Abstract
Medical records of 92 cats presented with clinical signs of spinal cord disease, which had undergone magnetic resonance imaging (MRI), were reviewed. The cats were grouped into seven categories based upon the diagnosis suggested by results of MRI, cerebrospinal fluid analysis and other diagnostic procedures: neoplastic (n=25), inflammatory or infectious (n=13), traumatic (n=8), vascular (n=6), degenerative (n=5), anomalous (n=3) and those with an unremarkable MRI (n=32). There were two independent predictors of abnormal MRI findings: severity of clinical signs and presence of spinal pain. Abnormal MRI findings and speed of onset of disease were significantly associated with survival. For the 32 cats with unremarkable MRI findings, only nine died due to spinal disease and, therefore, the median survival time (MST) was not reached (lower 95% confidence interval (CI)=970 days). For the 60 cats with abnormal MRI findings, 37 died due to their disease and the MST was 138 days (95% CI: 7–807).
Investigation of spinal cord disease can often represent a diagnostic challenge in cats. Several studies and case reports of specific disease processes that affect the spinal cord of cats have been published, including those on spinal lymphoma, 1–3 intervertebral disc disease, 4–8 and feline infectious peritonitis (FIP). 9–11 A large retrospective survey of neurological disease in cats using archived central nervous tissue 12 and a retrospective study of the frequency of spinal cord disease identified on post-mortem examination 13 exist, but the authors are not aware of any ante-mortem studies on the relative frequency of the different aetiologies responsible for feline spinal cord disease. Such a study is important in order that a more accurate frequency of devastating diseases such as spinal cord lymphoma can be ascertained and not misrepresented as the authors currently believe is the case.
Magnetic resonance imaging (MRI) of the spine and spinal cord has contributed substantially to the detection and characterisation of spinal diseases. MRI is the method of choice for imaging the spinal cord in human patients being a non-invasive tool that allows examination of the intervertebral discs, foraminae, nerve roots, extradural space and internal architecture of the cord. 14 To the authors' knowledge, there are no studies on the frequency of abnormal spinal MRI findings in cats. It is the authors' clinical opinion that MRI investigation of cats with clinical signs of spinal disease can frequently be normal, limiting the therapeutic and prognostic information which can be given to the owners. It is important that an attempt to quantify the frequency of ‘normal’ MRI studies in cats with clinical spinal cord disease be made, so that practitioners and owners alike are aware that it is a contributory diagnostic test rather than an absolute test. Information about the outcome of such cats may be clinically valuable and has not previously been investigated. Additionally, improving the diagnostic yield of MRI may be possible if risk factors for MRI abnormalities can be identified, important when diagnostic decision making is tempered by owner finances.
The aims of this study were to determine the frequency of occurrence of an abnormal MRI in cats with clinical signs of spinal cord disease; to examine the relationship between the patient's clinical characteristics and MRI findings; to identify potential predictors of a poor outcome and to investigate the outcome of cats with a normal MRI study. Our hypotheses were that most cats with clinical signs of spinal cord disease would present abnormalities on MRI and that these findings would not influence the outcome.
Materials and Methods
Medical records of cats, with neurological signs localising to the spinal cord, referred to the Animal Health Trust (1999–2004), were reviewed retrospectively. Inclusion in this study required documentation of a complete neurological examination on presentation and an MRI series of the suspected affected area being available for reassessment.
Data on signalment, speed of onset, duration and severity of clinical signs, presence of spinal pain, cerebrospinal fluid (CSF) analysis, corticosteroid administration prior to referral and patient outcome were recorded. Feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) status were also recorded where known. Speed of onset of clinical signs was defined as peracute (when clinical signs developed in less than 12 h), acute (12–48 h) or chronic (>48 h). Duration of clinical signs was recorded as the number of days between the start of clinical signs noted by the owner and the MRI investigation. For severity of clinical signs, a neurological score was developed using a six-point scale (Table 1). The results of the CSF analysis were recorded as normal or abnormal when increased protein concentration alone (albuminocytological dissociation) or pleocytosis was noted, with the latter classified according to the actual cell count (Table 1).
