Abstract
A 3-year-old, neutered male Tiffany cat was presented to the Animal Health Trust for investigation of pyrexia and a gastric lesion. Radiography and ultrasound showed severe thickening of the gastric wall and regional lymphadenopathy. There was altered gastric wall layering, predominately due to muscularis thickening. Histopathology confirmed eosinophilic fibrosing gastritis. The cat also had evidence of generalised Toxoplasma gondii infection, which may have been responsible for the gastric changes.
A 3-year-old Tiffany cat was referred to the Animal Health Trust for investigation of acute onset, unilateral head tilt and imbalance. There had been mild diarrhoea for 3 days, starting simultaneously with the head tilt but resolving prior to referral. Clinical (including ophthalmic) examination revealed a right-sided head tilt, otherwise the cat was normal. Neurolocalisation indicated a peripheral vestibular lesion. Magnetic resonance imaging, brainstem auditory evoked response (BAER) test and cerebrospinal fluid analyses (including coronavirus serology) were normal. Routine biochemistry and haematology were within normal limits. Serology for toxoplasma (IgG ELISA) was positive with a high titre (1:2048) and for feline coronavirus (1:1280). A provisional diagnosis of idiopathic vestibular disease was made, but to rule out otitis media/interna the cat was treated with enrofloxacin (Baytril; Bayer, 25 mg twice daily (bid) per os (PO)) for 6 weeks. The head tilt did not diminish and the owner reported the cat to be lethargic and that it licked its lips excessively. The antibiotic was changed to clindamycin (Antirobe; Pharmacia Animal Health, 15 mg/kg bid PO). There was no improvement in the head tilt or the cat's demeanour and the owner stopped the clindamycin after 8 days.
Six months later the cat presented to the referring veterinarian with vomiting and lethargy of 1 week's duration. Clinical examination by the referring vet revealed pyrexia and an irregular cranial abdominal mass, the cat was otherwise unremarkable. The head tilt was not evident and the owner was unable to state when it had resolved. A routine biochemical profile was normal. Radiography of the thorax was unremarkable, but abdominal radiography and ultrasound showed what appeared to be a large gastric mass. Exploratory laparotomy, performed on the same day, revealed poorly circumscribed thickening of the stomach and duodenum and enlargement of the drainage lymph nodes. Surgical biopsies of the stomach and lymph node were taken. Histological examination of the gastric biopsies showed marked fibrosis of the gastric wall accompanied by a mixed inflammatory infiltration including frequent eosinophils. The lymph node biopsy revealed hyperplasia and an eosinophilic lymphadenitis with marked thickening of the capsule, also associated with a predominately eosinophilic infiltrate. Based on the biopsy results a diagnosis of eosinophilic gastritis was made. The cat was treated with fenbendazole (Panacur; Intervet, 1.8 g once daily PO) for 3 days and prednisolone (Prednicare; Animalcare, 5 mg bid PO). The cat showed some improvement and stopped vomiting but remained lethargic. Repeat physical examination 11 days post-biopsy showed the gastric lesion to be enlarging.
Fourteen days after initial presentation the cat was referred to the Animal Health Trust for further investigation of the gastric lesion.
On admission, physical examination of the cat revealed a palpable (approximately 10 cm diameter) cranial abdominal mass and pyrexia (39.8°C). The cat weighed 3.8 kg and had a normal body condition score. Routine ophthalmic examination revealed areas of disseminated chorioretinitis (Fig. 1). The retinal arterioles were irregular (areas of dilation and constriction) with increased tortuosity. Routine haematological and biochemical analyses were performed and revealed a mild hypochromic anaemia (haematocrit 0.22 l/l, reference range 0.24–0.45 l/l, red cell count 3.9×1012 l/l, reference range 5.5–10×1012 l/l, mean corpuscular haemoglobin concentration 29.5 g/dl, reference range 32–35 g/dl), and mild increases in alanine aminotransferase (ALT) (205 U/l, reference range 0–80) and creatine kinase (Ck) (393 U/l, reference range 21–56).
Fig 1.
