Abstract
Introduction:
Renal colic affects 12% of the U.S. population, accounting for nearly 1% of emergency department (ED) visits. Current recommendations advocate narcotic-limiting multimodal analgesia regimens. The objective of this review is to determine if in patients with renal colic (Population), intravenous (IV) amide anesthetics (Intervention) result in better pain control, lower requirements for rescue analgesia, or less adverse medication effects (outcome) compared to placebo, non-steroidal anti-inflammatory drugs (NSAIDs), or opiates (Comparisons).
Methods:
Scholarly databases and relevant bibliographies were searched using a pre-designed systematic review protocol and registered with PROSPERO. Inclusion criteria were: (1) randomized clinical trial (RCT), (2) age ≥ 18 years, (3) confirmed or presumed renal colic, (4) amide anesthetic administered IV. Eligible comparison groups included: placebo, conventional therapy, acetaminophen, NSAID, or opiate. The primary outcome was pain intensity at baseline, 30, 60, and 120 minutes. Trial quality was graded, and risk-of-bias was assessed.
Results:
Of the 3930 identified references, 4 RCTs (479 participants) were included. One trial (n=240) reported improved analgesia with IV lidocaine (LidoIV) plus metoclopramide, compared to morphine. All other trials reported unchanged or less analgesia compared to placebo, ketorolac, or fentanyl. Very severe heterogeneity (I2= 88%) precluded pooling data.
Conclusion:
Current evidence precludes drawing a firm conclusion on the efficacy or superiority of LidoIV over traditional therapies for ED patients with renal colic. Evidence suggests LidoIV may be an effective non-opiate analgesic alliterative; however, it’s efficacy may not exceed that of NSAIDs or opiates. Further study is needed to validate the potential improved efficacy of LidoIV plus metoclopramide.
Key Words: Renal Colic, Kidney Calculi, Lidocaine, Analgesia, Emergency Service, Hospital
Introduction
Pain is the most common reason for emergency department (ED) visits in the United States (U.S.) (1), and its management requires mastery of multimodal approaches to achieve safe and effective analgesia. Nephrolithiasis and renal colic affect approximately 12% of the U.S. population (up to 5% in China) (2) and accounts for nearly 1% of ED visits and hospital admissions in the U.S. (2–5). For patients with prior stones, 10-year recurrence rates approach 50% (3,6). The pain of renal colic origin is multifactorial and is related to obstruction of urinary flow with subsequent increase in prostaglandin-mediate ureteral spasm (6,7).
As most renal calculi pass spontaneously, acute management should focus on rapid analgesia, diagnosis confirmation, and recognition of complications requiring immediate intervention (7). Approximately 85% of ED patients with renal colic are treated with analgesics (3). Whether used alone or in combination, NSAIDs and opioids constitute the primary therapeutic medications in ED management of renal colic (8). Each drug class possesses potentially unfavorable side effects and contraindications. Disadvantages to NSAIDs include lack of titratability, nausea, epigastric pain, and contraindications including renal insufficiency, peptic ulcer disease, the elderly (age >70 years), and concomitant use of anticoagulation or antiplatelet agents (9). Opioid administration in turn may lead to nausea, vomiting, pruritus, lethargy, bradycardia, hypotension, or respiratory depression, and may be relatively contraindicated for patients with a history of opioid abuse or dependence. Despite efficacy and possibly favorable side-effect profile of NSAIDS over opiates (6), 43% of patients are treated with an opiate, and 70% are prescribed an opiate on discharge (3). Moreover, ED opioid administration and prescription has been linked to an increased risk of recurrent opioid use (10). Current practice is moving towards recent U.S. Food and Drug Administration (FDA) goals that emphasize analgesia through multimodal regimens that decrease opiate use (11). Insufficient data regarding the efficacy of alternative regimens and their side-effect profiles have hampered efforts to move away from opiate heavy regimens (12). There is a need for identification and validation of safe and effective analgesia techniques for renal colic, which limit narcotic consumption and prescription, and provide treatment alternatives for patients who are unable to tolerate or have serious contraindications to NSAIDs or opiates (12).