Table 1.
Clinical characteristics of 92 cases of spinal cord disease in cats
| Clinical characteristic | Number | % |
|---|---|---|
| Speed of onset of clinical signs | ||
| Peracute, <12 h | 24 | 26.1 |
| Acute, 12–48 h | 25 | 27.2 |
| Chronic, >48 h | 43 | 46.7 |
| Neurological score | ||
| 1=Spinal pain only | 6 | 6.5 |
| 2=Ataxia±spinal pain | 22 | 23.9 |
| 3=Ambulatory paresis±spinal pain | 42 | 45.7 |
| 4=Non-ambulatory paresis±spinal pain | 10 | 10.9 |
| 5=Paralysis with intact nociception±spinal pain | 9 | 9.8 |
| 6=Paralysis with loss of nociception±spinal pain | 3 | 3.3 |
| CSF analysis – performed in 53 cats | ||
| Normal | 26 | 49.1 |
| Albuminocytological dissociation | 4 | 7.5 |
| Pleocytosis | 23 | 43.4 |
| Cell count 5–50 cells/μl | 17 | 73.9 |
| Cell count 50–500 cells/μl | 6 | 26.1 |
A 1.5-T GE Signa scanner (General Electric Medical Systems, Milwaukee, WI, USA) with a human extremity coil was used to image all the cats. Dorsal and sagittal T1-weighted, T2-weighted and T1-weighted post-contrast (gadobenate dimeglumine 0.05 mmol/kg) sequences were obtained; transverse images were obtained where considered appropriate by the operating radiologist. A board-certified veterinary radiologist reviewed all the images, recording the following MRI characteristics: spinal cord segment(s) affected, lesion characteristics, effects of the lesion on the cord and presence of osteolysis. The lesions identified on MRI were described according to their location relative to the spine and spinal cord parenchyma (paraspinal, extradural, intradural extramedullary or intramedullary) and to its effects on the spinal cord (compression, swelling and MRI signal alteration). According to the MRI findings, the spinal cord was subdivided into five vertebral segments for neurolocalisation: C1–C5, C6–T2, T3–L3, L4–L6 and L7–S3. The MRI findings together with the history, clinical signs, neurological evaluation and results from other diagnostic tests (CSF analysis, radiography, serology, cytology, histopathology, electrodiagnostic studies, surgical findings and post-mortem examination), generated a presumptive diagnosis. In view of these diagnoses, each case was assigned to one of the following seven categories: neoplastic, inflammatory or infectious, vascular, traumatic, degenerative, anomalous and normal MRI. Follow-up information on survival was sought by telephone interview with the referring veterinary surgeons or with the owner.
Statistical analysis
Data were entered into an Excel (Microsoft, 2000, Redmonds, WA, USA) spreadsheet and then imported into SPSS (Statistical Package for the Social Sciences version 14.0.1, SPSS Inc 1989–2005, Chicago, IL, USA) for analysis. Descriptive statistics are reported for continuous variables using mean (standard deviation) for approximately normally distributed variables and median (minimum–maximum) for variables with skewed distributions, and frequencies are reported for categorical variables.
Logistic regression was performed to examine the relationship between MRI findings and potential explanatory variables (age, gender, breed, duration of clinical signs, speed of onset of disease, severity of clinical signs, presence of spinal pain on presentation, cranial nerve involvement, CSF analysis, corticosteroid administration prior to referral and whether clinical signs progressed prior to MRI). Variables with P<0.30 in the univariable analysis were considered for inclusion in multivariable logistic regression. Variables were selected for inclusion in the final model if they significantly improved the fit (likelihood ratio χ2 statistic P<0.05). Cross-tabulation and a Fisher's exact test were used to examine the association between presence of osteolysis on MRI and the diagnosis of neoplastic disease. Results of logistic regression and cross-tabulation are presented as odds ratios (OR) and 95% confidence intervals (CIs). Kaplan–Meier survival analysis was performed to estimate median survival time (MST) with 95% CIs. Survival was determined from the date of diagnosis to the censor date of 15 November 2006. Cox proportional hazards regression was performed to evaluate the effects of potential explanatory variables on survival. Variables with P<0.30 in the univariable analysis were considered for inclusion in multivariable Cox proportional hazards regression. Variables were selected for inclusion in the final model if they significantly improved the fit (likelihood ratio χ2 statistic P<0.05). Results are presented as hazard ratios (HR) and 95% CIs. Significance was set at P<0.05 for all final models. The proportionality assumption was checked for the Cox model and the residuals were examined as an assessment of the fit of the final models to the data.