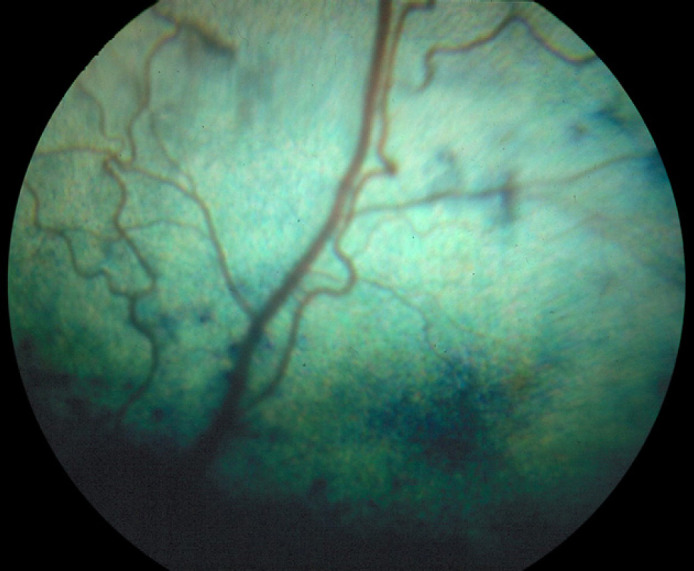
Photograph of the retina showing fundic lesions associated with a chorioretinitis. Retinal arterioles demonstrate increased tortuosity and irregularity. There are areas of abnormal choroidal pigmentation with a few bullae. Numerous bullae in addition to hyperreflectivity were present in other areas of the tapetal fundus.
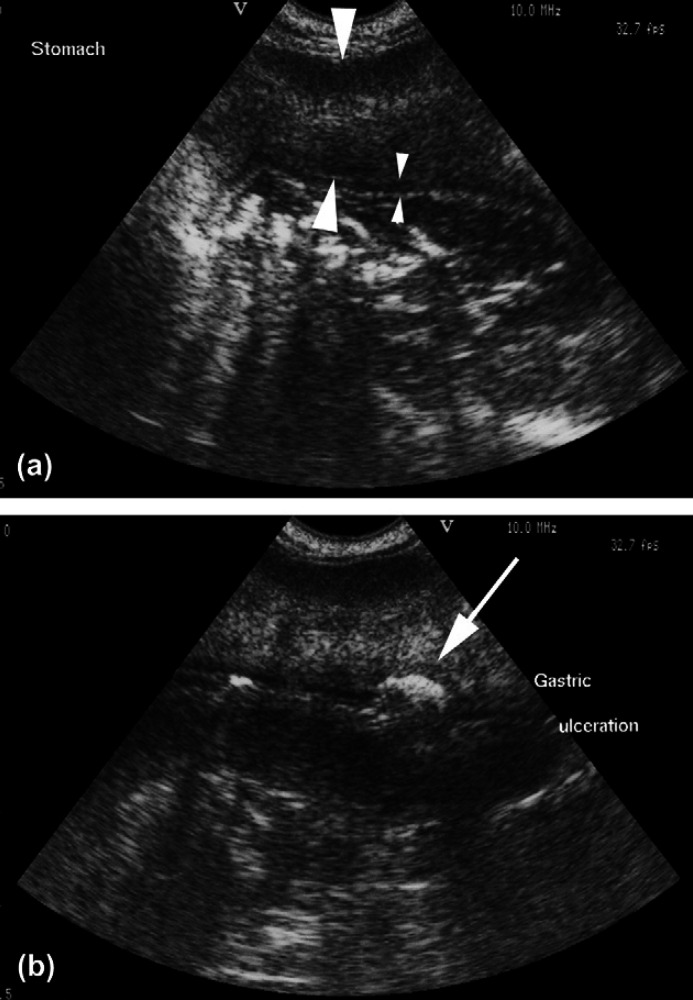
The cat was anaesthetised for abdominal ultrasound and biopsy of the stomach. Ultrasonographic examination of the abdomen showed severe, generalised and diffuse thickening of the stomach, predominately involving the muscularis and serosal layers of the gastric body and pylorus (Fig. 2). The stomach appeared rigid and amotile. The majority of the mucosa and submucosa had a normal layered appearance and subjectively appeared normal in thickness but there was massive, diffuse thickening throughout the muscularis layer. The muscularis was increased in echogenicity with a ‘streaky’ appearance resulting in reduced contrast with the submucosa. The serosal layer could not be clearly defined as a distinct layer but the external surface of the stomach was slightly irregular. In one area, there was a focal crater within the mucosal surface with a hyperechoic interface suggestive of gastric ulceration. No abnormalities were seen in the small intestine but there was generalised lymph node enlargement within the abdomen and the lymph nodes appeared hypoechoic and rounded. A small volume of anechoic free abdominal fluid was also seen. Ultrasound guided abdominocentesis and multiple (4+) percutaneous needle biopsies (18 gauge tru-cut) and fine needle aspirates of the muscularis and serosal layers of the stomach were performed.
