Abstract
Random peptide libraries and antigen-fragment libraries (also known as gene-fragment libraries) have been used to identify epitopes on protein antigens. These technologies promise to make significant contributions to diagnostic and vaccine development. Researchers in a number of labs have shown that phage selected from libraries with protective antibodies, raised against whole antigen, can be used as immunogens to stimulate antibody responses that bind native antigen and provide protection in vivo. Others have used the sera of patients with idiopathic diseases to screen libraries, and by this approach have identified candidate antigens involved in immune disease. These may prove useful for diagnosis and, possibly, in determining disease etiology.
Keywords: Random peptide libraries, phage display, combinatorial chemistry, antigen recognition, antibodies, antigen fragment libraries, vaccines
Nomenclature
- Ab – antibody
- AFL – Agn-fragment library
- Agn – antigen
- AHSV – African horsesickness virus
- anti-Id – anti-idiotypic
- CBR – critical-binding residue
- CSF – cerebral spinal fluid
- HCV – hepatitis C virus
- HEL – hen egg-white lysozyme
- HSV – herpes simplex virus
- IDDM – insulin-dependent diabetes mellitus
- LPS – lipopolysaccharide
- MAb – monoclonal Ab
- MS – multiple sclerosis
- PCAb – polyclonal antibody
- RPL – random peptide library
Introduction
Antibodies (Abs) play a crucial role in the immune system, especially in the defenses against extracellular pathogens (such as the encapsulated bacteria) and pathogens in extracellular phases of their life cycle (such as viral particles), and as such are a major immune component elicited by all vaccines currently in use for humans. Abs also have a variety of uses as biochemical and cellular markers, affinity reagents, diagnostics and therapeutics. Thus, our understanding of the principles governing the interactions of Abs with antigens (Agns) is of great significance to a wide spectrum of research and applications. Phage-display technology has been used to study Ab–Agn interactions with libraries of two types. Random peptide libraries (RPLs) have been used as a source from which peptide ligands can be identified for an Ab, without knowledge of the site on the Agn that the Ab recognizes (the epitope). Agn-fragment libraries (AFLs; also known as gene-fragment libraries) have served as a means of localizing epitopes to specific areas of protein Agns. In this review, we discuss recent progress with RPLs and AFLs in producing Ab-specific markers and epitope mimics, and as tools for mapping protein epitopes. Major focus is also given to the uses and limitations of these technologies in the development of vaccines and diagnostic tools. For brevity's sake, this review does not cover the significant body of work on peptide mimics of carbohydrate, and other non-protein Agns, nor on their development as vaccine and diagnostic leads. We briefly review Ab–protein-Agn interactions, phage-displayed RPLs and AFLs, and phage as immunogens. This is followed by a discussion of recent advances in the use of these libraries in epitope mapping, improvement of affinity, vaccine design, and in the search for pathogens involved in idiopathic disease.
The molecular basis of antigen recognition by antibodies
The humoral immune response is extremely versatile, having the ability to generate an extensive repertoire of molecular surfaces. Agn–Ab interactions have thus provided an excellent model for elucidating the principles of macromolecular recognition, and especially protein–protein recognition. The molecular surfaces mediating contact between an Ab and an Agn are called the paratope and epitope, respectively. The Ab repertoire has evolved to recognize a vast number of epitopes comprising proteins, carbohydrates, lipids, and even small chemical compounds (haptens), including man-made ones. As this review focuses primarily on protein Agns, it is important to distinguish between the general types of protein epitopes and the mechanisms by which Abs bind them. Protein epitopes usually bind Ab in three ways.
-
1.
Complementary fit between epitope and paratope.
-
2.
A larger number of lower-energy contacts (such as van der Waals interactions and H bonds) that define the surface of the epitope.
-
3.
A few (one to five) high-energy contacts (such as salt bridges) that confer a large component to the energy of binding.
Residues involved in these high-energy contacts are called critical-binding residues (CBRs), and amino-acid replacements at these sites usually reduce binding affinity greatly 1., 2.. The location of CBRs with respect to each other on the polypeptide chain of the antigen determines whether an epitope is linear or discontinuous. CBRs located within a short stretch of contiguous polypeptide sequence form linear epitopes, whereas discontinuous epitopes comprise CBRs located at distant sites on the polypeptide sequence that are brought together on the Agn surface by protein folding [3]. Epitopes whose affinity is affected by unfolding of the protein are said to be constrained; almost all discontinuous and many linear epitopes are constrained. Moreover, most protein epitopes occur at relatively protuberant and flexible sites on the protein surface [4].
Thus, structural principles are emerging that define the basis of Agn recognition by Abs. These principles are important in understanding the molecular basis by which peptides functionally mimic protein and non-protein epitopes (by binding the same paratope as the ‘native’ epitope) and structurally mimic them (by making identical contacts with the paratope to those made by the ‘native’ epitope). The concept of mimicry takes on added complexity in considering how peptide mimics may function as immunogenic mimics that elicit the same Ab as the corresponding epitope on the ‘native’ immunogen. Considering the simplest case of a peptide that is a structural mimic of much or all of a linear epitope that elicited a given Ab, such a peptide is expected to function as an immunogenic mimic in eliciting that same Ab, given the proper immunization conditions (e.g. structural format of the peptide, carrier protein [including helper-T cell epitopes], adjuvant, genetic background of the animal). However, one can also imagine the situation in which a peptide binds tightly and very specifically to a targeted Ab, producing, say, the same affinity as the native epitope, but via a different network of contacts with the Ab paratope. Would this peptide make a useful immunogenic mimic? The significance of peptides as structural mimics of an epitope versus purely functional mimics (that contact Ab through different interactions from the ‘native’ epitope) has yet to be clearly defined as it pertains to immunogenic mimicry.
The design and use of phage-displayed RPLs and AFLs
The field of phage display originated in 1985 when George P Smith showed that foreign DNA could be inserted into filamentous phage gene 3 to create a fusion protein in the amino-terminal domain of the minor coat protein pIII. He also demonstrated that the recombinant phage retained infectivity and could be enriched relative to ordinary phage by affinity purification on immobilized Ab, specific for the peptide insert [5]. Subsequently, in 1990, the first phage-display libraries were independently reported in three publications 6., 7., 8.. A comprehensive review of filamentous phage biology as well as instruction in a variety of phage-display technologies has been covered by Barbas III et al. [9], and will be described here briefly.
The filamentous phage consist of a long, cylindrical protein capsid encasing a circular, single-stranded DNA genome. The most commonly studied filamentous phage are the Ff class, which includes f1, fd and M13; these infect Escherichia coli cells that carry an F plasmid. Although all of the filamentous phage coat proteins have been used, with varying efficiency, to display foreign proteins and peptides, most of the currently used phage-display vectors fuse the foreign peptide or protein to be displayed to the amino terminus of pIII (a minor coat protein located at one tip of the phage) or pVIII (the major coat protein forming the tubular body of the phage). This allows phage to be produced that displays on its surface a foreign protein encoded by the genome it carries.
The phage vector can be designed such that the fusion protein is found on the phage genome or on a separate genome (i.e. a phagemid) that can be packaged, like the phage genome, into phage-like particles displaying pIII or pVIII fusions. Moreover, fusion can be to all or to only some copies of a given phage-coat protein. The pIII-display vectors can produce fusions with all four to five copies of pIII, or ‘hybrid phage’ that display less than one copy of the fusion per phage; the latter vectors use two copies of gene 3 (one wild-type gene, and one recombinant copy that encodes the fusion; Fig. 1 b). Only short peptides of six to eight amino acids can be displayed on all copies of pVIII without disruption of phage assembly 10., 11., 12.. Larger peptides can be displayed by hybrid phage via two copies of gene 8, with one copy encoding the wild-type protein, and the other the pVIII fusion protein. The copy number of fusion proteins to wild-type pVIII will vary depending on the vector and the sequence and length of the peptides or proteins displayed; however, for most pVIII-displayed, short peptides (of <25residues), the fusion copy number ranges from 5–15% of the total pVIII. The pIII- and pVIII-displayed peptides can be of varying lengths (typically pIII accommodates larger-sized fusions than pVIII), and conformational constraints can be imposed by the presence of one or more cysteine residues. Interestingly, pVIII-displayed peptide fusions containing two cysteine residues almost always form a constrained loop, whereas those containing one cysteine form homodimers [13•].