Results
A total of 92 cats fulfilled the inclusion criteria. Fifty-nine cats were male (one was entire) and 33 were female (two were entire). There was no breed predisposition noted, with 80% of the cats being domestic short- or longhairs and 20% purebred. The mean age at the time of MRI was 6.5 years (1 month–18 years).
The median duration of clinical signs before MRI was 21 days (0–951). Table 1 shows the clinical characteristics of the cases. Almost half of the cats presented with a chronic history of clinical signs. The condition was considered to be progressive in 63% of cats. In 39 cats, corticosteroids had been administered by the referring veterinary surgeon. The 55 cats tested for FIV and FeLV infection were all negative.
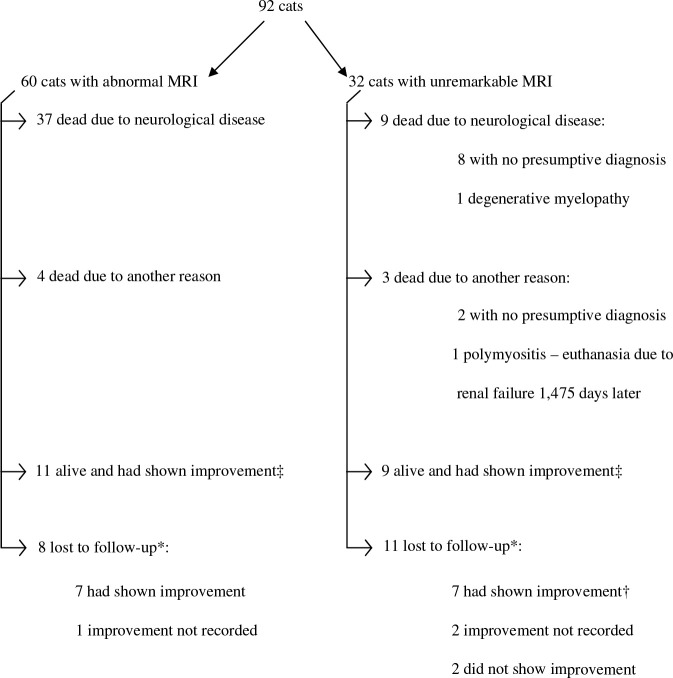
The MRI was unremarkable in 32 cases (35%) (Fig 1). A final diagnosis was obtained for three of these cats as a result of further diagnostic tests: one cat was diagnosed with polymyositis and one with polyneuritis through muscle and nerve biopsies (both cats were presented with tetraparesis and reduced segmental spinal reflexes in the fore limbs and were imaged because of the suspicion of caudal cervical lesions) and one cat was diagnosed with a degenerative myelopathy on post-mortem examination. The remaining 29 cats with no abnormalities found on MRI also had no identifiable presumptive diagnosis. The median duration of clinical signs before MRI in this group was 32 days (1–373) with 16 cases having undergone MRI more than 1 month after the onset of the clinical signs. Figure 1 summarises the outcomes of these cats. There was a history of trauma at the time of onset of the clinical signs in six cases (21%), with five of these cats still alive with an improved neurological status. Eleven cats had abnormalities on CSF analysis (three had increased protein only and in eight cases there was pleocytosis). In all these cats, serology titres and/or polymerase chain reaction (PCR) on the CSF for some of the most commonly diagnosed feline infectious diseases (FIV, FeLV, feline coronavirus and Toxoplasma species) were performed and were negative. Repeat CSF analysis was not performed in these cases. Fifteen cats had received corticosteroids within the week before presentation and in five cases CSF was not collected. Four cats (14%) deteriorated rapidly and had to be euthanased within days of presentation (two of these had CSF abnormalities). Four cases (14%) progressively deteriorated over a variable period of time (15–120 days) despite different treatment attempts (all with normal CSF analysis). Six cases (21%) responded to corticosteroid therapy (four of these had CSF abnormalities), and two responded to antibiotic therapy (both had CSF abnormalities).