Fig 2.
Transverse (a) and oblique (b) ultrasound images of the stomach. There is massive thickening and increased echogenicity of the muscularis layer (large arrowheads) resulting in loss of visualisation of the submucosal and serosal layers. The mucosal layer is normal (small arrowheads) and clearly defined except for focal cratering (arrow) (b) suggestive of ulceration.
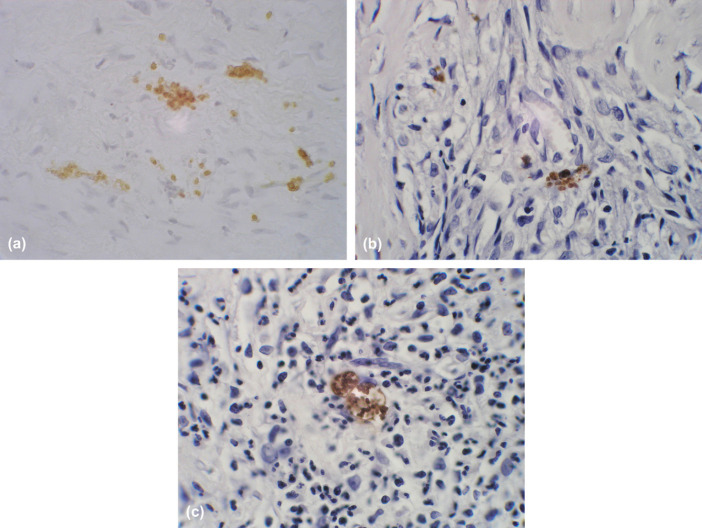
Analysis of the abdominal fluid showed elevated protein (62 g/l) and cell count (nucleated cell count 3410 cells/μl) consistent with a high protein modified transudate. The cells were a mixture of small lymphocytes, macrophages and eosinophils in roughly equal proportions with 7% neutrophils. Feline coronovirus serology of the fluid was negative. Protozoal tachyzoites were seen within the cytoplasm of a macrophage on one of the fine needle aspirate smears. The biopsy of the gastric wall showed disruption of the muscularis by bands of fibrovascular tissue infiltrated with large numbers of eosinophils and smaller numbers of lymphocytes, plasma cells and histiocytes. Examination with pinacyanol stain showed diffusely scattered well-differentiated mast cells consistent with an inflammatory infiltrate; there was no evidence of mast cell neoplasia. Ziehl–Neelsen staining for acid-fast bacteria was negative and special culture of fresh tissue obtained from the biopsy showed no growth of mycobacteria. Periodic acid-Schiff (PAS) staining did not reveal any evidence of fungal infection. Tissue cysts containing protozoal bodies were seen in one area of the biopsy. Immunostaining of the gastric biopsy showed the presence of multiple free organisms diffusely throughout the lesion (Fig. 3a) and confirmed the organisms to be Toxoplasma gondii. Subsequent immunostaining of the original gastric and lymph node biopsies revealed several cysts containing T gondii bradyzoites within the lymph node and free organisms, not apparent on haematoxylin and eosin stained sections, within inflammatory lesions in the gastric biopsy (Fig. 3b). Immunostaining proved negative for Neospora caninum organisms. Based on these results a diagnosis of eosinophilic fibrosing gastritis and toxoplasmosis was made. Blood tests for feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV) (by polymerase chain reaction) and serology for feline coronavirus were negative. Additional serological tests for T gondii were not performed.
Fig 3.
