Highlights
-
•
There are more than 100,000 confirmed COVID-19 cases, with spread to over 100 countries as of 2020-03-08.
-
•
Asymptomatic carriers during the incubation period can be a potential infection source of COVID-19.
-
•
Person-to-person transmission has been documented; asymptomatic carriers should be a focus for disease prevention.
Keywords: COVID-19, SARS-CoV-2, Asymptomatic carrier, Incubation period, Pneumonia
Abstract
Objectives
With the ongoing outbreak of COVID-19 around the world, it has become a worldwide health concern. One previous study reported a family cluster with an asymptomatic transmission of COVID-19. Here, we report another series of cases and further demonstrate the repeatability of the transmission of COVID-19 by pre-symptomatic carriers.
Methods
A familial cluster of five patients associated with COVID-19 was enrolled in the hospital. We collected epidemiological and clinical characteristics, laboratory outcomes from electronic medical records, and also verified them with the patients and their families.
Results
Among them, three family members (Case 3/4/5) had returned from Wuhan. Additionally, two family members, those who had not traveled to Wuhan, also contracted COVID-19 after contacting with the other three family members. Case 1 developed severe pneumonia and was admitted to the ICU. Case 3 and Case 5 presented fever and cough on days two through three of hospitalization and had ground-glass opacity changes in their lungs. Case 4 presented with diarrhea and pharyngalgia after admission without radiographic abnormalities. Case 2 presented no clinical nor radiographic abnormalities. All five cases had an increasing level of C-reactive protein.
Conclusions
Our findings indicate that COVID-19 can be transmitted by asymptomatic carriers during the incubation period.
1. Introduction
On Dec 31, 2019, the government of Hubei Province, China, first reported a group of confused patients with pneumonia (The Central Government of the People's Republic of China, 2020). Metagenomics sequencing analysis revealed a novel coronavirus, which was officially named SARS-CoV-2 and is the cause of the disease called COVID-19 (World Health Organization, 2020a). The National Health Commission (NHC) established COVID-19 as a category B infectious disease with A-class management on Jan 20 (The Central Government of the People's Republic of China, 2020). Furthermore, on Jan 30, the WHO issued a Public Health Emergency of International Concern (PHEIC) alarm and appealed to specialists all over the world to work together to control the rapid spread of COVID-19. As of Mar 8, there had been 80,859 confirmed COVID-19 patients in China, including 3,100 deaths. Internationally, over 100 countries have now reported laboratory-confirmed cases, including a total of 24,031 cases (World Health Organization, 2020b). As of March 27, 2020, the World Health Association reports that there are 465,915 confirmed cases of COVID-19 worldwide; 21,031 have died, a mortality rate of about 4.5%.
The first clinical data from 41 individuals with a confirmed diagnosis of COVID-19 from Wuhan, China, have been published (Huang et al., 2020). Most of them had been directly exposed to the Wuhan wholesale seafood market, which sells freshly slaughtered game animals that were the original infection source (Huang et al., 2020). The present data strongly suggest that game animals or mammals were probably intermediate hosts of SARS-CoV-2 that originated from the Chinese horseshoe bat (Zhou et al., 2020, Lu et al., 2020). The virus has preferential tropism to human airway epithelial cells through the same cellular receptor as that for SARS, angiotensin-converting enzyme 2 (ACE2), which is a central body receptor for the surface glycoprotein S of the virus (Munster et al., 2020). The way to ascertain disease depends on positive real-time reverse transcription-polymerase chain reaction (rRT-PCR) results for SARS-CoV-2 nucleic acid. The associated mortality rates are 2-3% (World Health Organization, 2020b, Chen et al., 2020). Currently, there are no definite antiviral therapies or vaccines for COVID-19, although some drugs are under investigation.