Fig 1.
Flowchart showing clinical characteristics and outcome for the 92 cases. ‡At date of censor (15 November 2006), *lost to follow-up after first interview (31 May 2004), and †one cat with polyneuritis lost to follow-up 197 days after diagnosis.
In the 60 cases with abnormal MRIs, the majority of lesions were extradural, and the spinal cord segment most commonly affected was T3–L3 (Table 2). A total of 29 out of the 60 cats with abnormal MRI had a confirmed diagnosis with histopathology from biopsy samples (nine), cytology from fine needle aspirates (five), post-mortem examinations (six), surgical intervention for intervertebral disc disease and trauma cases (six), serology for toxoplasmosis (one), and PCR for FIP (two) (Table 3). Figure 1 shows the outcome for the cases with abnormal MRI findings.
Table 2.
MRI findings in 60 cases with abnormalities detected on MRI
| Number | % | |
|---|---|---|
| Spinal cord segment affected | ||
| C1–C5 | 14 | 24 |
| C6–T2 | 5 | 8 |
| T3–L3 | 25 | 42 |
| L4–L6 | 8 | 13 |
| L7–S3 | 8 | 13 |
| Location of lesion | ||
| Paraspinal | 5 | 8 |
| Extradural | 30 | 50 |
| Intradural extramedullary | 5 | 8 |
| Intradural intramedullary | 20 | 34 |
| Effect of lesion on spinal cord | ||
| No spinal cord changes | 5 | 8 |
| Compression | 22 | 37 |
| Swelling | 1 | 2 |
| MRI signal alteration | 9 | 15 |
| Compression and signal alteration | 6 | 10 |
| Swelling and signal alteration | 15 | 25 |
| Compression, swelling and signal alteration | 2 | 3 |
Table 3.
Disease categories, presumptive diagnosis and age at diagnosis for 92 cats with clinical signs of spinal cord disease
| Disease category and presumptive diagnosis | Diagnosis confirmed * | Number | Age at diagnosis | ||
|---|---|---|---|---|---|
| Median | Minimum | Maximum | |||
| No abnormalities on MRI, n=32 (34.8%) | 4.7 | 0.3 | 13.6 | ||
| Polymyositis | 1HP | 1 | 10 | 10 | 10 |
| Polyneuropathy | 1HP | 1 | 8.7 | 8.7 | 8.7 |
| Degenerative myelopathy | 1PM | 1 | 2.1 | 2.1 | 2.1 |
| No final diagnosis, abnormal CSF only | 0 | 11 | 4.5 | 0.3 | 11.7 |
| No final diagnosis, normal CSF | 0 | 13 | 5 | 1.5 | 13.6 |
| No final diagnosis, no CSF analysis | 0 | 5 | 4 | 2.5 | 6 |
| Neoplasia, n=25 (27%) | 8 | 0.7 | 18 | ||
| Soft tissue lymphoma | 1HP, 1Cyt, 2PM | 4 | 6.4 | 3 | 18 |
| Intramedullary lymphoma | 1HP, 1Cyt, 1PM | 3 | 6 | 2 | 7 |
| Soft tissue sarcomas | 2HP, 1Cyt | 3 | 7.5 | 4 | 15 |
| Nerve root tumour | 1HP, 1Cyt | 3 | 8 | 7.6 | 11.5 |
| Meningioma | 1HP | 2 | 7.8 | 7.6 | 8 |
| Vertebral body tumour | 1HP, 1PM | 2 | 11 | 10 | 12 |
| Unknown neoplasia | 1Cyt | 8 | 9.5 | 0.75 | 12 |
| Inflammatory/infectious, n=13 (14%) | 6 | 0.8 | 14 | ||
| Soft tissue abscess | 1Sx, 1PM | 5 | 6.5 | 3 | 11.75 |
| FIP | 2PCR | 2 | 2 | 0.8 | 3.3 |
| Discospondylitis | 0 | 2 | 9.5 | 5.5 | 14 |
| Myelitis of unknown aetiology | 0 | 2 | 8 | 3 | 13 |
| Toxoplasmosis | 1Ser | 1 | 6 | 6 | 6 |
| Brachial plexus neuritis | 0 | 1 | 9 | 9 | 9 |
| Traumatic, n=8 (9%) | 1 | 0.1 | 6.2 | ||
| Fractures | 3Sx | 4 | 0.9 | 0.1 | 6.2 |