Fig. 1.
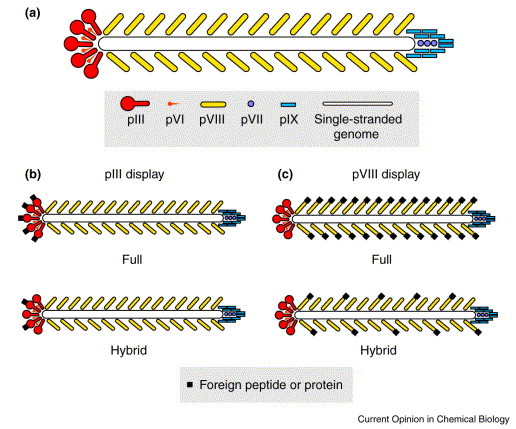
The filamentous phage for recombinant display of foreign peptide or protein. (a) Schematic of wild-type Ff phage and the major (pVIII) and minor (pIII, PVI, pVII, PIX) coat proteins. A comparison of full and hybrid display of foreign peptides or proteins via (b) pIII and (c) pVIII.The filamentous phage for recombinant display of foreign peptide or protein. (a) Schematic of wild-type Ff phage and the major (pVIII) and minor (pIII, PVI, pVII, PIX) coat proteins. A comparison of full and hybrid display of foreign peptides or proteins via (b) pIII and (c) pVIII.
RPLs display ‘randomly generated’ peptides that are genetically linked to coat-protein genes, such that each phage clone expresses one peptide in various copy numbers. The peptides are encoded by synthetic oligonucleotides, with each ‘randomized’ (X) residue being encoded by a degenerate codon (either NNK, or NNS in which N=A, C, T or G, K=G or T and S=G or C) that comprises codons for all 20 natural l-amino acids plus one stop codon. For example, the theoretical complexity of a 6-mer (X6) RPL is 64million (206) peptide sequences that are encoded from the ∼1billion (326) nucleotide sequences specified by (NNK)6. Thus, the linkage, via a soluble virion, of a protein readout to the DNA encoding it, allows the construction of libraries containing up to billions of virions, with each virion displaying and encoding a different peptide or protein fusion.
Typically, the affinity purification of phage bearing a ligand protein or peptide from a RPL (or an AFL, see below) involves multiple iterations of the following steps:
-
1.
An incubation period of the phage library with the target molecule(s).
-
2.
Washes to remove non-binding phage (the target molecule must be immobilized during this step).
-
3.
Amplification of target-binding phage in E. coli cells.
Purification of target-binding phage can be detected by increases in the yield of binding phage after each round of screening, and/or by testing the phage in a simple binding assay (e.g. an enzyme-linked immunosorbent assay [ELISA]). The sequence of the protein or peptide displayed by a binding phage is revealed simply by sequencing the viral DNA of selected clones in the region encoding the displayed peptide or Agn fragment. Conditions can be set during panning to influence the diversity and affinity of clones selected; these include stringency of the washes, the number of rounds of panning, and the concentration of the target molecule (if initial binding is done in solution) or the density of the immobilized target molecule (if panning involves direct binding of phage to immobilized target). In general, the diversity of phage clones decreases after multiple rounds of purification, and with higher-stringency selection conditions (i.e. with a low concentration or density of target molecule).
‘Sublibraries’ can also be constructed for the optimization of a lead peptide or a consensus sequence. This is significant for peptides selected from RPLs, because such libraries usually comprise from 108 to 109 independent phage clones, and hence, up to five amino-acid residues (comprising 205=3.2×106 quintapeptide sequences encoded by 325=3.4×107 (NNK)5 oligonucleotide sequences) can be redundantly selected from independent clones. Usually, consensus sequences comprise three to four residues (which are even more abundant in a given RPL). Thus, if a tighter-binding peptide is desired, further optimization can be obtained via sublibraries, in which a short consensus is fixed, and residues around it are randomized, as elegantly shown by Wrighton et al. [14]. More recently, Zhu et al. [15•] devised a technique to optimize peptide sequences that were selected with serum Abs (see ‘Vaccines: role of peptides and Agn fragments in anti-protein vaccines’, below).
AFLs differ from RPLs in that they consist of randomly generated fragments of a specific protein, which are normally fused to the amino terminus of the minor coat protein, pIII, or a truncated form of it. AFLs are also called ‘gene-fragment’ libraries, because they are constructed from cDNA fragments generated by digestion with a non-specific endonuclease [16]. Unlike RPLs, which can be used in a variety of screenings, a new AFL must be built for each target protein analyzed, and the gene for this protein must be cloned. To be useful, RPLs must be quite large, whereas AFLs made from a single gene can be quite effective at much lower library sizes, depending upon whether a single gene, or an entire genome (e.g. a fungal or bacterial genome) is used to construct the library.
Phage as immunogenic carriers for vaccination
Filamentous bacteriophage are commonly used as immunogenic carriers for generating Abs against recombinant peptides displayed at the amino terminus of phage coat proteins. The advantages of using phage over classical carrier proteins (e.g. ovalbumin, tetanus toxoid and keyhole limpet hemocyanin) are that the B cell epitopes on phage are limited to the outer 10–12 residues of pVIII [17•] and the ∼350-residue, outer domain of pIII (of which there are only 4–5copies). Thus, phage induce a restricted Ab response, as compared to the more typical carrier proteins.
The first use of recombinant phage as immunogens was by de la Cruz et al. [18], who tested the ability of recombinant phage to induce an Ab response against a pIII-displayed, antigenic peptide. Although they were able to generate a strong anti-phage Ab response, only marginal titers of Ab were elicited against the peptide, probably because of the low copy number of the peptide per phage. Indeed, Perham's group [10] showed that pVIII-display induces stronger anti-peptide responses upon immunization, even in the absence of an adjuvant. Meola et al. [19] showed that phage displaying a given peptide via fusion to pVIII produced better anti-peptide Ab responses than phage-borne fusion to pIII, recombinant fusion to human H ferritin or the hepatitis B core peptide, or synthetic peptide in the context of a multiple-antigenic peptide. More recently, our work has shown that the use of synthetic peptide conjugated to phage induces even stronger anti-peptide responses than pVIII-displayed recombinant phage, most probably as a result of an even higher copy number of peptide (NE van Houten, MB Zwick, PWHI Parren, DR Burton, JK Scott, unpublished data). However, the phage carrier did not produce as strong an Ab response as the peptide coupled to the classical carrier protein, ovalbumin.
Our work has also shown that a synthetic peptide conjugated to phage may not be structurally equivalent to its analog fused to the amino terminus of pVIII and displayed by hybrid phage [20]. On direct titration, the b12 Fab bound more tightly to recombinant phage bearing the peptide (100–200 copies per virion) than to phage to which synthetic peptide had been conjugated at a much higher copy number (see ‘RPLs and AFLs as a source of ligands for anti-protein Abs.’, below). Thus, perhaps a peptide tethered to a flexible linker is less constrained than the same peptide in recombinant form, linked to the phage coat through a less-flexible peptide bond. In support of this observation, Felici et al. [21] found that a MAb, specific for a discontinuous epitope on Bordetella pertusis toxin, would not bind to an RPL-derived synthetic peptide even though it bound tightly to the phage-displayed version of it. Further, a collaboration between Perham's and Opella's groups has revealed, for two different antigenic peptides, that the structure of the peptide fused to pVIII is stable enough to detect by NMR, whereas the synthetic analog of either peptide in solution gives no resolvable structure 22., 23.. Interestingly, the structural stabilization of both peptides appears to derive from only their ‘tethered’ state (i.e. their peptidyl linkage to the amino terminus of pVIII), and not from other interactions (such as hydrogen bonding) with the phage coat.