With the extensive outbreak of COVID-19, a mass of studies in the larger population have been reported. Obviously, the total number of cases and transmission events have far exceeded those of SARS and H1N1. Wu et al. (2020) found that the mean R0 of COVID-19 was approximately 2.68 (95% CI: 2.47-2.86). An increasing number of outbreaks of familial transmission stressed the possibility of person-to-person transmission (Chan et al., 2020, Rothe et al., 2020, Phan et al., 2020). The measures in which public health officials quarantine confirmed cases and isolate intimate patients are significant progress in controlling COVID-19. Another question for consideration is a group of asymptomatically infected individuals, which may propagate the virus and impede infection control (Zhou et al., 2020, Zhang et al., 2020). Bai et al. (2020) presented clinical data showing a familial cluster of five patients with COVID-19 and one asymptomatic family member, which presumed the possibility of infection from asymptomatic carriers.
In this study, we report the clinical features of a family cluster involving five COVID-19 cases in Luzhou. Three cases came from Wuhan, and the other two cases had not left Luzhou recently. Our data further verified the delivery of infection from asymptomatic carriers of COVID-19 during the incubation period.
2. Methods
On Feb. 4, a 50-year-old woman (Case 1) was admitted to the People's Hospital of Luxian County (Luzhou, Sichuan Province, China) with fever, dizziness, cough, and shortness of breath. On examination, her maximum temperature was 39. 1 °C, and her blood pressure was 152/93 mmHg. A chest CT scan revealed massive shadows of high density in the lungs. The local CDC performed rRT-PCR tests for SARS-CoV-2 nucleic acid, and a throat swab was positive (Ct values, 19). Case 1 was diagnosed and immediately isolated.
Subsequently, forty-four other intimate contacts were admitted to our hospital for a general physical examination on Feb. 5. The local CDC ascertained that the nasopharyngeal or oropharyngeal swabs were positive for four cases (Case 2/3/4/5) according to the SARS-CoV-2 nucleic acid test (Table 2). All cases were admitted to the hospital and treated in isolation. A detailed data collection for the five patients was executed, and all patients underwent chest CT imaging. All the laboratory procedures for clinical samples have been previously reported (To et al., 2010). Nasopharyngeal and oropharyngeal swabs and stool and urine samples were obtained and maintained viral transport medium. Plasma was separated from EDTA bottles, and serum was separated from clotted blood bottles. Specimens were held between 2 °C and 8 °C and sent to the CDC. RRT-PCR tests for SARS-CoV-2 nucleic acid were performed by the local CDC; the detection reagents were from Maccura Biotechnology Co., Ltd, and Sansure Biotechnology Co., Ltd.
Table 2.
Results of rRT-PCR test for SARS-CoV-2 nucleic acid.
| Specimen | Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Date |
||||||||||
| Feb. 4 | Feb. 14 | Feb. 5 | Feb. 12 | Feb. 5 | Feb. 15 | Feb. 5 | Feb. 15 | Feb. 5 | Feb. 15 | |
| Nasopharyngeal swab | NA | + (Ct 37) | + (Ct 30) | − | + (Ct 22) | + (Ct 33) | + (Ct 26) | − | NA | + (Ct 37) |
| Oropharyngeal swab | + (Ct 19) | + (Ct 35) | +(Ct 23) | +(Ct 38) | + (Ct 21) | +(Ct 31) | NA | − | + (Ct 21) | + (Ct 34) |
| Serum | ND | − | ND | NA | ND | − | ND | − | ND | NA |
| Urine | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Stool | ND | NA | ND | ND | ND | ND | ND | − | ND | ND |
Ct values for rRT-PCR presented in parentheses. Lower Ct values indicate higher viral loads. Ct = cycle threshold. + = positive. − = negative. ND = not detected. NA = not available.
The Ethics Committee of the People's Hospital of Luxian County approved this study. We obtained written consent from all the patients about clinical data, radiographic pictures, information, etc.
