Abstract
Interleukin-12 activates natural killer cells and promotes the differentiation of Th1 CD4+ cells; it is a critical factor in viral immunity. IL-12 is secreted by antigen presenting cells including dendritic cells, macrophages and astrocytes, both in tissues and in secondary lymphoid organs. Experimental studies have shown that administration of the cytokine rapidly activates both innate and specific immune responses; this results in enhanced host cellular responses and generally, promotes clearance of virus and host recovery from infection. The observations of many laboratories, studying viral immunity to both RNA and DNA based pathogens, are summarized.
Keywords: Interleukin-12, viral infection, signal transduction, innate immunity, acquired immunity
1. Introduction
The elaboration of cytokines by distinct cell types and their activities on either themselves (autocrine) or on neighboring or more distant cells (paracrine and systemic, respectively) has been well documented as a part of both specific and innate immune responses. The cell surface receptors and the intracellular signal transduction pathways have been elucidated for many of these molecules 1, 2, 3. The cytokines secreted in response to a variety of stimuli have been used to define distinct subsets (for example, CD4, Th2 cells are characterized to produce IL-4, IL-5, IL-10). However, almost exclusively, studies such as these have been performed using cells of the hematopoietic lineages (e.g. lymphocytes and macrophages). Many cytokines are produced or responded to by cells of other lineages. There is a growing body of literature of regulated cytokine gene expression in the CNS both by parenchymal cells in addition to inflammatory mononuclear cells [4]. This has been observed for autoimmune diseases such as Multiple Sclerosis and its animal model, experimental allergic encephalitis, as well as in response to bacterial and viral infections 5, 6, 7. Cytokines may be elaborated to recruit and activate circulating mononuclear cells, but it also appears that resident parenchymal cells both synthesize cytokines and respond to them 4, 8, 9. In order to respond to cytokines, cells must express receptors and also have the necessary signal transduction machinery for communicating receptor occupancy 1, 2, 3. Subsequently, there must be a change in the gene expression of the cell, in response to the cytokine ligand-receptor binding. In some cases, cytokines deliver a differentiating or activating signal. Alternatively, in the case of TNF-α action for many cells, for instance, the response may be to initiate the apoptosis cascade 10, 11, 12. There is abundant evidence of tissue pathology, including cell death, in the CNS associated with inflammatory cytokine synthesis 10, 13. This review is focused on the effects of one cytokine, interleukin-12 (IL-12).
2. IL-12 Is at the CUSP of Innate and Specific Immunity
IL-12 is a 70 kD heterodimer of 35 and 40 kD peptides [14]. It is synthesized by antigen (Ag) presenting cells such as macrophages, dendritic cells, B-lymphocytes, and astrocytes in response to stimulae which may include bacterial cell wall products 14, 15, 16, 17. IL-12 was initially characterized as a Natural Killer (NK) cell activator [18]and promotes the production of Th1 CD4 effector cells from Th0 precursors [15]. IL-12 has been shown to mediate a broad range of effects on both innate and acquired immunity. It induces IFN-γ production by NK, Th1 and CD8 cells, regulates T-cells proliferation, stimulates NK cell activity, and enhances CD8 CTL responses 18, 19, 20. IL-12 has been shown to induce IFN-γ and TNF-α levels in serum and in brain tissue homogenates in mice 21, 22, 23. Orange and co-workers showed that high levels of exogenous IL-12 induced strong acute phase responses and were associated with host toxicity through the activation of the hypothalamus-pituitary-adrenal axis [22], demonstrating an interaction of the immune and stress response systems. IL-12 has been shown to serve as a direct chemotactic factor for NK cell infiltration and increases its binding to vascular endothelium cells in vitro [24]. NK cells infiltrate the virus-infected CNS before T-cell infiltration 25, 26. NK cells have an important role in clearance of many viral infections that is independent of T-cell function 27, 28. NK cells secrete IFN-γ, which participates in a positive feed-back loop amplifying IL-12 production. IL-12 also induces Th1-specific immune responses by promoting the differentiation of TH1 cells from Th0 precursors at the expense of Th2 effector cell, inhibiting IL-4 production 29, 30. The Th1 subset secretes IL-2, IFN-γ, and lymphotoxin (LT, TNF-β). Th1 cells are also cytolytic, recognizing MHC Class II and Ag. They mediate delayed-type hypersensitivity, and are thus involved in inflammation. In vitro, IL-12 suppresses the synthesis of IgE by IL-4 stimulated B-cells [31]. In vivo, intraperitoneal injection of IL-12 resulted in the enhancement of IFN-γ and IL-10 gene expression, reduced levels of IL-3 and IL-4 gene expression, and increased serum IgG2a levels [32]. The in vivo effects of IL-12 on immunoglobulin isotypes were only partially mediated by IFN-γ. The administration of anti-IL-12 antibodies in vivo significantly blocked Th1 response to antigen, evaluated by either IFN-γ production or serum IgG2a antibody response 33, 34. Thus IL-12 production may antagonize the differentiation of Th2 cells and their expansion of B-cells switching to the epsilon heavy chain [32]; IgE readily sensitizes mast cells, thus early expression of IL-12 during sensitization can inhibit the development of immediate hypersensitivity such as allergic rhinitis and asthma. IL-12 knockout mice have been produced. It was also shown that IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses [35]. These results suggest that IL-12 has a role in antigen-induced Th1 differentiation in vivo and its effects on Ig isotypes. However, in spite of the lack of IL-12 secretion in the knockout mice, Th1 cells are able to develop and are active for alloantigen responses [36]and for responses to the coronavirus, mouse hepatitis virus [37]. This suggests that other factors or cytokines may regulate Th1 cell differentiation, possibly IL-18 (reviewed in [38]). IL-12 treatment of experimental animals has been found to modify the course of many infectious diseases and the response to tumors 39, 40. In short, where an inflammatory delayed hypersensitivity (Type IV hypersensitivity) or cytolytic T-cell response is beneficial to the host, IL-12 treatment generally promotes recovery from the infection or tumor challenge. Where inflammatory responses are disadvantageous, such as in several autoimmune diseases, IL-12 treatment does not promote recovery 41, 42. The effects of IL-12 on the hosts response to viral infection will be discussed in detail.