| Luxation | 1Sx | 1 | 2 | 2 | 2 |
| Cord contusion | 0 | 3 | 4 | 1.5 | 8 |
| Vascular, n=6 (6.5%) | 9.1 | 5.8 | 16 | ||
| Ischaemic myelopathy | 0 | 6 | 9.1 | 5.8 | 16 |
| Degenerative, n=5 (5.4%) | 8 | 0.7 | 12 | ||
| Intervertebral disc disease | 1Sx | 5 | 8 | 0.75 | 12 |
| Anomalous, n=3 (3.3%) | 1.5 | 1.3 | 7.5 | ||
| Vertebral stenosis | 0 | 3 | 1.5 | 1.3 | 7.5 |
Diagnosis based on: Cyt=cytology from fine needle aspirate; HP=histopathology from biopsy; PM=post-mortem examination; Ser=serology; Sx=surgery.
There were two independent predictors included in the final model predicting whether the MRI was abnormal: severity of clinical signs (P=0.03) and presence of spinal pain on presentation (P=0.006). Cats with the most severe clinical signs (grades 4–6) were eight times more likely (OR=0.121, 95% CI: 0.025–0.583) to have an abnormal MRI than cats with less severe (grade 2) clinical signs and 16 times more likely (OR=0.063, 95% CI: 0.007–0.554) than cats with the least severe (grade 1) clinical signs. Cats with spinal pain at presentation were four times more likely (OR=0.231, 95% CI: 0.081–0.659) to have an abnormal MRI compared to cats with no pain. It was also found that cats with osteolytic changes on MRI were 15.5 times more likely to have neoplastic disease (based on the presumptive diagnosis) than cats with no osteolysis (n=57, P=0.0003, 95% CI for OR: 3.0–79.8).
Survival analysis
The median follow-up time from onset of clinical signs was 331 days (1–2344). The median time from onset of clinical signs to date of diagnosis by MRI was 21 days (0–951). The median follow-up time from diagnosis by MRI was 267 days with a minimum of 0 for 11 cats that were euthanased on the day of MRI to a maximum of 5 years and 10 months (2118 days). A total of 46 cases died due to their disease. Outcome of cases is summarised in Fig 1. The overall MST of the 92 cats was 924 days (95% CI: 131–upper limit not estimated). There were two independent predictors of survival: identification of abnormalities on MRI (P=0.003) and speed of onset of clinical signs (P=0.008). Cats with abnormal MRI findings had a greater hazard of death compared to cats with an unremarkable MRI, and cats with chronic or peracute onset of clinical signs had a significantly lower hazard of death compared with cats with an acute onset of clinical signs. Ten of the 11 cats that underwent euthanasia on the day of MRI had an abnormal MRI. When these cats were excluded from analysis, the same two variables were associated with survival: identification of abnormalities on MRI (P=0.01) and speed of onset of clinical signs (P=0.03) with similar HR.
For the 32 cats with normal MRI findings, only nine died due to their disease and, therefore, the MST was not reached (lower 95% CI of 970 days). For the 60 cats with abnormal MRI findings, 37 died due to their disease and the MST was 138 days (95% CI: 7–807). Speed of onset of clinical signs was the only significant predictor of survival in these 60 cats with similar HR to those found in the survival model for all cats.