Effort has been focused on increasing the copy number of recombinant proteins on phage. Sidhu et al. [24••] identified regions in the 50-residue pVIII sequence of pVIII molecules displaying protein at their amino terminus, which could be altered to improve their copy number. The increase in copy number was achieved, presumably, as a result of better accommodation or packing of the recombinant pVIII fusions in the phage coat. Phage bearing increased numbers of pVIII fusions to both growth-hormone and streptavidin (residues 16–133) were selected on immobilized human growth hormone receptor (the extracellular domain) and anti-streptavidin PCAbs (polyclonal Abs), respectively. This same approach may also be effective in increasing the copy number of phage-displayed peptides of interest; this may be useful for increasing the immunogenicity of phage-displayed peptides and for structural studies of antigenic peptides.
The helper T cell response induced by phage is of great significance to the Ab responses against the phage and its displayed peptide. Initial work in Perham's laboratory [25] showed that phage induce Ab responses that are helper T cell-dependent. Our work using fairly small (10 μg) doses has shown that anti-peptide Ab production against a peptide conjugated at high copy number to phage levels off after four to five immunizations, as compared with the same peptide conjugated to ovalbumin (even after the eighth and final immunization, the anti-peptide Ab response had not reached a plateau), though the ratio of Abs against peptide to those against carrier is higher for phage. This result suggests that the T cell responses produced by phage are not as strong as against other, more typical carrier proteins. Phage are mainly composed of the major coat protein pVIII (∼2700 copies per phage), which is only 50 residues long, and each of the remaining (minor) coat proteins, pIII, pVI, pVII and pIX, which occur in low copy number (3–5 per phage; Fig. 1a). Only pIII is significantly large (∼400 residues) and thus may present a variety of T cell and B cell epitopes. Moreover, filamentous phage, being a parasite of E. coli, a commensal bacterium living in the gut of many vertebrates, including humans, may have evolved its proteins to induce a low-level response, so as to evade mucosally generated Ab responses.
Another indication of the low immunogenicity of the T cell epitopes on phage is found in the recent work of De Berardinis et al. [26••] who showed that phage can induce strong cytotoxic T cell responses against pVIII-displayed, foreign peptides. Thus, the filamentous phage have the potential to serve as a carrier for responses against both foreign T cell and B cell epitopes. To optimize the phage carrier, immunogenic peptides that are endogenous to phage should be minimized or removed altogether (so called ‘de-immunization’), so that the target epitopes, introduced by recombinant means, would be fully immunodominant. Exciting work by Le Doussal et al. [27••] suggests that it is possible for phage to display peptide–MHC complexes, and thus to be used directly in T cell selections. Libraries of peptides fused to MHC molecules should be useful for determining the spectrum of peptides that is recognized by a particular T cell receptor, and may complement the tetrameric MHC–peptide system for identifying specific T cell receptors [28]. Moreover, these peptide–MHC phage fusions may prove useful for inducing immune responses in vivo.
It was mentioned by Greenwood et al. [10] that adjuvant is not necessary for the induction of strong anti-phage Ab responses, and this has been more recently corroborated by Demangel et al. [29]. The strength of the Ab response against phage may be, in part, due to lipopolysaccharide (LPS) and the particulate nature of the filamentous virion. Significant amounts of E. coli LPS are associated with phage; it probably adheres to the phage coat during phage assembly and/or phage extrusion through the pIV-based outer-membrane pore, a gated ion channel [30]. LPS can be removed by subjecting the phage to detergent before precipitation with polyethylene glycol and sodium chloride. Depending on the size of the single-stranded genome it encapsulates, a phage particle can be ≥2 μm long (wild-type phage are ∼1 μm long). We have found that the Ribi Adjuvant System (Sigma#M6536) enhances the Ab response to fairly low doses of phage (e.g. 10–25 μg per subcutaneous injection). Small doses of phage may serve to induce higher-affinity Ab responses, because of more efficient competition for antigen in germinal centers.
Although phage are used as immunogen in almost all of the studies described below (see ‘Vaccines: role of peptides and Agn fragments in anti-protein vaccines’, below), it is possible that stronger and more focused anti-peptide Ab responses (as well as T cell responses) may be achieved using carriers other than phage (presumably because of the strength and variety of their T cell epitopes). Recently, Matthews et al. (LJ Matthews, R Davis, GP Smith, personal communication) found that low copy number, especially of long peptides, made it difficult to assess the immunogenicity of phage-displayed peptides; thus, they transferred the peptides from the amino terminus of pVIII to the amino terminus of IPIII, an internal protein of T4 bacteriophage. Immunization with fusions to IPIII elicited, on average, 10-fold higher Ab titers than the phage-borne peptides. Moreover, their transfer of the peptides to the maltose-binding protein of E. coli, following Zwick et al. [31], allowed them to assay the strength of Ab binding to monovalently displayed peptide, rather than of multivalently displayed phage-borne peptide, whose copy number can greatly vary.
RPLs and AFLs as a source of ligands for anti-protein Abs
With the appropriate Ab, peptide mimics of linear and discontinuous protein epitopes, as well as other non-protein Agn, can be affinity purified from RPLs. If a consensus sequence emerges among phage clones identified in a selection for protein epitope mimics, direct information regarding the critical binding residues is gained (usually 3–5 residues are identified). Moreover, if the mimicked epitope is linear, both its critical binding residues and the location of the epitope in the protein sequence can be easily obtained. Such information is useful in determining the minimal sequence required for Ab binding. RPLs can also be used to identify peptide mimics of discontinuous epitopes. The drawback of RPLs is that the phage clones tend to be of low affinity for the target molecule, and optimization of the peptides, through the construction and screening of sublibraries (see ‘The design and use of phage-displayed RPLs and AFLs’, above), is time consuming.
In contrast, a typical AFL affinity purification involves the screening of multiple, overlapping Agn fragments with Ab(s). A comparison of those fragments can be used to effectively map an epitope within a few residues. Moreover, if the epitope is present in a library, its structure is likely to be close or identical to that of the native epitope. Disadvantages are that certain discontinuous epitopes may not be formed from Agn fragments, Agn fragments do not provide information regarding critical binding residues, and they cannot be used to identify epitopes on non-protein antigens.
Bonnycastle et al. [32] screened a panel of 11 different phage-displayed RPLs with a set of MAbs (monoclonal Abs) and PCAbs against peptides, folded proteins and carbohydrates. They found that libraries having different disulfide-bridged constraints produced binding phage for different MAbs or PCAbs; however, no small subset of libraries was identified that produced all or most of the binding phage. Moreover, phage obtained from different libraries by the same MAb sometimes showed very different consensus sequences. They found that most of the MAbs against discontinuous epitopes isolated either weakly binding peptides or did not isolate binding peptides at all. One of the Abs used for screening was b12 [33], a human MAb that recognizes the CD4-binding site of the HIV-1 envelope protein gp120, neutralizes a broad spectrum of HIV-1 primary isolates in vitro, and protects macaques against mucosal challenge with a pathogenic SHIV strain (PWHI Parren, P Marx, AJ Hessell, A Luckay, J Harouse et al., personal communication). Only two weakly binding peptides were isolated by MAb b12; however, they shared a five-residue consensus sequence. Subsequently, Zwick et al. [20] used this sequence to make two sublibraries following the approach of Wrighton et al. [14], in which the consensus sequence was fixed, and residues around it were randomized. The tightest-binding peptide identified in the sublibrary screening was shown to be a homodimer that is bridged by the single cysteine residues on two identical peptides. In contrast, screening of a gp120-AFL with MAb b12 was unsuccessful, indicating that the epitope on gp120 is discontinuous and complex (PWHI Parren, personal communication).