3. IL-12 Receptor and its Signal Transduction Pathway
The IL-12 receptor (IL-12R) has been cloned from both human and murine lymphoid cells 43, 44, 45, 46, 47. They have termed the two chains β1 and β2, due to the sequence relatedness to other β chains of the hematopoietic growth factor receptor families. Th2 cells, which do not respond to IL-12, have been shown to express only one (β1) of the two IL-12R chains, which will bind IL-12 at low affinity 42, 44, 46. The signal transduction pathway has been defined in lymphoid cells to include activation of Janus kinase family members Tyk2 and Jak2, which in turn activate Signal Transducers and Activators of Transcription (STAT)3 and STAT4 48, 49. A model of the IL-12 receptor interaction with its transducers is shown in the accompanying figure. For contrast, a very well characterized receptor, is included. IL-2R is known to react with Ras which phosphorylates Mitogen Activated Protein kinases (MAP kinases) which trigger nuclear factor kappa-B (NF-kB) activation and signaling also through the phosphoinositol-3 (Pl-3) kinase pathway in addition to Jaks 1 and 3 and STATs 3 and 5. STATs are inactive in the cytoplasm until a ligand-induced activation of the cell takes place. Receptor-mediated cascades lead to phosphorylation of STATs by members of the Jak/Tyk family of tyrosine kinases and subsequent homo- or hetero- dimerization. The dimers are able to translocate to the nucleus where transcription is initiated 1, 2, 3, 50. Of all the cytokines and growth factors examined to date, STAT4 has uniquely been found to be phosphorylated by IL-12 [48]. Other stimuli are more promiscuous or overlapping in their STAT activation in lymphocytes 1, 2, 3. Mice deficient in STAT4 have been developed and initially examined for functional responses [51]. They appear to be deficient in Th1 responses and sufficient in Th2 responses to specific Ags. STAT6 knockout mice are able to mount Th1 responses but not Th2, from which it was concluded that STAT6 is essential for the IL-4 response [52]. Mice deficient in Jak2 have recently been described to be embryonic lethal; they have impaired erythropoiesis, so that the impact of this deficiency on cytokine signaling in lymphocytes cannot easily be defined 53, 54.
4. IL-12 and Viral Infections
Like bacterial infections of Ag presenting cells, viral infections rapidly induce IL-12 gene expression and immunoreactive material 55, 56, 57. In the CNS, infection rapidly induces IL-12 expression and also IL-12 treatment augments this induction (Fig. 2 Fig. 3 ), suggesting an autocrine pathway. During infection, IL-12Rβ1 and β2 mRNA is also increased (Fig. 4 ), however, at this time, since RNA was prepared from tissue homogenates, we cannot determine whether this gene expression is on IL-12-producing cells or by inflammatory NK and Th1 cells. There have been studies examining the role of IL-12 on the outcome from viral infection in many systems. In some experiments, investigators examined the change(s) in endogenous IL-12 gene expression. At times investigators have injected neutralizing Ab to IL-12 and in the majority of studies, IL-12 has been infused. The exact mechanisms by which IL-12 has its effects on the host may be distinct in each infection. We will attempt to put this into perspective.
Fig. 2.
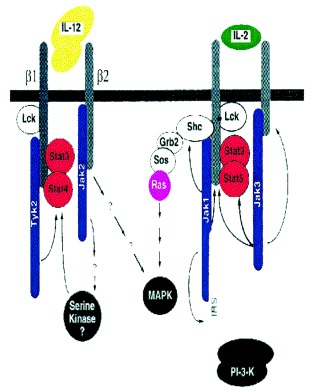
Fig. 3.