Intravenous local anesthetic use has emerged as an opiate-sparing alternative in treatment of renal colic. Local anesthetics halt impulse initiation and transmission processes in neuronal axons, and may be categorized into two major chemical classes: amino esters and amino amides (6). Amide local anesthetics are widely used for topical and local anesthesia, and as systemic antiarrhythmics (1). Lidocaine, an amino amide, has been described to have analgesic, anti-hyperalgesic, anti-inflammatory, and anti-bacterial properties (1,13,14). The analgesic effect from systemic administration affects both the peripheral and central nervous system (15). Lidocaine decreases excitability and conduction of unmyelinated C fibers, and intravenous lidocaine (LidoIV) suppresses post-synaptic reflexes in the spinal dorsal horn (15). Its mechanisms include reversible inhibition of voltage-gated open and inactivated sodium channels and G-protein-coupled receptors (3,13,15,16). Central anti-nociceptive effects are mediated through actions on muscarinic and nicotinic receptors, which in turn increase intraspinal acetylcholine release to reinforce the inhibitory descending pain pathway (15). Anti-hyperalgesic effects are mediated through the N-methyl-D-aspartate (NMDA) receptor (14,15).
LidoIV has a desirable pharmacokinetic profile, with a rapid onset and long duration-of-action (half-life 60-120 min), but the analgesic effects may last longer (1,17). Approximately, 90% of lidocaine is metabolized in the liver by dealkylation to lower potency active metabolites monoethylglycinexylidide (MEGX) and glycinexylidide (GX) (13,15), while ≤10% is excreted unchanged in the urine (15).
LidoIV application for ED patients with renal colic has been reported to improve pain intensity, time to pain relief, and nausea in randomized clinical studies (8,18–21), non-randomized clinical studies (22), and case series (17,23–25), and may be considered as a viable non-opioid addition or an alternative to traditional treatment modalities. We investigated the evidence on using intravenous amide anesthetics for analgesia and opioid sparing effects in patients with acute renal colic. The objective of this project is to address the following research question: In patients with renal colic (Population) do intravenous amide anesthetics such as lidocaine (Intervention) improve pain intensity, need for rescue analgesia, opiate consumption, or adverse events (Outcomes) compared to placebo, NSAIDs, or opiates (Comparisons)?
Methods
This systematic review followed the steps outlined in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (26). A systematic protocol was developed a priori and registered with PROSPERO (# CRD42019130355).
The primary outcome was pain intensity at baseline and 15, 30, 60, and 120 minutes post-treatment. The secondary outcomes were: (1) need for rescue analgesia at 30 or 60 minutes, (2) time to pain free, (3) treatment failure, and (4) adverse events.
A librarian-performed systematic search strategy was conducted (Supplemental Digital Content 1) in Cochrane CENTRAL, CINAHL, Embase, Latin American and Caribbean Health Sciences Literature (LILACS), Medline, Scopus, and Web of Science (WoS). Additional investigator-performed structured searches were conducted in: China National Knowledge Infrastructure (CHKD-CNKI), information/Chinese Scientific Journals database (CSJD-VIP), Directory of Open Access Journals (DOAJ), IEEE-Xplorer, Magiran, Scientific Information Database (SID), TÜBİTAK ULAKBİM, Russian Science Citation Index (RSCI), Korean Journal Database (KCI), and Scientific Electronic Library Online (SciELO). Relevant bibliographies were searched. Searches were not limited by date, language, or publication status. Clinical trial registries were searched to limit publication bias, including: ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), and the Australian New Zealand Clinical Trials Registry (ANZCTR). Abstracts of the conference proceedings of the relevant disciplines (emergency medicine, urology, nephrology, pain management) were searched (past 5 years). When the presented data were incomplete, the authors were contacted to obtain the missing information. These trials were only included if the authors responded to correspondence affirmatively with the requested information.
Inclusion criteria were: (1) randomized controlled human clinical trial, (2) patients aged ≥ 18 years, (3) presumed or confirmed renal colic, (4) amino amide anesthetic administered intravenously (eg. LidoIV) compared to placebo or another analgesic. Data of pain intensity that measured as either a 10 cm visual analogue scale (VAS) or 10-point numeric rating scale (NRS) were summarized. Significant improvement in pain intensity was defined as improvement in ≥ 3 cm or points on VAS or NRS, respectively. Rescue analgesia was defined as any analgesia medication administered following the study drug.