Discussion
The largest group of cats in this study was the one where no diagnosis was achieved when comparing the different categories of disease. This finding is similar to those of the two previous studies that did not involve the use of MRI. 12,13 There are several pathological processes that may be undetectable on MRI, including inflammatory, vascular or degenerative diseases. This group of cats presents a tremendous diagnostic, therapeutic and prognostic challenge. Further information on the cause(s) of spinal disease in this group of cats is evidently required. It is possible that the cats with abnormal CSF analysis, mainly the ones with pleocytosis, had underlying inflammatory diseases that remained undiagnosed. Some of these cats improved with antibiotic therapy, others with corticosteroid therapy, others deteriorated despite different treatment attempts and a last group improved without treatment. This suggests different underlying aetiologies that could not be diagnosed through the routinely available diagnostic procedures. In the cats with normal CSF findings, almost half of these cases (n=6) had a history of trauma and most of them recovered without treatment raising the possibility of subtle lesions that could not be visualised on MRI. Another section of these cats deteriorated slowly over a period of months despite different treatment attempts, suggesting possible degenerative disease. Finally, the possibility of erroneous neurolocalisation and subsequent inappropriate diagnostic evaluation cannot be ruled out. None of the cats that did not improve was re-evaluated to investigate this possibility or to assess if the progression of the clinical signs could aid in the diagnostic process.
The most common pathology among the 60 cats with abnormal MRI findings was neoplasia. This contrasts with previous studies that found inflammatory disease to be most common. 12,13 This may be related to geographic differences as both previous studies were performed in the USA, the fact that several of the feline inflammatory diseases can be diagnosed through serum titres and, therefore, these cats do not undergo advanced imaging or that a proportion of the cats with unremarkable MRI and abnormal CSF (n=11) had inflammatory diseases that remained unidentified. Lymphoma was the most frequent neoplastic process diagnosed and this agrees with what has been previously reported. 1–3,15 A positive FeLV status has been implicated as the most important factor associated with feline lymphoid tumours. 1,2 In our study, although all 55 of the cats tested for FeLV were negative, only three of the seven cats diagnosed with lymphoma were tested. Due to this small number, no conclusions can be made about the relationship between FeLV and spinal lymphoma. Nonetheless, it is possible that the widespread use of the FeLV vaccination over the past decade could account for a suspected reduction of FeLV associated lymphoma cases.
The segment of the spinal cord most affected was the thoracolumbar, concurring with previous literature. 16 The presence of lesions affecting the adjacent bone on MRI was found to be significantly associated with neoplastic disease, suggesting this may be used as an indicator of this pathology.
Age at diagnosis, breed, gender, onset and duration of clinical signs, use of corticosteroids, progression of disease and CSF analysis were not associated with finding of abnormalities on MRI. However, cats with severe clinical signs and the ones presenting with spinal pain were more likely to have abnormalities on MRI. Spinal pain is often caused by extradural compression of the spinal cord (eg, degenerative intervertebral disc disease), vertebral (eg, discospondylitis) or paraspinal lesions (eg, soft tissue abscess). Most of these lesions are easily identifiable on MRI and this may explain why abnormalities were more commonly found in the cats presenting pain. It also seems intuitive that animals with more severe clinical signs have a higher probability of having abnormalities detectable by MRI, wherever the causative lesion is located.
Two independent predictors of survival were identified: MRI findings and speed of onset. Cats with abnormal findings on MRI had significantly shorter survival times than the ones where nothing abnormal was detected. Cats with acute onset of disease had shorter survival times than the ones with peracute or chronic onset. The group of cats with peracute onset of the clinical signs included all the cats in the traumatic and vascular categories, pathologies that generally carry a good prognosis of recovery. In several of the cats with chronic onset of the clinical signs, these had been present for a long period before presentation and it was suspected that this led the owners to tolerate the clinical signs and even if no or little improvement was observed, this did not result in euthanasia.