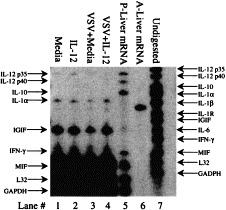
IL-12 gene expression is induced during infection or IL-12 treatment. BALB/cAnTac male mice (3/group) were untreated, injected with minimal media, or with 200 ng IL-12/mouse, infected with VSV treated with media or infected and injected with IL-12 on the day of infection. The CNS was removed on day 3 post infection and total RNA was collected from tissue homogenates of individual donors using the Ambion kit. A ribonuclease protection assay was performed on 50 μg samples of total RNA. Probes and kit were obtained from PharmingenTM and AmbionTM, respectively. Lane 2 shows an increase in the concentration of IL-12 p35 transcripts of those mice treated with IL-12, suggesting an autocrine pathway of IL-12 induction. The data shown represents the results of one individual per group and is representative of the three mice examined.
Fig. 4.
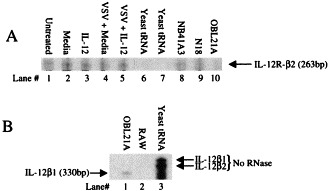
IL-12 receptor mRNA expression in induced by IL-122/ml (lanes 1 and 2 respectively), yeast tRNA (lane 3). The data show the expression of IL-12R β1 and β2 subunits both in vitro and in vivo as a result of IL-12 treatment.
4.1. EMC
Administration of a small, 20 ng dose of IL-12 protected mice from a lethal encephalomyelocarditis virus infection. This effect was not seen in mice deficient in the IFN-γR, and appeared to be acting through induction of endogenous IFN-γ secretion by NK and T cells [58].
4.2. Influenza
Endogenous IL-12 was induced during influenza pneumonia. The IL-12 attracted and activated NK cells, which secreted IFN-γ, inhibiting viral replication. In addition, there was a modest enhancement of the CD8 T cell response in response to IL-12 [59]. There was, however, no sensitivity of influenza to another downstream pathway [23].
4.3. LCMV
IL-12 administration was found to be either efficacious (at low doses) or quite toxic to mice infected with lymphocytic choriomeningitis virus. Low doses (1–10 ng) inhibited viral replication and enhanced host CD8 responses; in contrast, high doses (up to 1 mg) resulted in high levels of serum TNF-α, increased doses of virus and inhibited CD8 responses [21]. The toxicity, including thymic atrophy, of IL-12 was shown to be mediated by TNF-α and glucocorticoids [22].
4.4. VSV
IL-12 treatment promotes clearance of vesicular stomatitis virus from neurons in the CNS and survival of infected mice. This is accompanied by induction of GFAP, mac-1, MHC I and II, IFN-γ, TNF-α, NOS-1 -2 and -3, IL-12 [57](Fig. 3) and IL-12R (Fig. 4) 23, 60, 61, 62. IL-12 activity is not dependent on either IFN-γ or TNF-α in the IFN-γ-deficient mice [63]. However, IL-12 activity appears to require intracellular activity of NOS-1 in neurons for clearance of virus (not shown) and for host survival [23](Fig. 5 ). Nitric oxide (or its reaction product peroxynitrite) is a potent antiviral in many systems [64]. Most investigators have administered IL-12 either prior to or beginning on the day of infection. To be practical, however, if it were to be administered during human infections to viruses, IL-12 should be efficacious after infection has started, when symptoms are becoming apparent to the patient. IL-12 does have recovery-promoting activity even after the start of a lethal VSV encephalitis infection (Fig. 6 ).
Fig. 5.
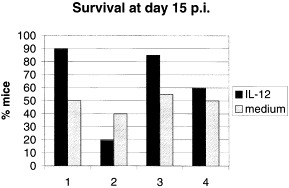
Survival of mice during experimental VSV encephalitis is promoted by IL-12 treatment and is dependent on NOS-1 expression and does not require IFN-γ gene expression. Groups of 10-40 mice (group 1: wild type = +/+; group 2: NOS-1 knockout; group 3: NOS-3 knockout; and group 4: IFN-γ knockout) were experimentally infected with vesicular stomatitis by the intranasal route. Half of the mice of each genotype were treated with 200 ng IL-12 by parenteral injection (solid bars), the others were treated with medium injections (hatched bars). Most mice succumb between days 7 and 10 post infection. IL-12 treatment of uninfected mice did not result in mortality. Data shown is pooled from five separate experiments. Survival of mice was noted over the course of a 15 day observation period.
Fig. 6.