3. Results
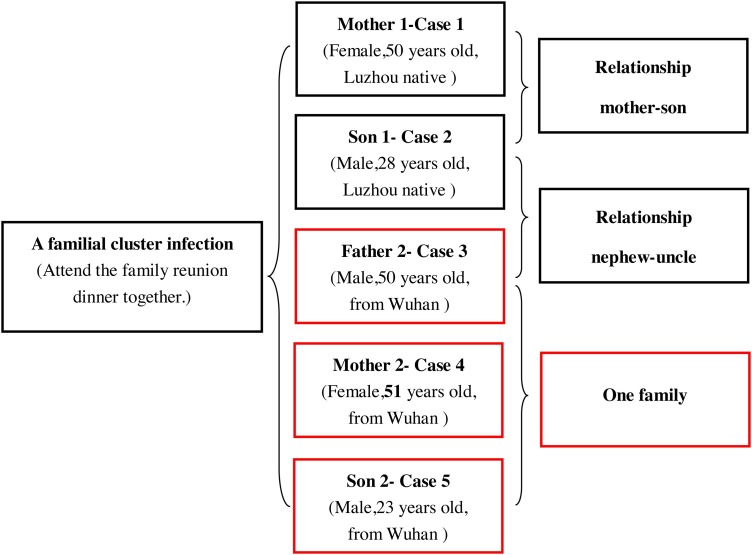
We documented and analyzed the clinical symptoms, laboratory results, and history of five cases with verified COVID-19, as presented in Table 1 . The relationship between the five cases and their general information is shown in Fig. 3 .
Table 1.
Summary of clinical features and laboratory results of a family cluster infected with COVID-19.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Relationship | Mother of Case 2 | Son of Case 1 | Father of Case 5 | Mother of Case 5 | Son of Case3 and 4 |
| Age (years) | 50 | 28 | 50 | 51 | 23 |
| Sex | Female | Male | Male | Female | Male |
| Occupation | Farmer | Worker | Trader | Trader | Trader |
| Chronic medical illness | Hypertension, emphysema | None | None | None | None |
| Presenting symptoms and signs | |||||
| Fever | + | − | + (hospital exposure) | − | + (hospital exposure) |
| Cough | + | − | + (hospital exposure) | − | + (hospital exposure) |
| Dizziness | + | − | − | − | − |
| Shortness of breath | + | − | − | − | − |
| Stuffiness | − | − | − | − | − |
| Diarrhoea | − | − | − | + (hospital exposure) | − |
| Pharyngalgia | − | − | − | + (hospital exposure) | − |
| Body temperature (°C) | 39.1 | 36.2 | 38.2 (hospital exposure) | 37.0 | 38.0 (hospital exposure) |
| Oximetry saturation (%) | low 90% (hospital exposure) | 94% | 92% | 92% | 98% |
| White blood cell count (×109 cells/L) (normal range 4.00–10.00) | 12.4 (↑) | 6.12 | 9.57 | 7.44 | 8.24 |
| Neutrophil count (×109 cells/L) (normal range 1.80–6.30) | 7.82 (↑) | 3.57 | 4.11 | 5.25 | 3.13 |
| Lymphocyte count (×109 cells/L) (normal range 1.10–3.20) | 0.84 (↓) | 1.03 (↓) | 1.07 (↓) | 1.70 | 2.46 |
| Haemoglobin (g/dL); (normal range 120–160) | 140 | 152 | 166 (↑) | 147 | 164 (↑) |
| Platelet count (×109 cells/L) (normal range 100–300) | 88 (↓) | 156 | 187 | 72 (↓) | 242 |
| C-reactive protein (mg/L) (normal range 0.0–5.0) | 189.2 (↑) | 10.5 (↑) | 9.0 (↑) | 43.5 (↑) | 24.7 (↑) |
NA = not available. + = positive. − = negative. ↑ = above normal range. ↓ = below normal range.
Fig. 3.
The relationship between the 5 cases and their general information.