Exclusion criteria were: (1) non-randomized study design, (2) studies enrolling patients aged < 18 years, (3) drug administration by routes other than intravenous, (4) studies published only in abstract form (or unpublished) for which the authors did not respond to correspondence by providing the requested information.
Reference management and application of inclusion/exclusion criteria was performed using Covidence (Covidence, Melbourne, Australia). Four authors reviewed the titles and abstracts to determine inclusion eligibility. Four authors extracted study data. Any disagreements were resolved by consensus.
Four authors independently assessed the risk-of-bias (RoB) using two validated tools: (1) Grading of Recommendations, Assessment, Development and Evaluations (GRADE) (27), and RoB 2.0: "Revised tool for Risk of Bias in randomized trials” (28). The authors considered methods of randomization and allocation, blinding (of treatment administrator, participants, and outcome assessors), selective outcome reporting (e.g. failure to report adverse events), incomplete outcome data, and sample size calculation. Each trial was graded as high, low or unclear risk of bias (RoB) for each criterion. Publication bias was assessed using both the Egger (29) and Begg-Mazumdar methods (30). Heterogeneity was evaluated using I² statistic. The confidence interval for I² was constructed using the iterative non-central chi-square distribution method of Hedges and Piggott (31). The threshold value for severe heterogenity was specified to be I² ≥50%, and very serious heterogenity was specified as I² >75%. Data pooling and meta-analysis was planned if I² <50%.
Results
The complete search was performed on December 19, 2018. The search strategy identified 3930 references, of which 4 RCTs (479 participants) met the inclusion criteria (8,18–20). Two ongoing studies were identified in clinical trial registries (32,33). See Figure 1 for the PRISMA flow diagram. The included studies are summarized in Table 1. One study took place in a high-income economy (USA) (8). Three were in a middle-income economy (Iran) (18–20). No studies were identified in low-income economies. Two published abstracts were excluded. The first was an RCT published only in abstract form for which the authors did not respond to correspondence (21). The second duplicated information available in a published manuscript (22). The primary reason for exclusion of full-text manuscripts was non-randomized study design. Two unpublished ongoing trials were identified (32,33). No additional studies were identified through bibliographic and conference abstracts analyses that were not previously identified through other search methods.
Figure 1.
PRISMA Flow Diagram
Table 1.
List of included studies
| Author [Year]; (Reference #) | Setting, design (N) | Intervention | Comparison |
Demographics:
Lidocaine (L) vs. no lidocaine (NL) |
Primary Endpoints | Secondary Endpoints |
|---|---|---|---|---|---|---|
| Firouzian [2016]; (18) | Iran, single center, RCT, double-blind (89) | Lidocaine 1.5 mg/kg IV plus morphine 0.1 mg/kg infusion over 2-4 minutes. | Morphine 0.1 mg/kg plus N.S. bolus infusion over 2-4 minutes. |
Age, years: (L) 37.91±10.76, (NL) 37.95±12.6 Sex, male: (L) 77%, (NL) 83% |
Pain intensity measured by VAS (0-10) at baseline, 5, 10, 30, 60, and 120 minutes. | (1) Time to pain free (2) Nausea intensity (3) Time to nausea free |
| Motamed [2017]; (19) | Iran, single center, RCT, double-blind (90) | Lidocaine 1.5 mg/kg IV infusion over 2 minutes. | Fentanyl 1.5 mcg/kg IV infusion over 2 minutes. |
Age, years: (L) 39.08±6.64, (NL) 34.08±8.87 Sex, male: (L) 86.7%, (NL) 93.3% |
Pain intensity measured by VAS (0-10) at baseline, 5, 10, 15, and 30 minutes. | Rescue medication at 15- & 30-minutes post-administration. |
| Motov [2019]; (8) |
USA, single center, RCT, double-blind (150) | Lidocaine 1.5 mg/kg IV infusion over 10 minutes. | (1) Ketorolac 30 mg IV push with 10 min N.S. infusion. (2) Ketorolac 30 mg IV push plus Lidocaine 1.5 mg/kg IV infusion over 10 minutes. |
Age, years: (L only) 39.34±10.95 (Combination) 42.92±10.36 (NL) 42.34±10.47 Sex, male: (L only) 54%, (Combination) 56%, (NL) 56% |
Pain intensity measured by numerical rating scale (0-10) at baseline, 5, 10, 30, and 60 minutes. | (1) Adverse effects (2) Use of diagnostic imaging |
| Soleimanpour [2012]; (20) | Iran, single center, RCT, double-blind, (150) | Lidocaine 1.5 mg/kg IV slow push from 10 cc syringe. | Morphine 0.1 mg/kg IV slow push from 10 cc syringe. |
Age, years: (L) 37.71±11.08, (NL) 35.23±12.37 Sex, male: (L) 28%, (NL) 72% |
Pain intensity measured by VAS (0-10) at baseline, 5, 10, 15, and 30 minutes. | (1) Pain resolution measured as VAS < 3/10 for 30 minutes. (2) Rescue medication at 30 minutes. (3) Adverse effects |
Abbreviations: ED means emergency department; IV means intravenous; N.S. means normal saline; RCT means randomized controlled trial; VAS means visual analogue scale.