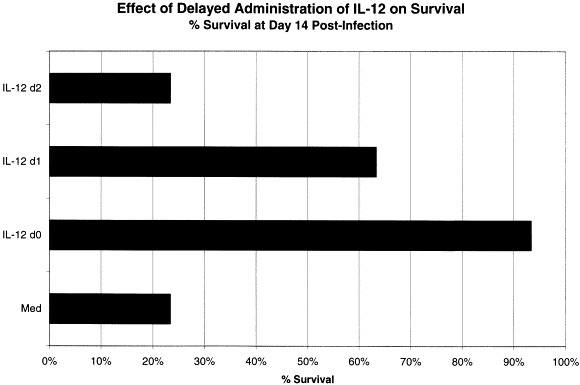
Delayed administration of IL-12 is efficacious. BALB/cAnTac male mice were infected on day zero and divided into four different groups of 10 mice: media treated beginning on the day of infection, 200 ng IL-12 on the day of infection, IL-12 on day 1 and IL-12 beginning on day 2 post infection. Survival was noted over the course of a 15 day observation period. These data represent the means of three replicate experiments.
4.5. MHV
In mice deficient of IFN-γR, mouse hepatitis virus infection results in increased susceptibility to liver injury and did not upregulate IL-12 mRNA. Exogenous IL-12 treatment of the IFN-γR knockout mice did not restore their resistance to MHV infection. However, normal mice could be protected by either IL-12 or by IFN-γ treatment [65]. IL-12 p40 or p35 knockout mice responded to MHV infection with a Th1 response like wild type mice, which was unexpected [37]. This suggests that IL-12 function may have been complemented by another cytokine or that viral infection served as a co-factor for IFN-γ-mediated activities. MHV is sensitive to IFN-γ and to another pathway, in vitro [23], but not in vivo [66].
4.6. Measles
Cell mediated immune suppression associated with measles virus infection was attributed to an inhibition in IL-12 production by infected macrophages. Measles virus receptor, CD46, also a complement receptor, is expressed on these monocytes; cross-linking of the cell surface molecule was found to diminish IL-12 production [75].
4.7. MAIDS
Murine acquired immunodeficiency syndrome, caused by infection with LP-BM5 virus, is associated with splenomegaly and lymphadenopathy as well as polyclonal B-cell activation. Treatment of MAIDS-infected mice with IL-12 resulted in diminished lymphoproliferation. This beneficial effect was not seen in IFN-γ deficient mice [67].
4.8. HIV
Co-incubation of HIV gp 120 and human macrophages/monocytes resulted in an IFN-γ-dependent production of IL-12. PBMC from HIV-infected patients were found to be deficient in production of IL-12, but not TNF-α, IL-1-β and IL-10 [20]. This led to the hypothesis that HIV-infected patients should receive IL-12 to supplement their responsiveness, overcoming the depletion of Th1 cells due to infection-related apoptosis. In addition, co-administration of genetic vaccines for HIV gp 160 and IL-12 resulted in enhanced cell mediated responses in mice [68].
4.9. HBV
Hepatitis B virus does not naturally infect mice. However, Chisari has developed a model of transgenic mice, which express virus in hepatocytes and develop immunopathology. IL-12 treatment induced IFN-γ, TNF-α, and IFN-α/β, and inhibited HBV replication in liver and kidney [69].
4.10. PRV
Th1 Pseudorabies virus vaccine responses were augmented by IL-12 administration in immunocompetent mice. However, mice which lacked IFN-γR were unresponsive to the IL-12 treatment and did not develop resistance to viral challenge [70].
4.11. MCMV
For many herpes virus infections, an early NK response is essential in recovery from infection [27]. For Murine Cytomegalovirus infection, NK-cell derived IFN-γ was shown to be required for the hostss response. This was augmented by IL-12 administration, increasing NK activity, increasing IFN-γ production, and diminishing viral titer [71].
4.12. HSV
Experimental corneal Herpes simplex virus infection induces the production of IL-12 p40 mRNA both in cornea and in draining lymph nodes. This may result in initiating inflammation to the site of infection [56], resulting in immunopathology. However, IL-12 exhibits potent antiviral activities for HSV, induced IFN-γ, and protected mice from lethal infection [72]. IL-12 treatment also protected mice from thermal injury and increased susceptibility to HSV-1 morbidity and mortality [73].
4.13. H. Sam.
Like Epstein–Barr virus transformation of human B-lymphocytes, Herpes saimiri virus can immortalize human γ δ T cells from peripheral blood. Treatment of transformed T-cells with IL-12 led to their activation, induced synthesis of perforin and granzyme B, and enhanced their CTL activity [74].