Photomicrographs following immunoperoxidase staining with a toxoplasma-specific antibody (400×). Disseminated toxoplasma organisms (brown staining) are present throughout inflammatory lesions within the tru-cut biopsy of the stomach wall (a) and the pre-treatment surgical biopsies of the stomach (b).
Pending the biopsy and cytology results, the cat was treated with 50 mg metronidazole (non-proprietary, 50 mg bid), clavulanate-potentiated amoxycillin (Synulox; Pfizer, 50 mg bid), cimetidine (non-proprietary, 20 mg tid) and sucralfate (non-proprietary, 0.2 g tid). Intravenous fluid therapy was given. Antibiotic therapy was changed to clindamycin (Antirobe; Pharmacia Animal Health, 50 mg bid PO) on receipt of the biopsy results 5 days later. Despite treatment, the cat remained persistently pyrexic (up to 40.3°C), although was clinically bright and eating normally. Eight days after referral the cat deteriorated acutely with tachypnoea and pale mucous membranes. Thoracic radiographs showed a mild generalised mixed broncho-interstitial lung pattern. In the periphery of the lungfields there were a few small, sparse ill-defined amorphous patches of an alveolar lung pattern. Abdominal radiographs demonstrated reduced serosal detail with wispy soft tissue/fluid superimposed over the falciform fat. The pylorus was enlarged with gross, irregular thickening of the gastric wall (Fig. 4). The transverse colon and small intestines were displaced caudally by the enlarged stomach. The serosal surface of the stomach could not be clearly seen due to a small volume of abdominal fluid. The haematocrit was reduced (0.10 l/l reference range 0.24–0.45 l/l) and as the cat was deteriorating, 50 ml of matched type A blood was transfused. There was no improvement in the cat's tachypnoea despite supplemental oxygen and he remained depressed. The owners requested euthanasia due to the poor response to treatment but refused permission for a necropsy.
Fig 4.
Lateral radiograph of the abdomen. There is severe circumferential thickening of the stomach wall (arrows) which displaces small and large intestines caudally.
Toxoplasma gondii is an obligate intracellular coccidian parasite with cats being the definitive host. The mode of transmission is through ingestion of infected tissues, ingestion of oocyst-contaminated food or water, or by congenital infection. Most cats are thought to become infected by ingesting intermediate hosts infected with tissue cysts (Dubey and Carpenter 1993, Dubey and Lappin 1998). In cats, Toxoplasma gondii undergoes an enteroepithelial cycle. Bradyzoites are released into the stomach and intestine from the tissue cysts when the cyst wall is dissolved by digestive enzymes. Bradyzoites penetrate the epithelial cells of the small intestine and initiate five asexual stages before oocysts are passed in faeces. An extraintestinal life cycle also occurs in all hosts. Bradyzoites (in tissue cysts) or sporozoites (in oocysts) excyst in the lumen of the small intestine and penetrate intestinal cells, including the cells of the lamina propria. The organism divides and transforms into tachyzoites, which can multiply in almost any cell in the body.
Infection with T gondii is widespread in cat populations, but in most cats, proliferation of the organism is contained by an immune response and the infection remains asymptomatic (Dubey 1986, Lappin et al 1991). When disease develops, clinical signs are variable and non-specific; but pyrexia, anorexia, lethargy and dyspnoea have been reported in generalised toxoplasmosis (Dubey and Lappin 1998). Gastrointestinal signs have been reported in up to 35% of cases of generalised disease (Petrak and Carpenter 1965), and involvement of the gastrointestinal tract (predominately intestinal) has been reported in up to 41.5% of clinical cases examined histologically (Dubey and Carpenter 1993). However, such information has been mainly derived from post-mortem examination of fatal cases of generalised feline toxoplasmosis, and there is evidence that ocular disease (uveitis and chorioretinitis) may be a more common and non-fatal manifestation of feline toxoplasmosis (Lappin et al 1989, Lappin 2000a).
