC43 and neonatal calf diarrhoea coronavirus. J Gen Virol (Lond) 1981;54:91–102. doi: 10.1099/0022-1317-54-1-91. [DOI] [PubMed] [Google Scholar]
- Gerna G, Passarani N, Cereda P.M, Battaglia M. Antigenic relatedness of human enteric coronavirus strains to human coronavirus OC43: a preliminary report. J Infect Dis. 1984;150:618–619. doi: 10.1093/infdis/150.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G, Passarani N, Battaglia M, Rondanelli E.G. Human enteric coronaviruses: antigenic relatedness to human coronavirus OC43 and possible etiologic role in viral gastroenteritis. J Infect Dis. 1985;151:796–803. doi: 10.1093/infdis/151.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann GS, Glass RI, Pereira HG, Monroe SS, Hightower RW, Bryan RT for the Enteric Opportunistic Infections Working Group. Enteric viruses and diarrhea in HIV-infected patients. New Engl J Med 1993;329(1):14–20. [DOI] [PubMed]
- Kern P, Muller G, Schmitz H, Racz P, Meigel W, Riethmuller G, Dietrich M. Detection of coronavirus-like particles in homosexual men with acquired immunodeficiency and related lymphadenopathy syndrome. Klin Wochenschrift. 1985;63:68–72. doi: 10.1007/BF01733070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans M, Horzinek M.C. Toroviruses of animals and humans: a review. Adv Virus Res. 1994;43:233–273. doi: 10.1016/S0065-3527(08)60050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M.R, Davies H.A. Human enteric coronaviruses. Arch Virol. 1981;70:301–313. doi: 10.1007/BF01320245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathan M, Mathan V.I, Swaminathan S.P, Yesudoss S, Baker S.J. Pleomorphic virus-like particles in human faeces. Lancet. 1975;1:1068–1069. doi: 10.1016/S0140-6736(75)91832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M.L, Ray C.G, Payne C.M, Friedman A.D, Minnich L.L, Rousseau C. Coronaviruslike particles in human gastrointestinal disease. Am J Dis Child. 1985;139:928–934. doi: 10.1001/archpedi.1985.02140110082036. [DOI] [PubMed] [Google Scholar]
- Resta S, Luby J.P, Rosenfeld C.R, Siegel J.D. Isolation and propagation of a human enteric coronavirus. Science. 1985;229:978–981. doi: 10.1126/science.2992091. [DOI] [PubMed] [Google Scholar]
- Rettig P.J, Altshuler G.P. Fatal gastroenteritis associated with coronaviruslike particles. Am J Dis Child. 1985;139:245–248. doi: 10.1001/archpedi.1985.02140050039017. [DOI] [PubMed] [Google Scholar]
- Rousset S, Moscovici O, Lebon P, Barbet J.P, Helardot P, Mace B, Bargy F, Vinh L.T, Chaney C. Intestinal lesions containing coronavirus-like particles in neonatal necrotizing enterocolitis: an ultrastructural analysis. Pediatrics. 1984;73:218–224. [PubMed] [Google Scholar]
- Schnagl R.D, Holmes I.J, Mackay-Scollay E.M. Coronavirus-like particles in Aboriginals and non-Aboriginals in Western Australia. Med J Aust. 1978;1:307–309. [PubMed] [Google Scholar]
- Schnagl R.D, Morey F, Holmes I.H. Rotavirus, coronavirus-like particles, bacteria and parasites in Central Australia. Med J Aust. 1979;2:115–118. doi: 10.5694/j.1326-5377.1979.tb142026.x. [DOI] [PubMed] [Google Scholar]
- Storz J, Rott R. Reactivity of antibodies in human serum with antigens of an enteropathogenic bovine coronavirus. Med Microbiol Immunol. 1981;169:169–178. doi: 10.1007/BF02123590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J, Rott R, Kaluza G. Enhancement of plaque formation and cell fusion of an enteropathogenic coronavirus by trypsin treatment. Infect Immun. 1981;31(3):1214–1222. doi: 10.1128/iai.31.3.1214-1222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucher Y.E, Ray C.G, Minnich L.L, Payne C.M, Beck D, Lowe P. Pleomorphic, enveloped, virus-like particles associated with gastrointestinal illness in neonates. J Infect Dis. 1982;145:27–36. doi: 10.1093/infdis/145.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.M, Herbst W, Kousoulas K.G, Storz J. Biological and genetic characterization of a hemagglutinating coronavirus isolated from a diarrhoeic child. J Med Virol. 1994;44:152–161. doi: 10.1002/jmv.1890440207. [DOI] [PMC free article] [PubMed] [Google Scholar]