The GRADE assessments are presented in Table 2. RoB assessment indicated that how each study ranked regarding the risk of selection bias, performance bias, detection bias, reporting bias, and “other” bias (8,18–20). Two studies were similarly at low risk for attrition bias (8,18), whereas 2 had unclear risk of attrition bias (19,20). Lastly, 3 studies had low risk of bias due to sample size (8,19,20), whereas one had unclear RoB (18). Additionally, all of the included studies were double-blind (8,18–20). Although all 4 studies had a control arm, but only one had a placebo arm (lidocaine + morphine vs. placebo + morphine) (18). One study reported no patient attrition (8), whereas one reported 19% attrition (18), and two did not report attrition data (19,20). Furthermore, each included study reported their intended primary outcomes. Moreover, one study reported adverse events (AE) (20), 2 reported no AEs (8,18), and one did not report on AEs (19). All but one study reported the method of sample size calculation (18).
Table 2.
GRADE quality of evidence ratings
|
Certainty assessment
|
Certainty | |||||||
| Variable | № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |
| Pain intensity | 4 | RCT | Not serious | Serious | Not serious | Not serious | None | ⨁⨁⨁◯ MODERATE |
| Rescue medication | 1 | RCT | Not serious | Not Serious | Not Serious | Not Serious | Publication bias strongly suspected | ⨁⨁⨁◯ MODERATE |
| Time to pain free | 1 | RCT | Not serious | Not Serious | Not Serious | Not Serious | Publication bias strongly suspected | ⨁⨁⨁◯ MODERATE |
| Treatment failure | 1 | RCT | Not serious | Not Serious | Not Serious | Not Serious | Publication bias strongly suspected | ⨁⨁⨁◯ MODERATE |
RCT means randomized controlled trial.
All included studies gave adequate information regarding diagnostic criteria, namely that patients were diagnosed with presumed or confirmed renal colic (8,18–20). Heterogeneity was noted in the methods and timing of pain intensity assessments. Three studies utilized a 10 cm VAS scale (18–20), and 1 utilized a 10-point NRS (8). Pain intensity was assessed at 15 minutes in 3 studies (8,19,20), at 30 minutes in 4 studies (8,18–20), at 60 minutes in 3 studies (8,18,20), and at 120 minutes in 2 studies (8,18). Only one study assessed time to pain-free (18), one assessed treatment failure (19), and one assessed the need for rescue analgesia (at 30 and 60 minutes) (8).
All four included trials gave adequate statistical descriptions; including appropriate use of statistical tests (8,18–20). No significant baseline differences were noted between groups (8,18–20). With the exception of one trial that reported better pain control with lidocaine (compared to IV morphine) at 10, 15, and 30 minutes post-administration (20), all other trials reported similar (or worse) pain intensity compared to placebo (18), ketorolac (8), or fentanyl (Figure 3) (19). Since not all trials reported immediate (10 minutes post-administration) or longer pain relief (60 or 120 minutes post-administration), it was not possible to draw a conclusion about the immediate or long-term analgesia effects of LidoIV. A forest plot depicting pain score comparisons between regimens with and without lidocaine is provided in Figure 2.