5. Conclusions
IL-12 treatment of experimental hosts generally has a profoundly beneficial outcome on the response to viral infection. Whether IL-12 acts directly or indirectly through IFN-γ or TNF-α and their downstream responses (RNAase L, NOS, caspases, for instance), as shown in Fig. 1 , the general effects are (1) the recruitment and induction of NK cells, (2) cytokine release by innate immune and T-cells, (3) facilitating the differentiation of Th1 cells which are both inflammatory and cytolytic as well as helpers, (4) amplifying CD8 responders, (5) enhancing the production of neutralizing IgG2a Ab, and ultimately (6) the inhibition of viral replication and clearance of virus from host cells. These activities appear both in peripheral and in CNS infections. Table 1 illustrates some of the cytokine-inducible proteins and their activities in virally infected cells. Since IL-12 works both at the time of infection, and also after symptoms and viral replication has begun (Fig. 6), it may be efficacious in treating humans. IL-12 treatment also enhances responses to vaccination, whether by co-administration of cytokine at the time of inoculation of protein Ags, or by genetic vaccination [68]. The cytokine is already in Phase II clinical trials for renal carcinoma and trials are under way for Mycobacterium tuberculosis infections, both long-term treatments; therefore, it may be relatively easy to develop clinical trials from human encephalitis (picornavirus, rabies), for Herpes infections, and for HIV. It may not be a magic bullet alone, but may substantially augment antiviral drug therapies.
Fig. 1.
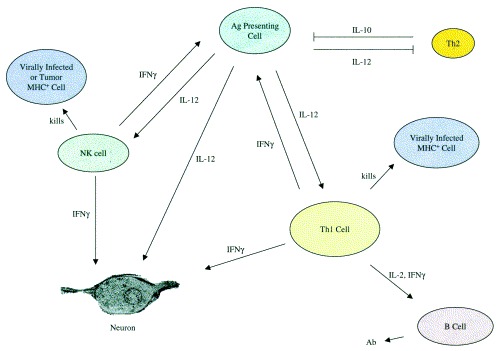
IL-12 regulation of immune responses. IL-12, produced by antigen presenting cells including dendritic cells, macrophages, and astrocytes, activates natural killer (NK) and CD4 T-cells. The latter produce IFN-γ which increases IL-12 production. IL-12 directly activates neurons to synthesize NOS-1, which is an antiviral molecule. CD4 cells help B-cells make Ab, especially isotypes which are able to neutralize virus or fix complement and lyse virally infected cells.
Table 1.
Effects of IL-12 on virally infected cells
| Action | Effector | Effector action | Example(s) | Outcome |
|---|---|---|---|---|
| Direct | IL-12 | IL-12 inducible genes | NOS 1, 2, 3, TNF-α, IFN-γ others? | Inhibition of viral infection, chemotaxis for NK cells |
| Indirect | IFN-γ | IFN-γ-inducible genes | IRF-1, Mx, GTPAses, RNAse L, 2′-5′A Synthase, RNA-dependent Protein Kinase (PKR), NOS 1, 2, 3, MHC, IP-10/crg-2 | Cytostasis, viral inhibition, increases sensitivity to CTL lysis, chemotaxis and angiostatic activities |
| Indirect | TNF-α | TNF-α-inducible genes and pathways | Caspases, NOS 1, 2, 3 | Apopotosis of virally infected cells, viral inhibition |
Acknowledgements
Acknowledgements:We gratefully acknowledge the availability of reagents and timely support from the Genetics Institute and ongoing support from the National Institute of Deafness and Communication Disorders DC03536 to CSR. Excellent artwork was provided by Ms Sophie Stein and Mr Andrew Mule. The original work included here built on studies done by Trinh Bang, Maria Barna, Zhengbiao Bi, Angela Christian, Nancy Chung, Andrew Mule, Vimal Patel, and Ilya Plakhov.
References
- 1.Levy DE. The house that Jak/STAT built. Cytokine and Growth Factor Rev 1997, 8, 81–90. [DOI] [PubMed]
- 2.OShea JJ. Jaks, Stats, cytokine signal transduction, and immunoregulation: are we there yet? Immunity 1997, 7, 1–11. [DOI] [PubMed]
- 3.Ihle JN. Cytokine receptor signaling. Nature 1995, 377, 591–594. [DOI] [PubMed]
- 4.Relton JK, Neuberger TJ, Bendele AM, Martin D, Russell D. Cytokines: neurotoxicity and neuroprotection. In Bar PR, Beal MF, eds. Neuroprotection in CNS Diseases. NY, Dekker, 1997, 225–241.
- 5.Ransahoff RM, Hamilton T.A, Tani M, Stoler M.H, Schick H.S, Major J.A, Estes M.L, Thomas D.M, Tuohy V.K. F.A.S.E.B. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 6.Fontana, Constam D, Frei K, Koedel U, Pfister W, Weller M. Cytokines and defense against CNS infection. Cytokines and the CNS. CRC Press, 1996, 188–219.
