To the Editor:
Coronavirus disease 2019 (COVID-19) pneumonia presents with severe hypoxemic respiratory failure, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The primary mode of transmission appears to be droplet-borne. Respiratory support and high levels of oxygen are required in the acute treatment of these patients. High-flow therapies have been included as part of the possible management of COVID-19.1 , 2 One such modality is high-flow therapy, including high-velocity nasal insufflation (HVNI), high-flow nasal oxygen (HFNO), and high-flow nasal cannulation (HFNC). HVNI shares characteristics with HFNC/HFNO, in that all reliably deliver high flows of oxygen-enriched gas at high Fio 2 to the patient via an open nasal interface. High-flow therapy has demonstrated the ability to help manage hypoxic or type I respiratory failure.3 , 4 All high-flow therapies also share the same issue of potential aerosol generation.
Recent correspondence has raised questions about health-care worker safety during the use of noninvasive ventilation and HFNC therapies.5 HFNC has been studied and found to have limited particle dispersion when the cannula is properly fitted.6 A recent recommendation has advised the use of a surgical mask over the face of the patient while wearing the high-flow therapy device to help reduce inadvertent aerosol.7 This is the initial report of a study using computational fluid dynamic (CFD) simulation to determine the ability of a mask to reduce the velocity of exhaled gas flow and capture particles during HVNI.
Methods
This study used CFD modeling to evaluate (1) the effect of the addition of a surgical mask over the patient’s face on the velocity of gas outflow into the room, (2) the consequence of leakage around the mask, and (3) the effect of the addition of a mask on the ability of HVNI to flush the upper airway deadspace. Two models were used to answer these questions.
For velocity and leak analysis an in silico simulation (Ansys, Inc.) modeled a three-dimensional head placed on a virtual bed positioned 736 mm above the floor of a virtual 43-m3 room (4.87 m × 3.65 m × 2.44 m), which included simulated inlet and outlet vents (two each, 0.305 m × 0.305 m) for modeling air handling in the room (six air exchanges per hour). A type I surgical mask was modeled over the face. Gaps in the mask-face interface were included to model the effect of poor fit on a patient: eight gaps totaling a 679-mm2 cross-section were modeled for all experiments (including a gap on both sides of the nose, simulating poor mask fit at the nose). This included six gaps and two inlets for HVNI cannula tubing. The mask was modeled to match EN14683 standards. HVNI therapy was modeled from CT imaging-derived architecture of a petite adult female, sinusoidal breathing of a 500-mL tidal volume at 32 breaths/min and a 1:1 inspiratory/expiratory ratio (the exaggerated tidal volume was intentional to model “worst-case” expiratory flow and velocity). HVNI flow was modeled at 40 L/min through a model of a Vapotherm adult small/pediatric cannula. Low-flow oxygen delivery was modeled with a similar cannula delivering a 6-L/min continuous flow (LFO2). A third scenario of “no therapy” on a patient breathing with the same dynamics was modeled for comparison.
A tetrahedral mesh geometry totaling 6 million elements with 1.1 million resulting polyhedra was used. Mesh density was set to achieve four elements through the thickness. Simulations are transient, and 5-h run times were simulated for the development of flow in the room. Particles, ranging from 0.1 to 100 μm, were simulated as coming from the airway. The model used a single particle generation rate across all scenarios. Particle mass disposition is reported as a proportion, as the actual volume of particulate generation in patients will vary.
The second experiment was performed with a different simulation, evaluating CO2 flush, performed using a CT imaging-derived anatomically accurate model of a petite adult airway and face. The model assumed exhalation of 8% CO2, with HVNI delivered at 40 L/min via a Vapotherm adult small/pediatric cannula. Flush was measured over a simulated complete breath (tidal volume, 500 mL), and washout was computed from the known remaining mass of CO2 in the modeled deadspace, with and without a mask.
Results
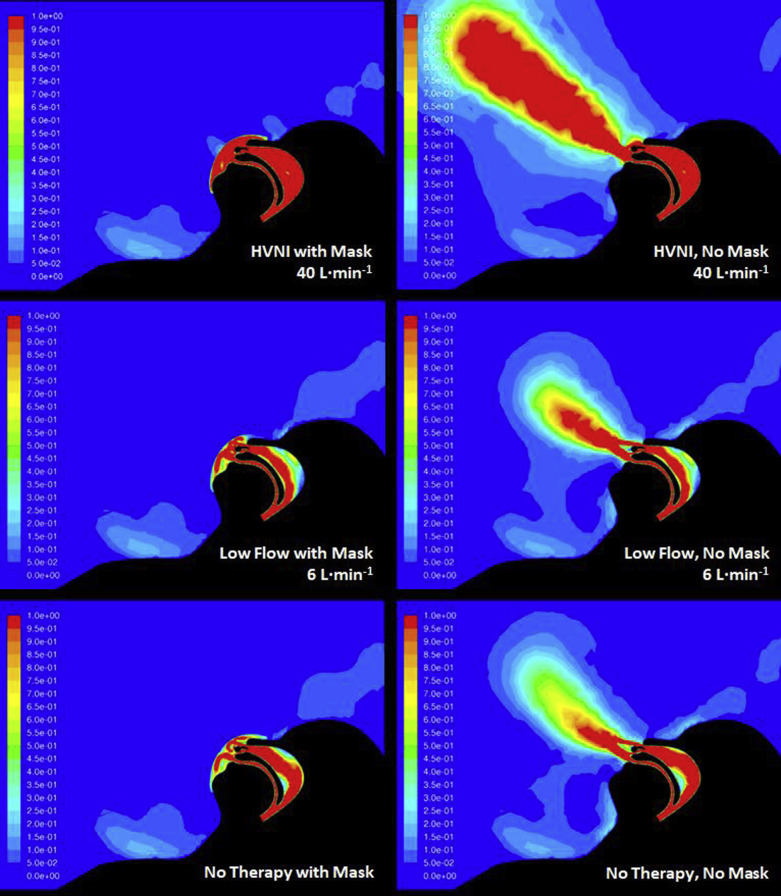
The first simulation showed that the persistence of high velocities (necessary for carrying nonairborne particles) was very low for all scenarios using the facemask (Fig 1 ). All scenarios showed the mask receiving the bulk of the breathing outflow, entering the matrix of the mask and rapidly losing velocity through diffusion into the mask. The intentional leak points showed greater leak with HVNI than with LFO2 or no therapy (16.5% vs 12.6% vs 11.6% leak, respectively). Other than flow through the intended leaks, the velocity of particles exiting the mask material was minimal. Simulated HVNI therapy through the mask does not have an exaggerated exiting velocity (with known capability of capturing and propelling droplets) and is comparable to that modeled for LFO2 or tidal breathing.