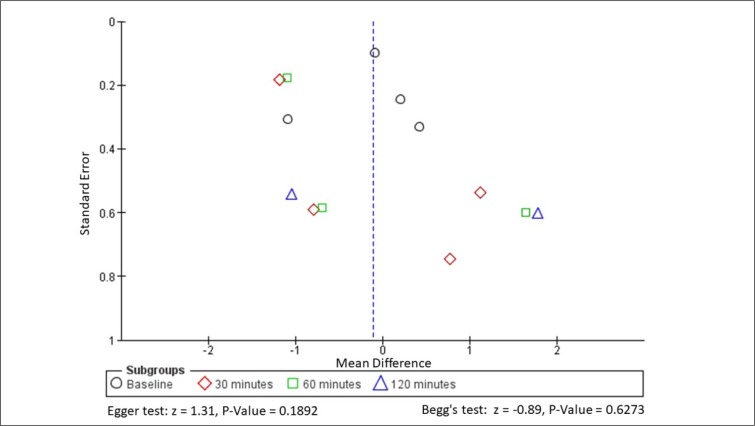
Figure 3.
Funnell plot and publication bias assessment
Figure 2.
Forrest plot of pain scores in lidocaine-containing versus no lidocaine regimens
Neither the Egger (z = 1.31, p-value = 0.1892) nor Begg-Mazumdar method (z = -0.89, P-Value = 0.6273) identified evidence of publication bias (Figure 3). The planned meta-analysis was not performed due to very severe heterogeneity (I2 >75%).
Discussion
This project aimed to clarify the clinical efficacy of LidoIV for decreasing the pain intensity and analgesic requirements associated with acute renal colic. Although substantial improvement was not noted over comparators, overall, some observations warrant further discussion. Of note, the methodology of the Soleimanpour et al. study that did report improvement over morphine differed from the other studies in a few important ways, namely: (1) coadministration with metoclopramide and (2) conservative IV fluid strategy (20).
Metoclopramide is a procaine amide structural analogue that may exert both antiemetic and analgesic effects. Proposed mechanisms include calcium channel-, opiate-, and prolactin-mediated mechanisms. The latter contributes to the analgesic action of the endogenous opioid system as evidenced by its reversibility by naloxone. Additionally, metoclopramide is a dopamine antagonist, increasing acetylcholine levels at neuro-effector junctions and post-ganglionic nerve terminals by inhibiting the action of acetylcholinesterase. Metoclopramide has been described to have antispasmodic effects on ureteral smooth muscle (34). Studies have described its analgesic efficacy in acute renal colic to exceed those of the isosorbide dinitrate (35), morphatropin (36), tenoxicam (37), and xintonding (38); however, findings were not significant for metoclopramide plus dipyrone vs. ketorolac alone (39), or metoclopramide plus pethidine vs. morphine alone (40). It has been described that combining lidocaine with metoclopramide may increase analgesia over lidocaine alone (41). It remains unclear whether an analgesia augmenting synergistic relationship exists between metoclopramide and the amide anesthetics.
The second way in which the study by Soleimanpour et al. differed from the others was fluid management strategy. In many regions, it is commonplace to treat patients with acute renal colic with forced IV fluids; however, this remains controversial. Large volumes of IV fluids are often administered to produce a diuresis that mechanically "flushes out" the stone. However, the benefit of this approach has not consistently borne out in clinical practice (42,43). The Soleimanpour et al. protocol did not administer forced IV fluids (20), whereas the other included studies did not specify the IV fluid management strategy (8,18,19). The influence of varied hydration strategies on the outcomes of these studies remains unclear.