Fack et al. [34] was the first group to compare an AFL with two RPLs (linear 6-mer and 15-mer libraries) for their ability to produce peptide ligands for a panel of four MAbs. They showed that the AFL yielded binding phage for all the MAbs, whereas the RPLs only produced binding peptides for two of the MAbs. More recently, Bentley et al. [35•] used three differently made AFLs for mapping an epitope from the VP2 outer capsid protein of African horsesickness virus (AHSV). Screening with chicken PCAbs against the virus produced tight-binding phage that allowed them to map several epitopes. In contrast, only three non-overlapping phage were isolated from the AFL when it was screened with a VP2-specific MAb.
One of these three clones was identified as carrying the true epitope, by further work with an RPL. Binding phage were isolated from an XCX15 library, and several of the phage shared one of two four-residue sequences that were present, separated by 36 residues, on one of the AFL clones. Thus, by screening both AFLs and RPLs, the authors determined elements of a discontinuous epitope.
The results of the studies described above extend the principle regarding RPLs, established by Bonnycastle et al. [32], to include AFLs; not only is it impossible to know a priori which type of RPL to screen with a given MAb, it is also impossible to know the type of library, RPL or AFL, that will produce the best ligands for a given anti-protein MAb. Very likely if the epitope is continuous, the construction and screening of an AFL may be useful, whereas if the epitope is discontinuous, selections from a panel of RPLs may be more effective.
Vaccines: role of peptides and Agn fragments in anti-protein vaccines
A major goal of RPL and AFL screening is to use a selected peptide ligand as an immunogenic mimic that will elicit in vivo Abs similar or identical to the Ab that isolated it. Successful immunogenic mimicry of peptides from AFLs implies that the native epitope has been closely mimicked by the selected Agn fragment, but not so for peptide ligands isolated from RPLs; these may bind regions in the Ab-combining site that do not contact the native epitope, as well as regions in the Ab-combining site that directly contact the native epitope. Thus, the mechanism of cross-reactivity of peptides isolated from RPLs may not fully reflect the structure of the Ab-native epitope interaction. Nevertheless, MAbs and PCAbs have been used to identify immunogenic-mimic peptides from both RPLs and AFLs.
As early as 1994, Schellekens et al. [36] showed that a peptide, identified from screening an RPL with a MAb specific for glycoprotein D of herpes simplex virus (HSV)-1, could be used to confer protective immunity to HSV-1 infection. Moreover, the affinity of the MAb for both the RPL-derived peptide and the 10-residue native linear epitope (mapped with the peptide) were approximately equal, suggesting that the CBRs had been identified. Bastien et al. [37] conferred immunity to respiratory syncytial virus by immunizing mice with phage displaying (via pIII) a previously identified, protective epitope (173–187) of its glycoprotein G. These experiments laid the foundation for the concept that linear epitopes, both from RPLs and AFLs, could induce neutralizing Ab production. Further evidence supporting this has been obtained by Grabowska et al. [38], who originally mapped a linear epitope on HSV-2 using an RPL and pepscanning. More recently, they showed that immunization of mice with the phage-displayed peptides conferred protective immunity against challenge with a lethal dose of HSV-2, and that survival was dependent on the amount of phage used for immunization [39•]. In similar work, Yu et al. [40•] used protective MAbs that recognize discontinuous (one MAb) and linear (two MAbs) epitopes on the surface-glycoprotein (surface glycoprotein) of murine coronavirus to screen a panel of RPLs. Although all three MAbs isolated binding phage, only one of the selected clones (which mimicked a continuous epitope on the surface-glycoprotein) induced a statistically-significant protective immune response. Interestingly, phage bearing the same consensus sequence as the protective clone, but different flanking sequences, did not induce protective immunity, indicating an important role for the regions flanking the consensus sequence in inducing cross-reactive Abs. Thus, protective Ab responses have been induced by phage-displayed peptides; however, in all the cases mentioned, the immunogenic-mimic peptides corresponded to linear protein epitopes on the cognate pathogens.
The PCAbs found in sera have also been used to identify a spectrum of peptides corresponding to different Abs in the response against an Agn or organism. Folgori et al. [41] were the first to show that the serum Abs from a panel of people with hepatitis C virus (HCV) infection could be used to identify peptide mimics of commonly recognized epitopes on HCV. Both mimics of linear and discontinuous epitopes were identified, and immunization of rabbits with some of these phage resulted in HCV-cross-reactive Ab production. Following this approach, Scala et al. [42•] screened two RPLs with serum Abs from HIV-1-infected people and identified phage that reacted with multiple sera. These phage were then used to immunize mice, and the sera from several mice was shown to stain immunoblots of the HIV-1 envelope proteins. Importantly, affinity-purified IgGs from the several mice neutralized HIV-1 primary isolates in an in vitro infection assay using human peripheral blood mononuclear cells.
In an extension of the work of Fack et al. [34], which compared the antigenicity of peptides derived from an RPL versus an AFL, Matthews et al. (LJ Matthews, R Davis, GP Smith, personal communication) compared the ability of a panel of RPLs and an AFL to produce immunogenic mimics of epitopes on the T4 bacteriophage, a model ‘pathogen’. Although they found peptide mimics of epitopes on T4 phage from both types of library, and both types of mimic were able to elicit significant Ab responses, only the AFL-derived peptides were able to elicit Abs that strongly bound T4 phage, when tested in a stringent in vitro assay. From this, the authors concluded that AFLs constitute a more useful source of immunogenic-mimic peptides than RPLs. However, as mentioned above, to bind with high affinity, peptides derived from RPLs may require affinity-optimization, which was not done in this case.
Of importance is the relevance of RPLs and AFLs to the development of vaccines for human and animal use; under what circumstances should one use a single peptide from an RPL or a protein fragment from an AFL for immunization, rather than the whole antigen, or a killed or attenuated microbe? In many cases, there are multiple sites of neutralization that are immunodominant on the Agn or organism, making neutralizing Abs relatively straightforward to elicit. However, if the protein or the organism is difficult to produce, or if neutralization sites are not immunodominant, then the approach of identifying epitope-mimic peptides to serve as immunogenic mimics may be useful. In these cases, it would be helpful to use neutralizing MAbs for the search (rather than PCAbs), so as to limit the epitope-mimics obtained to those involved in neutralization. HIV-1 infection is such a case; although a strong Ab response is elicited against the viral envelope proteins, rarely are broadly neutralizing specificities produced in significant amounts. Moreover, many of the anti-envelope-protein Abs produced bind the envelope proteins in free form, but will not bind them in the context of the viral envelope [33].
These peptide immunogenicity studies would benefit greatly from the approach taken by Demangel et al. [29], who prepared hybridomas from the phage immunizations, so that the elicited Abs could be directly compared with the ‘target’ MAb that initially isolated the phage (see ‘Conclusions’, below). Such comparisons should demonstrate, at both structural and functional levels, the degree to which peptide mimics can elicit Abs with binding properties, and perhaps V-gene and/or CDR-H3 sequences, similar to those of the MAb used for library screening.
Screening serum antibodies to identify disease-specific peptides
RPLs have been used for ‘epitope discovery’ with human serum (or panels of sera), even without prior knowledge of the Agn, such as in the cases of emerging and autoimmune diseases and allergies. The screening of RPLs with sera from individuals who have been exposed to the same etiological agent may allow the discovery of common features in the Ab response of different individuals, and perhaps even lead to the identification of the agent itself. Whereas, if the disease-causing agent is known, screening with sera enables the identification of disease-specific peptides that could be useful for diagnosis and/or prognosis of the disease; this may be of use if the target antigen is difficult to obtain, or reacts with high background with ‘normal’ sera.