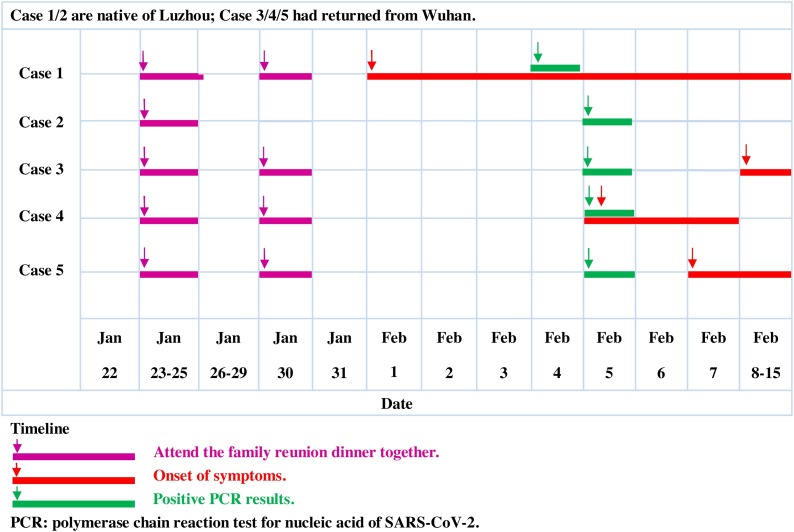
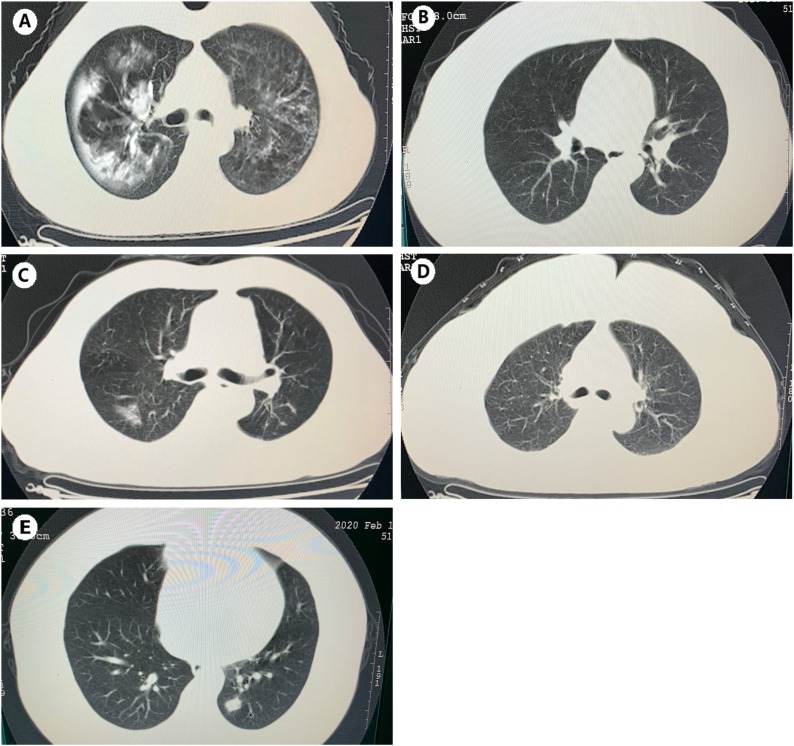
Case 1, a 50-year-old woman, had lived in Luzhou year-round and had hypertension and chronic emphysema disease. Between Jan 23 and Jan 25, she attended the family reunion dinner with her family members (Case 2/3/4/5). On Jan 30, they celebrated the Spring Festival together. Because of the limits on access, Case 1 could not leave her hometown in the countryside, where there had been no previous reports of COVID-19. Furthermore, she had not been in contact with any person returning from Wuhan (except Case 3/4/5). In particular, Case 1 did not present any symptoms until Feb. 1. She became ill with fever and a non-productive cough; she took drugs at that time. By the evening, Case 1 started feeling better and continued to enjoy the Spring Festival. However, two days later, a fever of 38.6 °C recurred, along with dizziness, non-productive cough, and shortness of breath. She was brought to the hospital by her son on Feb. 4 (Fig. 2). The laboratory examination revealed an increased level of white blood cell count (12.4 × 109/L), neutrophil count (7.82 × 109/L), C-reactive protein level (189.2 mg/L) and a low lymphocyte count (0.84 × 109/L) and platelet count (88 × 109/L) (Table 1). CT images showed massive shadows of high density in the lungs (Fig. 1 ). Case 1 developed severe pneumonia and was placed in the ICU as dyspnoea increased, with hypoxemia on day two of hospitalization. As of Feb. 15, her clinical condition had improved, and her oxygen saturation values recovered to 94% on supplemental oxygen. A nasopharyngeal and an oropharyngeal swab obtained on admission day ten remained positive for SARS-CoV-2 (Table 2 ).