In some situations, LidoIV doses may need to be modified. With commonly recommended doses, lidocain’s therapeutic index remains very high and plasma concentrations stay largely below the cardiotoxic and neurotoxic threshold levels (15). For renal colic, the recommended dose of LidoIV is 1.5 mg/kg (maximum 200 mg/dose) administered over 20-30 minutes (17,23,25). For patients being admitted, repeat dosing or an infusion may be used to maintain a steady-state plasma concentration: 1.5 mg/kg IV bolus, then 50 μg/kg/min (3.0 mg/kg) IV infusion for one hour, then 25 μg/kg/min (1.5 mg/kg) IV infusion for the second hour (h), then 12 μg/kg/min (0.7 mg/kg) IV infusion for the next 22 h, and finally 10 μg/kg/min (0.6 mg/kg) IV infusion from 24 to 48 h (15). Without a loading dose, it takes >60 min for LidoIV to achieve a therapeutic steady-state plasma concentration (15). Although continuous lidocaine infusion might theoretically lead to toxicity over time, blood concentrations reported in clinical studies have remained below toxic levels ( 5 µg/ml), except for cardiac surgery trials in which higher doses were used for longer durations (15).
As hepatic blood flow appears to be a limiting factor for lidocaine metabolism (13), the reduction in hepatic blood flow in patients with congestive heart failure may prolong the elimination half-life (T1/2) (44). No dose adjustment is necessary in patients with moderate liver cirrhosis; however, the dose should be decreased by 50% in patients with severe cirrhosis (Child score C) (45). Additionally, first- and second-degree heart blocks could be exacerbated and progress to a higher degree block with lidocaine administration, and both cardiovascular instability and concomitant use of alpha-agonists or beta-blockers are relative contraindications (46). Moreover, lidocaine clearance is linearly altered with kidney impairment, thus the elimination T1/2 of lidocaine and GX (but not MEGX) is doubled in case of severe renal insufficiency (47).
Volume of distribution is also an important factor when considering LidoIV dose and metabolism. Elderly patients have an increase in apparent volume of distribution, and consequently a significantly longer elimination T1/2 compared to younger patients (2.7 vs. 1.6 h) (48). For elderly patients, the initial loading dose should be the same, but any continuous infusion rate should be decreased by approximately 35% (15). The increased volume of distribution similarly accounts for the prolonged clearance seen in obese patients compared to non-obese patients (48). For obese patients, the bolus or loading dose should be calculated based on the patient’s total body weight, but the continuous infusion rate should be based on the ideal body weight (15). Lastly, lidocaine crosses the placenta and the blood–brain barriers via simple passive diffusion, and is excreted in breast milk (15). Thus, the clearance rate should be taken into consideration for breastfeeding mothers to avoid toxicity in the breast-fed infant (15).
Acknowledgements
We thank Dr. Seyed M. Hosseininejad from Mazandaran University of Medical Sciences (Sari, Iran) (18), Dr. Hassan Soleimanpour from Tabriz University of Medical Sciences (Tabriz, Iran) (20), and Dr. Mohammadreza Maleki Verki from Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran) (19) for supplying unpublished summary data from their respective studies (18). We also thank Mr. Jefferson Drapkin from Maimonides Medical Center in Brooklyn, NY, USA for responding to inquiries for data clarification (8). The authors attest to the originality of this previously unpublished work, that all listed authors contributed meet International Committee of Medical Journal Editors (ICMJE) criteria for authorship, and that all persons meeting ICMJE authorship criteria are credited.
Limitations
Since the data from some studies was unavailable, some risk of publication and selective reporting bias exists. Additionally, all included studies were conducted in Asia and North America. It is possible that genetic differences in drug metabolism or response may account for some of the observed variation in response. We identified no other systematic reviews or meta-analyses on this topic for comparison.
Conclusion
While we cannot draw a firm conclusion on the efficacy or superiority of intravenous lidocaine over traditional therapy for the treatment of renal colic in the emergency department, the available evidence indicates that the analgesic effects of intravenous lidocaine may not exceed that of NSAIDS or opiates. Although evidence exists to suggest the efficacy of intravenous lidocaine as an alternative treatment modality for acute renal colic, additional study is needed to clarify its role as compared to other traditional treatment modalities.
Author’s contributions
Concept generation and project supervision was performed by ACM. Literature searches were performed by ACM and KAS. Studies were screened for inclusion by ACM, CF, AACB and AMK. Data was abstracted by ACM, CF, AACB and AMK. Unpublished data was acquired from corresponding authors by ACM, AVA and SZ. Risk-of-bias analysis and evidence grading was performed by ACM, CF, AACB and AMK. Statistics and figure generation was performed by ACM, AVA and SZ. Manuscript preparation was performed by ACM, CF, AACB, AMK, AVA and SZ. Manuscript revision was performed by ACM and SZ.