Clinical signs caused by generalised toxoplasmosis are uncommon in infected cats and may be associated with immunosuppression (Dubey and Lappin 1998). Despite investigations, an underlying or predisposing cause could not be conclusively identified in this cat. Immunosuppressive drugs can result in recrudescence and generalised toxoplasmosis (Bernsteen et al 1999), but experimentally, the use of high doses of prednisolone in cats with evidence of exposure did not result in development of generalised toxoplasmosis (Dubey and Frenkel 1974). The initial serology results 6 months earlier had showed exposure to T gondii and recrudescence is possible. In this case, steroid administration was not thought to be a contributing factor as there was evidence of active infection in the biopsies taken prior to treatment with steroids. Inflammatory doses of prednisolone are used widely without resulting in recrudescence of toxoplasmosis, and are recommended in cases of ocular toxoplasmosis. In the largest reported series of naturally occurring cases, the use of corticosteroids was not thought to be a predisposing factor (Dubey and Carpenter 1993).
A presumptive diagnosis of toxoplasmosis may be made on the basis of appropriate clinical signs, serological testing and appropriate response to therapy (Lappin et al 1989). In this case, previous serology had demonstrated a high IgG antibody titre to T gondii and a definitive ante mortem diagnosis of active infection was made on the basis of histology and immunohistochemistry.
Gastric changes are uncommon with toxoplasmosis, and in this case a direct causal link to the toxoplasmosis cannot be shown, but the two conditions were thought to be linked. Gastric involvement in naturally occurring generalised toxoplasmosis is rarely reported (Petrak and Carpenter 1965, Peterson et al 1991). In one experimental study, where cats were infected with T gondii and then killed, no gastric involvement was reported (Dubey and Frenkel 1972). In another study, in which infected cats were given high doses of corticosteroids, gastric involvement was found in six of 24 kittens, with the organism preferentially affecting the muscularis (Dubey and Frenkel 1974). Acute toxoplasmosis typically results in necrosis within the intestinal wall (Dubey 1986, Peterson et al 1991). There are, however, few detailed reports describing the gastrointestinal changes seen in naturally occurring chronic toxoplasmosis (Meier et al 1957, Hirth and Nielsen 1969). There are occasional reports of intestinal granulomas, and chronic and proliferative lesions secondary to toxoplasmosis (Hulland 1956, Meier et al 1957, Hirth and Nielsen 1969, Feeney et al 1981). Unfortunately, detailed histopathological descriptions are often lacking (Meier et al 1957, Hirth and Nielsen 1969). Further studies are required to determine if naturally occurring toxoplasmosis can result in chronic gastrointestinal granulomatous disease. This case differs from previously published reports in that a marked eosinophilic component and chronic inflammatory response was seen. It is possible that the chronic inflammatory response seen in this case reflects a prolonged infection with T gondii, with incomplete treatment early in the course of the disease.
Eosinophilic fibrosing gastritis has not been previously reported with toxoplasmosis. Eosinophilic gastritis is an uncommon condition and may be secondary to foreign bodies, neoplasia, hypersensitivity, fungal, viral (feline infectious peritonitis), parasitic disease and hypereosinophilic syndrome (HES) (Guildord and Strombeck 1996). HES was excluded, in this cat, on the basis of haematology. Careful histopathological review with special staining did not reveal any other underlying infectious or neoplastic disease although tumours, such as mast cell tumour, may be difficult to identify (Howl and Peterson 1995).
Many of the findings in this case (eg, mesenteric lymphadenopathy, anaemia, biochemical changes, pyrexia) are similar to those reported in generalised toxoplasmosis, but are non-specific findings (Dubey and Lappin 1998). The increased serum concentrations of ALT and CK may have been the result of hepatic and muscle involvement, although this was unconfirmed. Anaemia is commonly seen in cases of generalised toxoplasmosis but is a non-specific finding. In this case the cause of the anaemia is unknown but the acute decrease in haematocrit was thought to be due to bleeding from a gastric ulcer (based on ultrasonographic findings), although other causes such as haemolytic anaemia cannot be ruled out. Toxoplasmosis has been tentatively associated with gastric ulceration in one cat (Liptak et al 2002). The non-regenerative anaemia present at admission was consistent with gastrointestinal blood loss but is very non-specific.