Fig. 2.
Timeline of exposure to the asymptomatic carrier of COVID-19 which leads to a familial cluster infection.
Fig. 1.
Respectively, the chest CT images of five Cases. (A) Massive shadows of high density of Case 1. (B/D) No abnormalities of Case 2 and Case 4. (C/E) Ground-glass changes of Case 3 and Case 5.
Case 2, a 28-year-old man, was living in Luzhou. He had not come to an area where COVID-19 was spreading or had contact with any fever or pneumonia patients. He had just attended the family reunion dinner with his family members, including his mother (Case 1)/uncle (Case 3)/aunt (Case 4)/cousin(Case 5), on Jan 23 through 25. After his diagnosis of COVID-19, he never had an elevated temperature, and there were no abnormal symptoms except an increased level of C-reactive protein (10.5 mg/L) and a reduced lymphocyte count (1.03 × 109/L). Chest CT images showed no significant abnormalities (Fig. 1).
On Jan. 22, Case 4, a migrant who did business in Wuhan, drove back to Luzhou with Case 3 and Case 5 for the Spring Festival. On Feb 5, she also was admitted to the hospital, and a nasopharyngeal swab was positive for SARS-CoV-2 (Ct values, 26). Her chest CT images were standard (Fig. 1). From Jan 22 to Feb. 4, Case 4 did not present any symptoms until 7 PM on Feb 5. She started having slight pharyngalgia and diarrhea (3–5 times per day) without an increased body temperature or cough.
Laboratory examination indicated an elevated C-reactive protein (43.5 mg/L), and decreased platelet count (72 × 109/L). Case 4 was started on oxygen support at 2 L/min and was treated with oseltamivir (75 mg every 12 h), dioctahedral smectite powder (3 g every 8 h) and cefixime (100 mg every 12 h). Two days later, she felt better. Her rRT-PCR results were negative on Feb 15 (Table 2).
Case 3 and Case 5 are previously healthy men without a history of hypertension or type 2 diabetes. On days three through five of hospitalization, they developed fever and cough symptoms. Chest CT scans showed ground-glass opacities in the lungs (Fig. 1), and other laboratory examinations showed mildly decreased lymphocyte counts (Table 1) and increased C-reactive protein (9.0/24.7 mg/L). Their infections were moderate. Although their symptoms had resolved except for the cough, they remained hospitalized because their rRT-PCR test results were positive as of Feb. 15 (Table 2).