Work by Cortese et al. [43] provides an early example of the use of serum Abs to screen RPLs for elucidating the etiology and pathogenesis of a suspected autoimmune disease. They screened an RPL with the cerebral spinal fluid (CSF) of multiple sclerosis (MS) patients, which often contains high concentrations of oligoclonal Abs, whose specificity and role in MS is unknown. Phage displaying the selected peptides were shown to react with both the serum and the CSF of the same patient, as well as with the serum of other MS patients and normal patients, but only sometimes showed cross-reactivity with the CSF of other MS patients. Such reactivity indicates that the CSF Abs from different MS patients did not have the same specificity (the Abs probably resulted from nonspecific immunodysregulation), and that the peptides may be mimicking ubiquitous Agns, not essential to the disease state at the time of the analysis. In further work, Cortese et al [44••] studied a phage-displayed peptide, representative of a family of peptides selected with the common motif Lys–Pro–Pro–Asn–Pro. As this was the dominant motif identified, the sera were further tested for their ability to bind a panel of neurotropic viruses. Interestingly, a phage clone, selected for its good reactivity to CSF Abs and sera of MS patients, was shown to cross-react with an epitope present in the envelope glycoprotein B of HSV-1. Moreover, Abs generated against this clone identified a brain-specific protein by Western blot. This brain protein, thus, may be a putative target for HSV-1-induced autoimmune Ab responses. Importantly, this approach may reveal evidence supporting the long-held hypothesis that common viral infections trigger the production of self-reactive CSF Abs in MS.
RPLs and cDNA libraries have been used in conjunction to identify antigenic and immunogenic epitope mimics of autoimmune-inducing Agns. Fierabracci et al. [45•] screened two RPLs with sera from patients with insulin-dependent diabetes mellitus (IDDM). Synthetic peptide (representative of one identified mimitope) coupled to a poly-l-lysine matrix was then used to raise serum Abs in rabbits, and these Abs were used to screen a human, pancreatic-islet-cell cDNA library; from this a protein was identified that bore significant homology to osteopontin. The anti-peptide rabbit serum stained human islet cells with the same pattern as some of the IDDM sera that had reacted with the peptide. This indicated that both patient and rabbit sera contained similar islet-cell-staining reactivities. A novel islet-related autoantigen was thus identified in this study.
Serum Abs may be used to identify peptides that specifically bind Abs that target the pathogen responsible for a given disease, and thus could be developed into a fast, specific, and inexpensive diagnostic tool. Kouzmitcheva et al. [46•] used serum Abs (the IgG fraction of the serum Abs was isolated) from patients with Lyme disease, along with a panel of negative sera, to select binding phage from 12 RPLs. The selected peptides were all characterized as being mimics of discontinuous epitopes, as none matched contiguous segments of protein from the disease-causing agent, Borrelia burgdorferi. Serum Abs from a number of patients, who had had Lyme disease, which were not used in the RPL selections, were also shown to bind the selected phage. This suggests that peptides may be developed as a simple diagnostic for infection by the pathogen causing Lyme disease.
It is also possible to improve the affinity of serum-selected peptides, so as to enhance their use in disease detection. Bartoli et al. [47] found lead peptides for a diagnostic against HCV. In further work by Zhu et al. [15•], two such diagnostic leads were improved in their ability to detect HCV-specific serum Abs. The authors built phage sublibraries in which the DNA encoding binding peptides was randomly mutated by error-prone PCR, with further diversity being added by flanking each peptide on either side with five X residues. Screening of these libraries with a panel of HCV-positive sera identified tight-binding clones that showed broader reactivity with a larger panel of positive sera than the original clones. The authors also found that different variants were the best binders for different sera, which probably reflects variation (perhaps somatic mutations) in similar Abs that were produced by different people.
Conclusions
Phage-display technology has been used in a wide spectrum of research, and has made significant contributions to the characterization of Agn–Ab interactions. Both RPLs and AFLs have been used successfully to map protein epitopes [35•] and to identify peptide mimics or protein fragments that have functioned as immunogenic mimics of target Agn epitopes ([39•], LJ Matthews, R Davis, GP Smith, personal communication). These peptides/protein fragments may prove to be useful leads for the diagnosis and/or prognosis of disease [46•], and as a means of fine-tuning the Ab response against neutralizing epitopes on pathogens [40•].
Recent advances have been made in optimizing various components of phage-display systems. Techniques have been devised to improve the affinity and broaden the reactivity of selected phage clones, both for MAbs [14] and the serum Abs from diseased individuals [15•]. Other approaches have identified phage-coat modifications that increase the copy number of a particular recombinant protein [24••]; this could be useful in NMR studies of antigenic peptide structure, and in vaccination strategies that use recombinant phage. It is apparent that the use of different RPLs (comprising peptide inserts of various length, and cyclic versus linear peptides) increases the probability of identifying Ab-binding ligands in a selection [32], and that the combined use of RPLs and AFLs can lead to discoveries, such as the identification of novel autoantigens [45•].
Protection against viral challenge has been achieved in murine model systems using recombinant phage as immunogens 39•., 40•.. Thus, Abs elicited against a phage-displayed, epitope-mimic peptide cross-react with the native epitope in the context of the whole Agn or pathogen. The basis of such cross-reactivity is unclear. It is apparent, however, that the structure of recombinant peptide is influenced by fusion to the phage coat, and as such, synthetic peptides conjugated to carrier may not be as effective as recombinant phage in inducing cross-reacting Abs [20]. More stringent assays of cross-reactivity are required in assessing the success of an epitope-mimic peptide, whether it be from a RPL or an AFL. Studies should be undertaken to analyze the V genes and structure of the Abs that have been induced by immunization with an epitope-mimic peptide, to allow its comparison with the MAb used to select it. Also needed are structures of the screening MAb, both free and bound to native Agn and to peptide mimic, as well as the corresponding structures of the bound and free MAbs elicited by the immunogenic-mimic peptides. Such work should establish whether peptides can indeed be used to elicit the same or similar Abs as those used in the initial peptide selection. This type of analysis has most closely been achieved by Demangel et al. [29] and Goldbaum et al. [48].
Demangel et al. [29] compared MAb D14-3 (raised against a carboxy-terminal fragment of the major merozoite surface protein 1 of Plasmodium vivax), used in their library screening, with MAbs elicited by selected peptide, and from this showed some similarities in V gene usage and the hypervariable-loop canonical structures [49] of the two types of MAb. Lower sequence homology, however, was found in the hypervariable regions (especially in CDR-H3), and preliminary structural models suggested significant differences in the Ab combining sites. The best example of immunogenic mimicry of a discontinuous protein epitope comes from Goldbaum et al. [48]. Their work, however, involved not peptides, but anti-idiotypic Abs raised against a MAb. Following the network theory of Jerne and others [50], they used the well-characterized murine MAb D1.3, which binds a discontinuous epitope on hen egg-white lysozyme (HEL), to immunize mice. An anti-idiotypic (anti-Id) MAb was shown by crystallographic study to behave similar to HEL, in binding the D1.3 paratope via many of the same contacts as HEL. Further, anti-anti-Id Abs raised in mice using rabbit PCAbs against D.13 were also shown to behave as D1.3, in binding HEL, and the anti-Id MAb, via many of the same contacts as D1.3. The K d of the D1.3-HEL interaction was 1×10−8M, whereas those of HEL with the two anti-anti-Id MAbs were 1.8×10−6M and 3.8×10−6M; moreover, the V gene usage, including the HCDR-3 region, of the anti-anti-Id MAbs were very similar to those of D.13. Thus, even though there was significant affinity of the anti-anti-Id MAbs for HEL, it was still lower than that of D1.3 for HEL, and the affinity of the anti-anti-Id MAbs for the epitope-mimic (anti-Id) MAb was greater than the cross-reactivity with HEL. This study constitutes an elegant and significant contribution to the development and analysis of immunogenic-mimics.