The thoracic radiographic changes in this case, with poorly demarcated foci of interstitial and alveolar pneumonia, were assumed to be associated with disseminated toxoplasmosis. Similar changes are reported in cases of pulmonary toxoplasmosis (Dubey and Carpenter 1993) but may be seen with any lower respiratory tract disease. The presence of chorioretinitis, which is commonly seen in cases of feline toxoplasmosis (Lappin et al 1989), also provided circumstantial evidence for disseminated disease in this case.
The ultrasonographic and radiographic appearance of gastrointestinal toxoplasmosis has not been previously reported. The severe gastric wall thickening seen in this case was compatible with diffuse gastric neoplasia. The ultrasonographic appearance of gastric tumours in small animals usually includes loss of normal layering and wall thickening with regional lymphadenopathy, but a pseudolayered appearance may be seen (Penninck et al 1994, Kaser-Hotz et al 1996, Penninck et al 1998, Lamb and Grierson 1999). In this case, the thickening primarily involved the muscularis, which is atypical of gastric lymphoma but could be compatible with a smooth muscle tumour but these are usually eccentrically located (Penninck et al 1994, Myers and Penninck 1994). Hypertrophy of the muscularis layer of the intestines may also be seen secondary to inflammatory bowel disease (Bettini et al 2003). As has been previously reported, the ultrasonographic findings of gastrointestinal masses may be non-specific and definitive diagnosis relies on biopsy (Penninck 2002). This case shows that histopathological examination of ultrasound guided biopsy samples may demonstrate T gondii in infected viscera but immunohistochemical staining may be necessary.
The cat was treated for generalised toxoplasmosis with clindamycin at a recommended dose rate and route (Lappin 2000b). Higher dose rates and other routes of administration are also recommended (Dubey and Carpenter 1993). In this case the use of other drugs such as trimethoprim–sulphonamides or pyrimethamine may have been beneficial given the severity of the cat's signs. However, it is not uncommon for severe cases of generalised toxoplasmosis to die despite appropriate treatment. The cytology results were delayed for unknown reasons, but if they had been available treatment could have been started sooner, which may have altered the outcome of the case. Surgery may be beneficial in some cases of gastric masses (Liptak et al 2002), but the extensive nature of the lesions at diagnosis made this unfeasible. It is possible that earlier in the course of the disease surgery may have been an option for the gastric lesion.
This case documents eosinophilic fibrosing gastritis and generalised toxoplasmosis in a cat. The association between granulomatous gastrointestinal disease and toxoplasmosis and the spectrum of histopathological changes in chronic cases is unknown and further studies are required. It is possible that eosinophilic fibrosing gastritis is a rare manifestation of toxoplasmosis.
Acknowledgements
We would like to acknowledge the referring veterinary surgeons N. Mee, H. Scott and T. Smith for their help in this case.
References
- Bernsteen L., Gregory C.R., Aronson L.R., Lirtzman R.A., Brummer D.G. Acute toxoplasmosis following renal transplantation in three cats and a dog, Journal of the American Veterinary Medicine Association 215, 1999, 1123–1126. [PubMed] [Google Scholar]
- Bettini G., Muracchini M., Salda L. Della, Preziosi R., Morini M., Gugliemini C., et al. Hypertrophy of the intestinal smooth muscle in cats, Research in Veterinary Science 75, 2003, 43–53. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Frenkel J.K. Cyst-induced toxoplasmosis in cats, Journal of Protozoology 19, 1972, 155–177. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Frenkel J.K. Immunity to feline toxoplasmosis; modification by administration of corticosteroids, Veterinary Pathology 11, 1974, 350–379. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasmosis in cats, Feline Practice 16, 1986, 12–45. [Google Scholar]