Conflict of interest
The authors have no conflicts of interest to disclose. There were no sources of funding or support for this project.
Differences between protocol and review
Due to significant methodological heterogeneity between studies, the planned meta-analysis was not performed.
Funding and support
No funding was received for this work.
References
- 1.e Silva LOJ, Scherber K, Cabrera D, Motov S, Erwin PJ, West CP, et al. Safety and efficacy of intravenous lidocaine for pain management in the emergency department: A systematic review. Ann Emerg Med. 2018 ;72(2):135–144. doi: 10.1016/j.annemergmed.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Liu J. Comparative observation of analgesic effect of dizocine and diclofenac lidocaine combined with progesterone in the treatment of acute renal colic ureterolithiasis. J Clin Ration Drug Use. 2016;28:24–5. [Chinese] [Google Scholar]
- 3.Motov S, Drapkin J, Butt M, Monfort R, Likourezos A, Marshall J. Pain management of renal colic in the emergency department with intravenous lidocaine. Am J Emerg Med. 2018;36(10):1862–4. doi: 10.1016/j.ajem.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Ghani KR, Roghmann F, Sammon JD, Trudeau V, Sukumar S, Rahbar H, et al. Emergency department visits in the United States for upper urinary tract stones: trends in hospitalization and charges. J Urol. 2014;191(1):90–6. doi: 10.1016/j.juro.2013.07.098. [DOI] [PubMed] [Google Scholar]
- 5.Brown J. Diagnostic and treatment patterns for renal colic in US emergency departments. Int Urol Nephrol. 2006;38(1):87–92. doi: 10.1007/s11255-005-3622-6. [DOI] [PubMed] [Google Scholar]
- 6.Golzari SE, Soleimanpour H, Rahmani F, Zamani Mehr N, Safari S, Heshmat Y, et al. Therapeutic approaches for renal colic in the emergency department: a review article. Anesthesiol Pain Med . 2014;4(1):e16222. doi: 10.5812/aapm.16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ. 2004;328(7453) doi: 10.1136/bmj.38119.581991.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motov S, Fassassi C, Drapkin J, Butt M, Hossain R, Likourezos A, et al. Comparison of intravenous lidocaine/ketorolac combination to either analgesic alone for suspected renal colic pain in the ED. Am J Emerg Med. 2019;S0735-6757(19):30070–1. doi: 10.1016/j.ajem.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 9.Day RO, Graham GG. Non-steroidal anti-inflammatory drugs (NSAIDs) BMJ. 2013;346:f3195. doi: 10.1136/bmj.f3195. [DOI] [PubMed] [Google Scholar]
- 10.Hoppe JA, Nelson LS, Perrone J, Weiner SG. Prescribing Opioids Safely in the Emergency Department (POSED) Study Investigators Opioid prescribing in a cross section of US emergency departments. Ann Emerg Med. 2015;66(3):253–259. doi: 10.1016/j.annemergmed.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones MR, Viswanath O, Peck J, Kaye AD, Gill JS, Simopoulos TT. A brief history of the opioid epidemic and strategies for pain medicine. Pain Ther. 2018;7(1):13–21. doi: 10.1007/s40122-018-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller AC, Khan AM, Castro Bigalli AA, Sewell KA, King AR, Ghadermarzi S, et al. Neuroleptanalgesia for acute abdominal pain: a systematic review. J Pain Res. 2019;12:787–801. doi: 10.2147/JPR.S187798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberg L. Pharmacokinetics and pharmacodynamics of lignocaine: A review. World J Anesthesiol. 2015;4(2):17. [Google Scholar]
- 14.Koppert W, Zeck S, Sittl R, Likar R, Knoll R, Schmelz M. Low-dose lidocaine suppresses experimentally induced hyperalgesia in humans. Anesthesiology. 1998;89(6):1345–53. doi: 10.1097/00000542-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Beaussier M, Delbos A, Maurice-Szamburski A, Ecoffey C, Mercadal L. Perioperative use of intravenous lidocaine. Drugs. 2018;78(12):1229–46. doi: 10.1007/s40265-018-0955-x. [DOI] [PubMed] [Google Scholar]