In future, phage-displayed RPLs and AFLs should make significant contributions where there is a need for targeting the Ab response to specific epitopes on a toxin, virus or organism, and in the diagnosis, and potentially, the etiology of certain idiopathic autoimmune and inflammatory diseases. Careful study of the structural and functional features of ligand-peptides and the Abs they bind should clarify the underlying mechanisms behind the development of epitope-targeted vaccine and diagnostic approaches.
Acknowledgement
We thank Sharmini Thiagarajah for producing Fig. 1.
References and recommended reading
Papers of particular interest, published within the annual period of review,have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.Geysen H.M, Tainer J.A, Rodda S.J, Mason T.J, Alexander H, Getzoff E.D, Lerner R.A. Chemistry of antibody binding to a protein. Science. 1987;235:1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- 2.Davies D.R, Cohen G.H. Interactions of protein antigens with antibodies. Proc Natl Acad Sci USA. 1996;93:7–12. doi: 10.1073/pnas.93.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow D.J, Edwards M.S, Thornton J.M. Continuous and discontinuous protein antigenic determinants. Nature. 1986;322:747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- 4.Getzoff E.D, Geysen H.M, Rodda S.J, Alexander H, Tainer J.A, Lerner R.A. Mechanisms of antibody binding to a protein. Science. 1987;235:1191–1196. doi: 10.1126/science.3823879. [DOI] [PubMed] [Google Scholar]
- 5.Smith G.P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 6.Devlin J.J, Panganiban L.C, Devlin P.E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990;249:404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- 7.Scott J.K, Smith G.P. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 8.Cwirla S.E, Peters E.A, Barrett R.W, Dower W.J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci USA. 1990;87:6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbas C.F.I.I.I, Burton D.R, Scott J.K, Silverman G.J. Phage Display: A Laboratory Manual. Cold Spring Harbour Laboratory Press; Plainview, New York: 2001. [Google Scholar]
- 10.Greenwood J, Willis A.E, Perham R.N. Multiple display of foreign peptides on a filamentous bacteriophage. Peptides from Plasmodium falciparum circumsporozoite protein as antigens. J Mol Biol. 1991;220:821–827. doi: 10.1016/0022-2836(91)90354-9. [DOI] [PubMed] [Google Scholar]
- 11.Iannolo G, Minenkova O, Petruzzelli R, Cesareni G. Modifying filamentous phage capsid: limits in the size of the major capsid protein. J Mol Biol. 1995;248:835–844. doi: 10.1006/jmbi.1995.0264. [DOI] [PubMed] [Google Scholar]
- 12.Malik P, Terry T.D, Gowda L.R, Langara A, Petukhov S.A, Symmons M.F, Welsh L.C, Marvin D.A, Perham R.N. Role of capsid structure and membrane protein processing in determining the size and copy number of peptides displayed on the major coat protein of filamentous bacteriophage. J Mol Biol. 1996;260:9–21. doi: 10.1006/jmbi.1996.0378. [DOI] [PubMed] [Google Scholar]
- 13•.Zwick M.B, Shen J, Scott J.K. Homodimeric peptides displayed by the major coat protein of filamentous phage. J Mol Biol. 2000;300:307–320. doi: 10.1006/jmbi.2000.3850. The authors randomly selected phage clones from libraries containing one or two fixed cysteine residues and found that phage clones whose recombinant peptide bore a single cysteine tended to form dimers on the phage coat, whereas those with two cysteine residues usually did not (they formed intramolecular disulfide bridges). Interestingly, it was shown that dimerization of single cysteine-containing recombinant pVIII peptides occurs in the host cell and prior to its assembly on the phage coat. [DOI] [PubMed] [Google Scholar]
- 14.Wrighton N.C, Farrell F.X, Chang R, Kashyap A.K, Barbone F.P, Mulcahy L.S, Johnson D.L, Barrett R.W, Jolliffe L.K, Dower W.J. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273:458–464. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 15•.Zhu Z.Y, Minenkova O, Bellintani F, De Tomassi A, Urbanelli L, Felici F, Monaci P. In vitro evolution’ of ligands for HCV-specific serum antibodies. Biol Chem. 2000;381:245–254. doi: 10.1515/BC.2000.031. A protocol that introduces mutations and extends the size of a target sequence, selected from phage-displayed RPLs, was developed to identify improved ligands for HCV-specific serum Abs. The addition of residues at both the peptide amino- and carboxy-terminus (closer to the phage surface) was shown to improve the interaction between peptide and HCV-specific serum Abs; these additions may increase the accessibility of the target sequence at the phage surface, increase the number of residues involved in the binding interaction, contribute to the peptide conformation, or improve phage viability. [DOI] [PubMed] [Google Scholar]
- 16.van Zonneveld A.J, van den Berg B.M, van Meijer M, Pannekoek H. Identification of functional interaction sites on proteins using bacteriophage-displayed random epitope libraries. Gene. 1995;167:49–52. doi: 10.1016/0378-1119(95)00614-1. [DOI] [PubMed] [Google Scholar]
- 17•.Kneissel S, Queitsch I, Petersen G, Behrsing O, Micheel B, Dubel S. Epitope structures recognised by antibodies against the major coat protein (g8p) of filamentous bacteriophage fd (Inoviridae) J Mol Biol. 1999;288:21–28. doi: 10.1006/jmbi.1999.2676. The authors mapped the accessible surface of filamentous bacteriophage fd particles using polyclonal rabbit serum and three mouse MAbs raised against complete phage. By Western blot analysis, their study identified the major structural component of phage, pVIII (the major coat protein), to be the antigen. (The amino terminus of the pVIII subunit is located at the surface of the virion and consists of an amphipathic helix.) Further, by spot synthesis of overlapping peptides (covering the sequence of pVIII) on cellulose membranes they showed that the three mouse MAbs interacted with a core of 10 amino acid residues located near the amino terminus of pVIII and that extension of the amino terminus did not inhibit binding. Similarly, the rabbit polyclonal Abs recognized a core of 12 amino acids near the amino terminus of pVIII. Subsequently, to define the minimal length of each epitope, a set of amino-terminal peptides containing individual amino acids substituted with glycine, were synthesized. Interestingly, the essential residues identified corresponded with the helical conformation of the amino terminus of pVIII. [DOI] [PubMed] [Google Scholar]
- 18.de la Cruz V.F, Lal A.A, McCutchan T.F. Immunogenicity and epitope mapping of foreign sequences via genetically engineered filamentous phage. J Biol Chem. 1988;263:4318–4322. [PubMed] [Google Scholar]
- 19.Meola A, Delmastro P, Monaci P, Luzzago A, Nicosia A, Felici F, Cortese R, Galfre G. Derivation of vaccines from mimotopes. Immunologic properties of human hepatitis B virus surface antigen mimotopes displayed on filamentous phage. J Immunol. 1995;154:3162–3172. [PubMed] [Google Scholar]
- 20.Zwick MB, Bonnycastle LC, Menendez A, Irving MB, Barbas CF III, Parren PWHI, Burton DR, Scott JK: Identification and characterization of a peptide that specifically binds the broadly HIV-1-neutralizing human antibody, b12.J Virol 2001, in press. [DOI] [PMC free article] [PubMed]
- 21.Felici F, Luzzago A, Folgori A, Cortese R. Mimicking of discontinuous epitopes by phage-displayed peptides, II. Selection of clones recognized by a protective monoclonal antibody against the Bordetella pertussis toxin from phage peptide libraries. Gene. 1993;128:21–27. doi: 10.1016/0378-1119(93)90148-v. [DOI] [PubMed] [Google Scholar]