- 7.Irani DN, Lin K-I, Griffin D. Regulation of brain-derived T cells during acute CNS inflammation. Immunol 1997, 158, 2318–2326. [PubMed]
- 8.Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CF. Distribution and characterization of TNF-α-like immunoreactivity in the murine CNS. J Comp Neuro 1993, 337, 543–567. [DOI] [PubMed]
- 9.Ilyin SE, Plata-Salaman CR. In vivo regulation of the IL-1β system and TNF-α mRNAs in specific brain regions. Biochem and Biophys Res Comm 1996, 227, 861–867. [DOI] [PubMed]
- 10.Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G. TNF-α transgenic and knockout models of CNS inflammation and degeneration. J NeuroImmunol 1997, 72, 137–141. [DOI] [PubMed]
- 11.Wallach D. Suicide by order: some open questions about the cell killing activities of the TNF-α ligand and receptor families. Cytokine and Growth Factor Rev 1996, 7, 211–221. [DOI] [PubMed]
- 12.Wisniewski H-G, Hua JC, Poppers DM, Naime D, Vilcek J, Cronstein BN. TNF-IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-α-inhibitor and exerts strong anti-inflammatory effect in vivo. J Immunol 1996, 156, 1609–1616. [PubMed]
- 13.Bruce AJ, Bolling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF-R. Nat Med 1996, 2, 788–794. [DOI] [PubMed]
- 14.Wolf SF, Diebarth S, Sypek J. IL-12 a key modulator of immune function. Stem Cell 1994, 12, 154–168. [DOI] [PubMed]
- 15.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, OGarra A, Murphy KM. Development of CD4 T cells through IL-12 produced by Listeria-induced macrophages. Science 1993, 260, 547–551. [DOI] [PubMed]
- 16.Constantinuescu C, Frei K, Malipiero U, Rostami A, Fontana A. Astrocytes and microglia produce IL-12. Ann N York Acad Sci 1996, 795, 328–333. [DOI] [PubMed]
- 17.Stalder AK, Pagenstecher A, Yu NC, Kincaid C, Chiang CS, Hobbs MV, Bloom FE, Campbell IL. Lipopolysaccharide-induced IL-12 expression in the CNS and cultured astrocytes and microglia. J Immunol 1997, 159, 1344–1351. [PubMed]
- 18.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf S, Young D, Clark SC, Trinchieri G. Induction of IFN-γ production by NK stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med 1991, 173, 869–879. [DOI] [PMC free article] [PubMed]
- 19.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med 1989, 170, 827–846. [DOI] [PMC free article] [PubMed]
- 20.Chehimi A, Valiante N, DAndrea A, Rengaraju M, Rosado Z, Kobayashi M, Perussia B, Wolf SF, Starr SE, Trinchieri G. Enhancing effect of natural killer cell stimulatory factor (NKSF/interleukin-12) on cell-mediated cytotoxicity against tumor-derived and virus-infected cells. Eur J Immunol 1993, 23, 1826–1830. [DOI] [PubMed]
- 21.Orange JS, Wolf SF, Biron CA. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol 1994, 152, 1253–1264. [PubMed]
- 22.Orange JS, Salazar-Mather TP, Opal SM, Spencer RL, Miller AH, McEwen BS, Biron CA. Mechanism of interleukin-12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med 1995, 181, 901–914. [DOI] [PMC free article] [PubMed]
- 23.Komatsu T, Bi Z, Reiss CS. IFN-γ induced NOS-1 activity inhibits viral replication in neurons. J NeuroImmunol 1996, 68, 101–108. [DOI] [PubMed]
- 24.Allavena P, Paganin C, Zhou D, Bianchi G, Sozzani S, Mantovani A. Interleukin-12 is chemotactic for natural killer cells and stimulates their interaction with vascular endothelium. Blood 1994, 84, 2261–2268. [PubMed]
- 25.Williamson JS, Sykes KC, Stohlman SA. Characterization of brain-infiltrating mononuclear cells during infection with mouse hepatitis virus strain JHM. J NeuroImmunol 1991, 32, 199–207. [DOI] [PMC free article] [PubMed]
- 26.Christian AY, Barna M, Bi Z, Reiss CS. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BI/6 mice. Viral Immunol 1996, 9, 195–205. [DOI] [PubMed]
- 27.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Eng J Med 1989, 320, 1731–1735. [DOI] [PubMed]
- 28.Welsh R.M, Brubaker J.O, Vargas-Cortes M, O Donnell C.L. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991;173:1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, OGarra A, Murphy KM. Development of Th1 CD4 T cells through IL-12 produced by Listeria-induced macrophages. Science 1993, 260, 547–549. [DOI] [PubMed]