- Dubey J.P., Carpenter J.L. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952–1990), Journal of the American Veterinary Medicine Association 203, 1993, 1156–1566. [PubMed] [Google Scholar]
- Dubey J.P., Lappin M.R. Toxoplasmosis and Neosporosis. Greene C.E. Infectious Diseases of the Dog and Cat, 2nd edn, 1998, W.B. Saunders: Philadelphia, USA, 493–509. [Google Scholar]
- Feeney D.A., Sautter J.H., Lees G.H. An unusual case of acute disseminated toxoplasmosis in a cat, Journal of the American Animal Hospital Association 17, 1981, 311–314. [Google Scholar]
- Guildord W.G., Strombeck D.R. Chronic gastric diseases. Guidford W.G., Center S.A., Strombeck D.R., Williams D.A., Meyer D.J. Strombeck's Small Animal Gastroenterology, 3rd edn, 1996, W.B. Saunders: Philadelphia, USA, 275–302. [Google Scholar]
- Hirth R.S., Nielsen S.W. Pathology of feline toxoplasmosis, Journal of Small Animal Practice 10, 1969, 213–221. [DOI] [PubMed] [Google Scholar]
- Howl J.H., Peterson M.G. Intestinal mast cell tumour in a cat: presentation as eosinophilic enteritis, Journal of the American Animal Hospital Association 31, 1995, 457–461. [DOI] [PubMed] [Google Scholar]
- Hulland T.J. Toxoplasmosis in Canada, Journal of the American Veterinary Medicine Association 128, 1956, 74–79. [PubMed] [Google Scholar]
- Kaser-Hotz B., Hauser B., Arnold P. Ultrasonographic findings in canine gastric neoplasia in 13 patients, Veterinary Radiology and Ultrasound 37, 1996, 51–56. [Google Scholar]
- Lamb C.R., Grierson J. Ultrasonographic appearance of primary gastric neoplasis in 21 dogs, Journal of Small Animal Practice 40, 1999, 211–215. [DOI] [PubMed] [Google Scholar]
- Lappin M.R. Protozoal and miscellaneous infections. Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine, 5th edn, 2000a, W.B. Saunders: Philadelphia, USA, 408–413. [Google Scholar]
- Lappin M.R. Feline infectious uveitis, Journal of Feline Medicine and Surgery 2, 2000b, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M.R., Greene C.E., Winston S., Toll S.L., Epstein M.E. Clinical feline toxoplasmosis. Serological diagnosis and therapeutic management of 15 cases, Journal of Veterinary Internal Medicine 3, 1989, 139–143. [DOI] [PubMed] [Google Scholar]
- Lappin M.R., Dawe D.L., Lindl P.A., Greene C.E., Prestwood A.K. The effect of glucocorticoid administration on oocyst shedding, serology, and cell-mediated immune responses in cats with recent or chronic toxoplasmosis, Journal of the American Animal Hospital Association 27, 1991, 625–632. [Google Scholar]
- Liptak J.M., Hunt G.B., Barrs V.R., Foster S.F., Tisdall P.L., O'Brien C.L., et al. Gastroduodenal ulceration in cats: eight cases and a review of the literature, Journal of Feline Medicine and Surgery 4, 2002, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier H., Holzworth J., Griffiths R.C. Toxoplasmosis in the cat – fourteen cases, Journal of the American Veterinary Medicine Association 131, 1957, 395–413. [PubMed] [Google Scholar]
- Myers N.C., Penninck D.G. Ultrasonographic diagnosis of gastrointestinal smooth muscle tumours in the dog, Veterinary Radiology and Ultrasound 35, 1994, 391–397. [Google Scholar]
- Penninck D.G. Gastrointestinal tract. Nyland T.G., Mattoon J.S. Small Animal Diagnostic Ultrasound, 2nd edn, 2002, W.B. Saunders: Philadelphia, USA, 207–230. [Google Scholar]
- Penninck D.G., Moore A.S., Tidwell A.S., Matz M.E., Freden G.O. Ultrasonography of alimentary lymphoma in the cat, Veterinary Radiology and Ultrasound 35, 1994, 299–304. [Google Scholar]
- Penninck D.G., Moore A.S., Gliatto J. Ultrasonography of canine gastric epithelial neoplasia, Veterinary Radiology and Ultrasound 39, 1998, 342–348. [DOI] [PubMed] [Google Scholar]
- Peterson J.L., Willard M.D., Lees G.E., Lappin M.R., Dieringer T., Floyd E. Toxoplasmosis in two cats with inflammatory intestinal disease, Journal of the American Veterinary Medicine Association 199, 1991, 473–476. [PubMed] [Google Scholar]
- Petrak M., Carpenter J. Feline toxoplasmosis, Journal of the American Veterinary Medicine Association 146, 1965, 728–734. [PubMed] [Google Scholar]