Figure 1.
Velocity map of gas flow for all tested settings. HVNI = high-velocity nasal insufflation.
The simulated surgical mask during HVNI at 40 L/min captured 83.2% of particles; LFO2 at 6 L/min captured 73.6% of particles; and tidal breathing (no therapy) captured 87.2% of particles. It is important to note that the proportion of droplets (ie, ≥ 5 μm) that are captured in the mask with HVNI therapy is 85.9%, as compared with 75.9% while receiving LFO2, and 89.9% during tidal breathing. The greater HVNI capture is likely due to the rapid incorporation of high-velocity particles into the mask material, as compared with the lower velocity LFO2 therapy gas stream. The minority of particles (15.9%) that escaped in the HVNI simulation showed a travel distance greater than 1 m as compared with 6.9% on LFO2. This was overwhelmingly attributed to mask leak. For comparison, simulation of tidal breathing without a mask showed 31% of particles leaving the nose and mouth with travel greater than 1 m from the face.
In the second experiment, the simulation showed a flush efficacy of 52% at 40 L/min under the mask. This is a moderate reduction in CO2 clearance. This is slightly lower than the flush calculated from the model run at 35 L/min without a mask (62.1% flush at the same ventilatory parameters—16% difference). A drop in flush efficiency should therefore be accounted for with increased flow if the patient exhibits increased work of breathing.
Discussion
These simulations suggest that (1) the velocity of exhaled gas flow of patients receiving LFO2 or HVNI therapy can be substantially slowed by using a surgical facemask in place—with the attendant reduction in particulate dispersal; (2) the simulated mask showed capture of the majority of particle mass, with slightly better capture than LFO2, and leakage occurring primarily at the points of intentional leak; and (3) a moderate reduction in flush capability occurs with a surgical mask in place, suggesting increasing flow rate if the patient is displaying increased work of breathing.
These preliminary findings suggest that the addition of a simple type I surgical mask may provide an effective tool to further reduce droplet deposition due to exhaled gas flow, except at mask leaks. A properly fitted mask may be a reasonable tool with which to further manage particulate contamination of the room for patients with droplet-borne disease. Note that all scenarios (HVNI, LFO2, tidal breathing) resulted in particulate and airflow escape, and personal protective equipment/environmental precautions must be considered when treating patients receiving HVNI, even with the surgical mask. Further high-definition simulations are underway to determine the geometry of deposition as well as to refine particulate dynamics.
Acknowledgments
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: Reid Prichard, PhD student, Liberty University, Lynchburg, VA; Leonithas I. Volakis, MS, PhD, Research Scientist, Vapotherm Inc., Exeter, NH; Maria Carrasquillo, Sr. Compliance Specialist, Vapotherm Inc., Exeter, NH.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: The authors have reported to CHEST the following: C. W. A. has received fees from Vapotherm, Inc for clinical research consultation. J. S. W. has received fees for clinical research consultation and a speaker honorarium from Vapotherm, Inc. S. L., R. J. DeB., and G. C. D. are employees of Vapotherm, Inc. None declared (B. K. W., W. S.).
FUNDING/SUPPORT: Funding provided from Vapotherm to Liberty University in a fee for service contract for support from computational fluid dynamics laboratory.
References
- 1.World Health Organization Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance. https://apps.who.int/iris/handle/10665/330893 WHO/nCoV/Clinical/2020.3, March 13, 2020. (accessed April 9, 2020)
- 2.Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. In press. [DOI] [PMC free article] [PubMed]
- 3.Doshi P., Whittle J.S., Bublewicz M. High-velocity nasal insufflation in the treatment of respiratory failure: a randomized clinical trial. Ann Emerg Med. 2018;72(1):73–83.e5. doi: 10.1016/j.annemergmed.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Frat J.P., Thille A.W., Mercat A. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 5.Cheung J.C., Ho L.T., Cheng J.V., Cham E.Y.K., Lam K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. 2020;8(4):e19. doi: 10.1016/S2213-2600(20)30084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui D.S., Chow B.K., Lo T. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4):1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 7.Respiratory Care Committee of Chinese Thoracic Society [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia] [article in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2020;17(0):E020. doi: 10.3760/cma.j.issn.1001-0939.2020.0020. [DOI] [PubMed] [Google Scholar]