- 30.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer stimulatory factor (interleukin 12) induces T helper type-1 (Th1)-specific immune responses and inhibits the development of IL-4-producing cells. J Exp Med 1993, 177, 1199–1204. [DOI] [PMC free article] [PubMed]
- 31.Kiniwa M, Gately M, Gubler U, Chizzonite R, Fargeas C, Delespesse G. Recombinant interleukin-12 suppresses the synthesis of immunoglobulin E by interleukin-4 stimulated human lymphocytes. J Clin Invest 1992, 90, 262–266. [DOI] [PMC free article] [PubMed]
- 32.Morris SC, Madden KB, Adamovicz JJ, Gause WC, Hubbard BR, Gately MK, Finkelman FD. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol 1994, 152, 1047–1056. [PubMed]
- 33.McKnight AJ, Zimmer GJ, Fogelman I, Wolf SF, Abbas AK. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol 1994, 152, 2172–2179. [PubMed]
- 34.Williamson E, Garside P, Bradley JA, More IA, Mowat AM. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes and cytokine profile toward a Th2-type alloimmune response and confers long term protection from disease. J Immunol 1997, 159, 1208–1215. [PubMed]
- 35.Magram J, Connaughton SE, Warrier RR, Carvajal D, Wu C, Ferrante J, Steward C, Sarmiento U, Faherty DA, Gately MK. IL-12 deficient mice are defective in IFN-gamma production and type 1 cytokine responses. Immunity 1996, 4, 471. [DOI] [PubMed]
- 36.Piccotti JR, Li K, Chan SY, Ferrante J, Magram J, Eichwald EJ, Bishop DK. Alloantigen-reactive TH1 development in IL-12 deficient mice. J Immunol 1998, 160, 1132–1138. [PubMed]
- 37.Schijns VE, Haagmans BL, Wierda CM, Kruithof B, Heijnen IA, Alber G, Horzinek MC. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J Immunol 1998, 160, 3958–3964. [PubMed]
- 38.Gillespie MT, Horwood NJ. IL-8: prespectives on the newest interleukin. Cytokine and Growth Factor Rev 1998, 9, 109–116. [DOI] [PubMed]
- 39.Chen L, Chen D, Block E, ODonnell M, Kufe DW, Clinton SK. Eradication of murine bladder carcinoma by intratrumor injection of a bicistronic adenoviral vector carrying cDNAs of the IL-12 heterodimer and its inhibition by the IL-12 p40 subunit homodimer. J Immunol 1997, 159, 351–359. [PubMed]
- 40.Coughlin CM, Wysocka M, Trinchieri G, Lee WMF. The effects of IL-12 desensitization on the antitumor efficacy of rIL-12. Cancer Res 1997, 57, 2460–2467. [PubMed]
- 41.Leonard JP, Waldberger KE, Goldman NS SJ. Prevention of EAE by antibodies against IL-12. J Exp Med 1995, 181, 368–381. [DOI] [PMC free article] [PubMed]
- 42.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. IL-12/IL-12R system: role in normal and pathologic immune responses. Ann Rev Immunol 1998, 16, 495–521. [DOI] [PubMed]
- 43.Chua AO, Wilkinson VL, Presky D, Gubler U. Cloning and characterization of a mouse IL-12R β component. J Immunol 1995, 155, 4286–4294. [PubMed]
- 44.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the IL-12R β2 subunit expression in developing Th1 and Th2 cells. J Exp Med 1997, 185, 817–824. [DOI] [PMC free article] [PubMed]
- 45.Wu C-Y, Warrier R.R, Wang X, Presky D.H, Gately M.K. European J Immunol. 1997;27:147–154. doi: 10.1002/eji.1830270122. [DOI] [PubMed] [Google Scholar]
- 46.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of IL-12R by human Th1 cells. J Exp Med 1997, 185, 825–831. [DOI] [PMC free article] [PubMed]
- 47.Showe L.C, Wysocka M, Wang B, Line-man-Williams D, Peritt D, Showe M.K, Trinchieri G. In IL- 1996;795:413–415. doi: 10.1111/j.1749-6632.1996.tb52708.x. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Murphy KM. IL-12 signaling in Th1 cells involves tyrosine phosphorylation of Stat 3 and 4. J Exp Med 1995, 181, 1755–1762. [DOI] [PMC free article] [PubMed]
- 49.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Development commitment to the TH2 lineage by extinction of IL-12 signaling. Immunity 1995, 2, 665–675. [DOI] [PubMed]
- 50.Leonard WJ, OShea JJ. Jaks STATs: Biological implication. Ann Rev Immunol 1998, 16, 295–322. [DOI] [PubMed]
- 51.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat-4-deficient mice. Nature 1996, 382, 174–177. [DOI] [PubMed]
- 52.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for the development of the Th2 cells. Immunity 1996, 4, 313–319. [DOI] [PubMed]