4. Discussion
COVID-19 has been proven to be transmitted through the respiratory tract, digestive system, and mucosal surfaces (such as the conjunctiva) (Chan et al., 2020, To et al., 2020). Nosocomial infections in healthcare facilities also occur and highlight the significance of effective infection control (Zhang et al., 2020). At the onset, the symptoms are usually fever, cough, shortness of breath, and pharyngalgia or diarrhea (National Health Commission of the People's Republic of China, 2020). The emblematic radiologic characteristic is ground-glass opacities in the lungs. Laboratory data present as lymphocytopenia, hypoxemia, thrombocytopenia, and even liver and kidney dysfunction in severe pneumonia patients (Huang et al., 2020). At present, verified patients are the primary sources of infection, however asymptomatic carriers can also be a source to propagate the outbreak. These patients are not easy to detect when initially infected but may have abnormal symptoms later (Huang et al., 2020).
In this familial cluster of five patients associated with COVID-19 in Luzhou, China, Case 1 had contact with only her family members (Case 2/3/4/5), some of them must be asymptomatic carriers of COVID 19. It is certain that Case 2 through 5 had no abnormal symptoms before the outbreak of Case 1. Notably, COVID-19 is highly infectious and may be transmitted by asymptomatic carriers during the incubation period. The timeline events suggest that Case 4 was the most likely initial infection source, as the time of the onset of symptoms and negative rRT-PCR results was relatively earlier than Case 3 and Case 5 (Fig. 2 ). Case 2 was afebrile without any clinical signs, and his chest CT images showed no abnormalities on Feb. 5 and Feb. 15 (Fig. 1), which also proved the existence of asymptomatic carriers. Our findings provide evidence that asymptomatic carriers can be a latent source of COVID-19 infection. As the spread of COVID-19 is worsening worldwide, it is pressing to provide more information for an improved understanding of both the transmission and precautions to take to deal with COVID-19. Further studies on the mechanism in which asymptomatic carriers can acquire and transmit COVID-19 are warranted.
Ethical approval
The Ethics Committee of People's Hospital of Luxian County approved this study. We obtained written consent from all the patients about clinical data, radiographic pictures, information, etc.
Availability of data and materials
All data and materials used in this work are publicly available.
Funding
This work was not funded and did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
All of the authors collected epidemiological and clinical data and discussed the results. F.Y. drafted the article. S.X. contributed to summarizing all clinical data. Z.R. and P.D. analyzed all epidemiological data. X.X and R.X. revised the final manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the Luzhou CDC for performing SARS-CoV-2 nucleic acid tests and thank AJE Editing Service (https://www.aje.com) for editing this manuscript.
Contributor Information
Feng Ye, Email: yefeng19930@126.com.
Shicai Xu, Email: xsc691092906@163.com.
Zhihua Rong, Email: doctorrzh@126.com.
Ronghua Xu, Email: 1442416311@qq.com.
Xiaowei Liu, Email: 625289749@qq.com.
Pingfu Deng, Email: 743371203@qq.com.
Hai Liu, Email: liuhai6233@163.com.
Xuejun Xu, Email: docxxj@163.com.
References
- Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with the 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E: A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020 doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- National Health Commission of the People's Republic of China . 2020. Diagnosis and treatment of novel coronavirus infected pneumonia (The sixth edition) http://www.nhc.gov.cn [accessed 19.02.20] [Google Scholar]
- Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020 doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020 doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Central Government of the People's Republic of China. Available from: http://www.gov.cn [accessed 09.02.20]
- To K.K., Chan K.H., Li I.W., Tsang T.Y., Tse H., Chan J.F. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J Med Virol. 2010;82:1–7. doi: 10.1002/jmv.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.-W., Tsang O.T.Y., Chik-Yan Yip C., Chan K.-H., Wu T.-C., Chan J. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February. Available from: https://www.who.int/home [accessed 11.02.20] [Google Scholar]
- World Health Organization . 2020. Coronavirus disease 2019 (COVID-2019) Situation Report-48 (WHO, 2020) Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200308-sitrep-48-covid-19.pdf?sfvrsn=16f7ccef_4 [accessed 08.03.20] [Google Scholar]
- Wu J.T., Leung K., Leung GM: Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modeling study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020 [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials used in this work are publicly available.