Due to the retrospective nature of this study, it was not possible to standardise the diagnostic procedures performed in all cats, so there is considerable variation regarding which tests were undertaken in each case. Furthermore, information regarding the history prior to referral is somewhat inconsistent. Also, as this was an ante-mortem study, in a substantial number of cases (n=63) no confirmation of the suspected disease process was obtained, raising the possibility that in some cases the presumptive diagnosis was not correct.
In summary, MRI appears to be a valuable diagnostic tool in the investigation of spinal cord disease in cats, especially when used in conjunction with other diagnostic procedures. Two variables (presence of pain and severity of clinical signs) were identified that may help improve the yield of MRI. It was found that MRI findings and speed of onset of disease were significantly associated with survival. In a large number of cats with clinical signs of spinal cord disease, no diagnosis was achieved. The findings of this study, suggest that traumatic, inflammatory and degenerative conditions should be considered as possible underlying pathologies in these cats, which still present an immense diagnostic and therapeutic challenge.
References
- 1.Spodnick G.J., Berg J., Moore F.M., Cotter S.M. Spinal lymphoma in cats: 21 cases (1976–1989), J Am Vet Med Assoc 200, 1992, 373–376. [PubMed] [Google Scholar]
- 2.Lane S.B., Kornegay J.N., Duncan J.R., Oliver J.E. Feline spinal lymphosarcoma: A retrospective evaluation of 23 cats, J Vet Int Med 8, 1994, 99–104. [DOI] [PubMed] [Google Scholar]
- 3.Noonan M., Kline K.L., Meleo K. Lymphoma of the central nervous system: A retrospective study of 18 cats, Compend Contin Educ Pract Vet 19, 1997, 497–503. [Google Scholar]
- 4.King A.S., Smith R.N., Kon V.M. Protrusion of the intervertebral disc in the cat, Vet Rec 70, 1958, 509–515. [Google Scholar]
- 5.King A.S., Smith R.N. Degeneration of the intervertebral disc in the cat, Acta Orthop Scandinavica 34, 1964, 139–158. [DOI] [PubMed] [Google Scholar]
- 6.Heavner J.E. Intervertebral disc syndrome in the cat, J Am Vet Med Assoc 159, 1971, 425–427. [PubMed] [Google Scholar]
- 7.Munana K.R., Olby N.J., Sharp N.J.H., Skeen T.M. Intervertebral disc disease in 10 cats, J Am Anim Hosp Assoc 37, 2001, 384–389. [DOI] [PubMed] [Google Scholar]
- 8.Rayward R.M. Feline intervertebral disc disease, Vet Comp Orthop Traumatol 15, 2002, 137–144. [Google Scholar]
- 9.Kornegay J.N. Feline infectious peritonitis: The central nervous system form, J Am Anim Hosp Assoc 14, 1978, 580–584. [Google Scholar]
- 10.Kline K.L., Joseph R.J., Averill D.R. Feline infectious peritonitis with neurological involvement: Clinical and pathological findings in 24 cats, J Am Anim Hosp Assoc 30, 1994, 111–118. [Google Scholar]
- 11.Legendre A.M., Whitenack D.L. Feline infectious peritonitis with spinal cord involvement in two cats, J Am Vet Med Assoc 167, 1995, 931–932. [PubMed] [Google Scholar]
- 12.Bradshaw J.M., Pearson G.R., Gruffydd-Jones T.J. A retrospective study of 286 cases of neurological disorders of the cat, J Comp Pathol 131, 2004, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marioni-Henry K., Vite C.H., Newton A.L., van Winkle T.J. Prevalence of diseases of the spinal cord of cats, J Vet Int Med 18, 2004, 851–858. [DOI] [PubMed] [Google Scholar]
- 14.Lane B. Practical imaging of the spine and spinal cord, Top Magn Reson Imaging 14, 2003, 438–443. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler S.J. Spinal tumours in cats, Vet Annu 29, 1989, 270–277. [Google Scholar]
- 16.LeCouteur R.A., Grandy J.L. Diseases of the spinal cord. Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine, 6th edn, 2005, Elsevier: Missouri, 842–887. [Google Scholar]