- 53.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, Grosveld G, Ihle JN. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 1998, 93, 385–395. [DOI] [PubMed]
- 54.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 1998, 93, 397–409. [DOI] [PubMed]
- 55.Coutelier JP, Broeck JV, Wolf SF. Interleukin-12 gene expression after viral infection in the mouse. J Virol 1995, 69, 1955–1958. [DOI] [PMC free article] [PubMed]
- 56.Kanangat S, Thomas J, Grangappa S, Babu J.S, Rouse B.T. Implications in immunopathogenesis and protection. J Immunol. 1996;156:1110–1116. [PubMed] [Google Scholar]
- 57.Barna M, Komatsu T, Reiss CS. Cytokine-induced activation of type III nitric oxide synthase in astrocytes following a neurotropic viral infection in the CNS. Virol 1996, 233, 331–343. [DOI] [PubMed]
- 58.Ozmen L, Aguet M, Trinchieri G, Garotta G. The in vivo antiviral activity of IL-12 is mediated by IFN-γ. J Virol 1998, 69, 8147–8150. [DOI] [PMC free article] [PubMed]
- 59.Monteiro JM, Harvey C, Trinchieri G. Role of interleukin-12 in primary influenza virus infection. J Virol 1998, 72, 4825–4831. [DOI] [PMC free article] [PubMed]
- 60.Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol 1995, 69, 6466–6471. [DOI] [PMC free article] [PubMed]
- 61.Bi Z, Quandt P, Komatsu T, Barna M, Reiss CS. IL-12 promotes enhanced recovery from vesicular stomatitis virus infection of the central nervous system. J Immunol 1995, 155, 5684–5689. [PubMed]
- 62.Komatsu T, Barna M, Reiss CS. IL-12 promotes recovery from viral encephalitis. Viral Immunol 1997, 10, 35–47. [DOI] [PubMed]
- 63.Komatsu T, Reiss CS. IFN-γ is not required in the IL-12 response to VSV infection of the olfactory bulb. J Immunol 1997, 159, 3444–3452. [PubMed]
- 64.Reiss CS, Komatsu T. Does nitric oxide play a critical role in viral infections? J Virol 1998, 72, 4547–4551. [DOI] [PMC free article] [PubMed]
- 65.Schijns V, Wierda CMH, Hoeij MV, Horzinek MC. Exacerbated viral hepatitis in IFN-γ receptor-deficient mice is not suppressed by IL-12. J Immunol 1996, 157, 815–821. [PubMed]
- 66.Lane TE, Paoletti AD, Buchmeier MJ. Dissociation between in vitro and in vivo effects of nitric oxide on a neurtropic murine coronavirus. J Virol 1997, 71, 2202–2210. [DOI] [PMC free article] [PubMed]
- 67.Gazzinelli RT, Giese NA, Morse III HC. In vivo treatment with interleukin-12 protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome (MAIDS). J Exp Med 1994, 180, 2199–2208. [DOI] [PMC free article] [PubMed]
- 68.Tsuji T, Hamajima K, Fukushima J, Xin K-Q, Ishii N, Aoki I, Ishigatsubo Y, Tani K, Kawamoto S, Nitta Y, Miyazaki J, Koff WC, Okubo T, Okuda K. Enhancement of cell-mediated immunity against HIV-1 induced by coinoculation of plasmic-encoded HIV-1 antigen with plasmid expressing IL-12. J Immunol 1997, 158, 4008–4013. [PubMed]
- 69.Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol 1997, 71, 3236–3243. [DOI] [PMC free article] [PubMed]
- 70.Schijns, VECJ, Haagmas BL, Horzinek MC. IL-12 stimulates an antiviral type 1 cytokine response but lacks adjuvant activity in IFN-γ-receptor deficient mice. J Immunol 1995, 155, 2525–2532. [PubMed]
- 71.Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell-produced interferon-γ in defense against murine cytomegalovirus infection and enhancement of this defense pathway by Interleukin-12 administration. J Exp Med 1995, 182, 1045–1056. [DOI] [PMC free article] [PubMed]
- 72.Carr JA, Rogerson J, Mulqueen MJ, Roberts NA, Booth RFG. Interleukin-12 exhibits potent antiviral activity in experimental herpesvirus infections. J Virol 1997, 71, 7799–7803. [DOI] [PMC free article] [PubMed]
- 73.Matsuo R, Kobayashi M, Herndon DN, Pollard RB, Suzuki F. Interleukin-12 protects thermally injured mice from herpes simplex virus type I infection. J Leuk Biol 1996, 59, 623–630. [DOI] [PubMed]
- 74.Klein JL, Fickenscher H, Holliday JE, Biesinger B, Fleckenstein B. Herpesvirus saimiri immortalized γ δ T cell line activated by IL-12. J Immunol 1996, 156, 2754–2760. [PubMed]
- 75.Karp CL, Wysocka M, Whal LM, Ahern JM, Cuomo PJ, Sherry B, Trinchieri G, Griffin DE. Mechanism of suppression of cell mediated immunity by measles virus. Science 1996, 273, 228–231. [DOI] [PubMed]