- 22.Jelinek R, Terry T.D, Gesell J.J, Malik P, Perham R.N, Opella S.J. NMR structure of the principal neutralizing determinant of HIV-1 displayed in filamentous bacteriophage coat protein. J Mol Biol. 1997;266:649–655. doi: 10.1006/jmbi.1996.0821. [DOI] [PubMed] [Google Scholar]
- 23.Monette M, Opella SJ, Greenwood J, Willis AE, Perham RN: Structure of a malaria parasite antigenic determinant displayed on filamentous bacteriophage determined by NMR spectroscopy: implications for the structure of continuous peptide epitopes in proteins.Protein Sci 2001, in press. [DOI] [PMC free article] [PubMed]
- 24••.Sidhu S.S, Weiss G.A, Wells J.A. High copy display of large proteins on phage for functional selections. J Mol Biol. 2000;296:487–495. doi: 10.1006/jmbi.1999.3465. Five 10-residue libraries,that together spanned the 50-residue pVIII sequence of M13, were constructed such that most residues were fully or partially randomized. Only the lysine residues, believed to interact with the negatively charged DNA of the phage particle, were not mutated. Interestingly, very few wild-type residues were highly conserved in the first 30 residues at the amino terminus of pVIII, with only Ala10 being completely conserved. The last 20 residues of pVIII, however, appeared intolerant to mutation as no selectants were identified from the libraries spanning those residues (only contaminants from other libraries were identified). Variants of pVIII were shown to increase the display of two different recombinant proteins. [DOI] [PubMed] [Google Scholar]
- 25.Willis A.E, Perham R.N, Wraith D. Immunological properties of foreign peptides in multiple display on a filamentous bacteriophage. Gene. 1993;128:79–83. doi: 10.1016/0378-1119(93)90156-w. [DOI] [PubMed] [Google Scholar]
- 26••.De Berardinis P, Sartorius R, Fanutti C, Perham R.N, Del Pozzo G, Guardiola J. Phage display of peptide epitopes from HIV-1 elicits strong cytolytic responses. Nat Biotechnol. 2000;18:873–876. doi: 10.1038/78490. The authors showed that bacteriophage displaying RT2, a peptide epitope of the reverse transcriptase of HIV-1, were able to prime an Agn-specific CTL response both in vitro and in vivo. Successful priming also required a T helper epitope; they used pep23, an immunodominant B cell and T helper epitope, also found on HIV-1 reverse transcriptase. It was shown that the T helper epitope could by supplied either on the same or a separate bacteriophage. As expected, their experiments showed that priming of CTLs required expression of pep23 and RT2 on the surface of the same APC. It is unknown, however, how the phage virions were able to target both class I and class II MHC compartments. [DOI] [PubMed] [Google Scholar]
- 27••.Le Doussal J, Piqueras B, Dogan I, Debre P, Gorochov G. Phage display of peptide/major histocompatibility complex. J Immunol Methods. 2000;241:147–158. doi: 10.1016/s0022-1759(00)00211-8. The authors report for the first time the expression of single-chain peptide–MHC polypeptides in the periplasm of E. coli and their display on the minor phage coat protein pIII. It was determined that about 1% of the produced phage displayed at least one peptide–MHC complex. Also demonstrated was specific binding of the phage-displayed peptide–MHC complex to immobilized recombinant T cell receptors (TCRs) and the TCRs of T-cell hybridomas. Because of the low affinity of the interaction between TCRs and peptide–MHC complexes, and their rapid dissociation, achieving a higher copy number of phage-displayed peptide–MHC complex will be critical for the successful screening of libraries on immobilized TCR. [DOI] [PubMed] [Google Scholar]
- 28.Altman J.D, Moss P.A, Goulder P.J, Barouch D.H, McHeyzer-Williams M.G, Bell J.I, McMichael A.J, Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 29.Demangel C, Rouyre S, Alzari P.M, Nato F, Longacre S, Lafaye P, Mazie J.C. Phage-displayed mimotopes elicit monoclonal antibodies specific for a malaria vaccine candidate. Biol Chem. 1998;379:65–70. doi: 10.1515/bchm.1998.379.1.65. [DOI] [PubMed] [Google Scholar]
- 30.Russel M. Filamentous phage assembly. Mol Microbiol. 1991;5:1607–1613. doi: 10.1111/j.1365-2958.1991.tb01907.x. [DOI] [PubMed] [Google Scholar]
- 31.Zwick M.B, Bonnycastle L.L, Noren K.A, Venturini S, Leong E, Barbas C.F.I.I.I, Noren C.J, Scott J.K. The maltose-binding protein as a scaffold for monovalent display of peptides derived from phage libraries. Anal Biochem. 1998;264:87–97. doi: 10.1006/abio.1998.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnycastle L.L, Mehroke J.S, Rashed M, Gong X, Scott J.K. Probing the basis of antibody reactivity with a panel of constrained peptide libraries displayed by filamentous phage. J Mol Biol. 1996;258:747–762. doi: 10.1006/jmbi.1996.0284. [DOI] [PubMed] [Google Scholar]
- 33.Parren P.W, Moore J.P, Burton D.R, Sattentau Q.J. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. Aids. 1999;13:S137–S162. [PubMed] [Google Scholar]
- 34.Fack F, Hugle-Dorr B, Song D, Queitsch I, Petersen G, Bautz E.K. Epitope mapping by phage display: random versus gene-fragment libraries. J Immunol Meth. 1997;206:43–52. doi: 10.1016/s0022-1759(97)00083-5. [DOI] [PubMed] [Google Scholar]
- 35•.Bentley L, Fehrsen J, Jordaan F, Huismans H, du Plessis D.H. Identification of antigenic regions on VP2 of African horsesickness virus serotype 3 by using phage-displayed epitope libraries. J Gen Virol. 2000;81(Pt 4):993–1000. doi: 10.1099/0022-1317-81-4-993. A filamentous phage-display library (comprising three different sublibraries to maximize peptide diversity) was constructed from the fragmented cDNA of VP2, an outer capsid protein of African horsesickness virus (AHSV), in order to locate its antigenic regions. The library was screened with AHSV-specific polyclonal chicken IgY, polyclonal horse immunoglobulins and a MAb capable of neutralizing AHSV. The peptides selected with the chicken and horse Abs mostly differed in their minimal overlapping residues, possibly because of intrinsic genetic differences between the two immune systems, but they both identified the same stretch of amino acids as having significant immunogenicity. [DOI] [PubMed] [Google Scholar]
- 36.Schellekens G.A, Lasonder E, Feijlbrief M, Koedijk D.G, Drijfhout J.W, Scheffer A.J, Welling-Wester S, Welling G.W. Identification of the core residues of the epitope of a monoclonal antibody raised against glycoprotein D of herpes simplex virus type 1 by screening of a random peptide library. Eur J Immunol. 1994;24:3188–3193. doi: 10.1002/eji.1830241241. [DOI] [PubMed] [Google Scholar]
- 37.Bastien N, Trudel M, Simard C. Protective immune responses induced by the immunization of mice with a recombinant bacteriophage displaying an epitope of the human respiratory syncytial virus. Virology. 1997;234:118–122. doi: 10.1006/viro.1997.8632. [DOI] [PubMed] [Google Scholar]
- 38.Grabowska A, Jameson C, Laing P, Jeansson S, Sjogren-Jansson E, Taylor J, Cunningham A, Irving W.L. Identification of type-specific domains within glycoprotein G of herpes simplex virus type 2 (HSV-2) recognized by the majority of patients infected with HSV-2, but not by those infected with HSV-1. J Gen Virol. 1999;80:1789–1798. doi: 10.1099/0022-1317-80-7-1789. [DOI] [PubMed] [Google Scholar]
- 39•.Grabowska A.M, Jennings R, Laing P, Darsley M, Jameson C.L, Swift L, Irving W.L. Immunisation with phage displaying peptides representing single epitopes of the glycoprotein G can give rise to partial protective immunity to HSV-2. Virology. 2000;269:47–53. doi: 10.1006/viro.2000.0185. Phage clones selected with anti-HSV-2 glycoprotein G MAbs were used as immunogens in mice to give partial protection against HSV-2. Interestingly, the most antigenic clone, when used as an immunogen, was the least able to induce cross-reacting Abs with the native Agn (glycoprotein G). The phage preparations were administered without adjuvant and the overall levels of Ab were lower in mice given phage preparations that had been pre-absorbed against polymixin B (to remove LPS) than those given samples that hadn't been pre-absorbed; this suggests that LPS provides adjuvant activity to the phage immunogen. The authors noted a variability in the survival of mice between experiments, and suggest that minor differences in the age and size of the animals, the phage preparation used, and the dose of virus used for lethal challenge may influence survival. [DOI] [PubMed] [Google Scholar]
- 40•.Yu M.W, Scott J.K, Fournier A, Talbot P.J. Characterization of murine coronavirus neutralization epitopes with phage-displayed peptides. Virology. 2000;271:182–196. doi: 10.1006/viro.2000.0310. A panel of 12 RPLs was used to map immunologically relevant epitopes on the surface glycoprotein of murine coronavirus with three neutralizing mAbs. Analysis of the amino acid sequences of selected phage showed a clear consensus sequence for the mAbs recognizing continuous epitopes (phage selected by one mAb were recognized by both mAbs) but it was more difficult to identify a consensus sequence for the mAb which recognized a discontinuous epitope. Only one clone selected with a continuous epitope-binding MAb, when used as an immunogen, induced a protective immune response; it was presumed that the peptides selected by the mAb specific for a discontinuous epitope did not mimic the complete epitope sufficiently and/or did not bind with adequate strength to elicit detectable cross-reactivity with the viral antigen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folgori A, Tafi R, Meola A, Felici F, Galfre G, Cortese R, Monaci P, Nicosia A. A general strategy to identify mimotopes of pathological antigens using only random peptide libraries and human sera. EMBO J. 1994;13:2236–2243. doi: 10.1002/j.1460-2075.1994.tb06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Scala G, Chen X, Liu W, Telles J.N, Cohen O.J, Vaccarezza M, Igarashi T, Fauci A.S. Selection of HIV-specific immunogenic epitopes by screening random peptide libraries with HIV-1-positive sera. J Immunol. 1999;162:6155–6161. Abs play an important role in protection against HIV challenge. However, the high variability of the HIV envelope sequences and its complex oligomeric structure have hindered the development of a prophylactic vaccine. In this study, the authors sought to identify epitopes that are specifically recognized by Abs generated by HIV-1 infected individuals. Thus, RPLs were thus screened with HIV-1 positive sera (and counterscreened with HIV-1 negative sera) to select peptides; the phage-displayed peptides were shown to behave as both antigenic and immunogenic mimics of linear and discontinuous HIV-1 epitopes. Of significance, the peptides, when used as immunogens in mice, elicited HIV-1 specific Abs that neutralized HIV-1 isolates in vitro. Also of interest is that the peptides were recognized by the sera of simian HIV-infected monkeys and the sera from long-term nonprogressor individuals showed higher Ab titers to certain epitopes, suggesting that these epitopes confer some degree of protection against disease progression. [PubMed] [Google Scholar]
- 43.Cortese I, Tafi R, Grimaldi L.M, Martino G, Nicosia A, Cortese R. Identification of peptides specific for cerebrospinal fluid antibodies in multiple sclerosis by using phage libraries. Proc Natl Acad Sci USA. 1996;93:11063–11067. doi: 10.1073/pnas.93.20.11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Cortese I, Capone S, Luchetti S, Cortese R, Nicosia A. Cross reactive phage-displayed mimotopes lead to the discovery of mimicry between HSV-1 and a brain-specific protein. J Neuroimmunol. 2001;113:119–128. doi: 10.1016/s0165-5728(00)00398-2. To overcome the limitation of low Ab concentrations in the CSF of MS patients the authors raised rabbit sera against a phage-displayed peptide (MS17), selected from screening with the CSF of MS patients. MS CSF samples that recognized MS17 were shown also to bind and immunoprecipitate HSV-1 envelope glycoprotein B (gB), as were immunopurified anti-MS17 IgG (from the rabbit sera), suggesting that MS17 is both an antigenic and immunogenic mimic of HSV-1 gB. [DOI] [PubMed] [Google Scholar]
- 45•.Fierabracci A, Biro P.A, Yiangou Y, Mennuni C, Luzzago A, Ludvigsson J, Cortese R, Bottazzo G.F. Osteopontin is an autoantigen of the somatostatin cells in human islets: identification by screening random peptide libraries with sera of patients with insulin-dependent diabetes mellitus. Vaccine. 1999;18:342–354. doi: 10.1016/s0264-410x(99)00204-2. The authors used sera from IDDM patients to screen an RPL in an attempt to identify novel pancreatic islet-related epitopes associated with IDDM. Their screening involved three steps: first, screening with a single serum; second, screening of the enriched phage with two additional serum with different autoantibody specificities (so that the enriched phage are commonly recognized by Abs in all 3 sera); and third, screening and counter-screening with sera from other patients and normal controls. One identified clone, CH1p, was detected by 70% of newly diagnosed IDDM patient sera compared with only 10% of normal sera. Rabbit sera raised against the MAP-conjugated CH1p synthetic peptide selected three clones from a cDNA-expression library constructed from human islet cells; the sequence of this peptide shared 99% homology with that of human osteopontin. However, a radioimmunoassay using recombinant human osteopontin from bone revealed that only ∼7% of IDDM sera may contain anti-osteopontin Abs. On the basis of the data presented in the paper, osteopontin is probably an autoantigen, but it is not involved in a common pathogenic mechanism underlying IDDM. [DOI] [PubMed] [Google Scholar]
- 46•.Kouzmitcheva G.A, Petrenko V.A, Smith G.P. Identifying diagnostic peptides for Lyme disease through epitope discovery. Clin Diagn Lab Immunol. 2001;8:150–160. doi: 10.1128/CDLI.8.1.150-160.2001. The authors demonstrated the usefulness of screening RPLs with serum Abs from infected individuals, even without prior knowledge of the pathogen or its antigenic structure, for ‘epitope discovery’ and the identification of diagnostic peptides. Twelve RPLs were screened to select peptides recognized by Lyme-disease related Abs but not by background Abs present in negative sera samples. The 22 potential diagnostic peptides identified in the study fell into eight sequence motifs. Interestingly, peptides of the same motif showed similar binding patterns of reactivity with different sera samples but this was not true for peptides with different motifs; this suggests that the sequence motifs represented functional motifs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartoli F, Nuzzo M, Urbanelli L, Bellintani F, Prezzi C, Cortese R, Monaci P. DNA-based selection and screening of peptide ligands. Nat Biotechnol. 1998;16:1068–1073. doi: 10.1038/3525. [DOI] [PubMed] [Google Scholar]
- 48.Goldbaum F.A, Velikovsky C.A, Dall'Acqua W, Fossati C.A, Fields B.A, Braden B.C, Poljak R.J, Mariuzza R.A. Characterization of anti-anti-idiotypic antibodies that bind antigen and an anti-idiotype. Proc Natl Acad Sci USA. 1997;94:8697–8701. doi: 10.1073/pnas.94.16.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lara-Ochoa F, Almagro J.C, Vargas-Madrazo E, Conrad M. Antibody-antigen recognition: a canonical structure paradigm. J Mol Evol. 1996;43:678–684. doi: 10.1007/BF02202116. [DOI] [PubMed] [Google Scholar]
- 50.Jerne N.K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974;125C:373–389. [PubMed] [Google Scholar]