- 16.Hosseininejad SM. Can the addition of low dose lidocaine improve the effectiveness of narcotics in reducing renal colic pain? Am J Emerg Med. 2018;36(4):721–2. doi: 10.1016/j.ajem.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Makhoul T, Kelly G, Schult RF, Acquisto NM. Intravenous lidocaine for renal colic in the emergency department (ED) Am J Emerg Med. 2019;37(4):775. doi: 10.1016/j.ajem.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 18.Firouzian A, Alipour A, Rashidian Dezfouli H, Zamani Kiasari A, Gholipour Baradari A, Emami Zeydi A, et al. Does lidocaine as an adjuvant to morphine improve pain relief in patients presenting to the ED with acute renal colic? A double-blind, randomized controlled trial. Am J Emerg Med. 2016;34(3):443–8. doi: 10.1016/j.ajem.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 19.Motamed H, Maleki Verki M. Intravenous lidocaine compared to fentanyl in renal colic pain management; A randomized clinical trial. Emergency. 2017;5(1):e82. doi: 10.22037/emergency.v5i1.18894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soleimanpour H, Hassanzadeh K, Vaezi H, Golzari SEJ, Esfanjani RM, Soleimanpour M. Effectiveness of intravenous lidocaine versus intravenous morphine for patients with renal colic in the emergency department. BMC Urol. 2012;12:13. doi: 10.1186/1471-2490-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gani H, Hoxha B, Xhani R, Dredha H, Karamitri G, Lenjani B, et al. Comparison of intravenous lidocaine versus intravenous morphine for patients with renal colic. Eur Urol Suppl. 2016;15(10):e1285. [Google Scholar]
- 22.Drapkin J, Motov S, Likourezos A, Monfort R, Butt M, Hossain R, et al. A randomized trial comparing the combination of intravenous lidocaine and ketorolac to either analgesics alone for emergency department patients with acute renal colic. Ann Emerg Med. 2018;72(Suppl) [Google Scholar]
- 23.Sin B, Effendi M, Bjork C, Punnapuza S. The use of intravenous lidocaine for renal colic in the emergency department. Ann Pharmacother. 2016;50(3):242. doi: 10.1177/1060028015625661. [DOI] [PubMed] [Google Scholar]
- 24.Sin B, Cao J, Yang D, Ambert K, Punnapuzha S. Intravenous lidocaine for intractable renal colic unresponsive to Standard therapy. Am J Ther. 2019;26(4):e487–8. doi: 10.1097/MJT.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 25.Soleimanpour H, Hassanzadeh K, Mohammadi DA, Vaezi H, Esfanjani RM. Parenteral lidocaine for treatment of intractable renal colic: a case series. J Med Case Rep. 2011;5:256. doi: 10.1186/1752-1947-5-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1 Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Jørgensen L, Paludan-Müller AS, Laursen DRT, Savović J, Boutron I, Sterne JAC, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: Overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. 2016;5:80. doi: 10.1186/s13643-016-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 31.Hedges L V, Pigott TD. The power of statistical tests in meta-analysis. Psychol Methods. 2001;6(3):203–17. [PubMed] [Google Scholar]
- 32.Nouira S. Treatment of renal colic in the Emergency Department (ED). Clinicaltrials.gov. 2017. [ [cited 2019 Jun 16]]. Available from: https://clinicaltrials.gov/ct2/show/NCT03199924?term=Treatment+of+Renal+Colic+in+the+Emergency+Departement+%28ED%29&rank=1.
- 33.Sin BW. Lidocaine vs ketorolac for management of renal colic in the Emergency Department. Clinicaltrials.gov. 2017. [[cited 2019 Jun 19]]. Available from: https://clinicaltrials.gov/ct2/show/NCT03137498?term=Treatment+of+Renal+Colic+in+the+Emergency+Departement+%28ED%29&rank=6.
- 34.Berman DJ, Firlit CF. Effect of metoclopramide on ureteral motility. Urology. 1984;23(2):150–6. doi: 10.1016/0090-4295(84)90010-4. [DOI] [PubMed] [Google Scholar]
- 35.Qiwei L, Tan Y, Wengue W, Zhenying W, Chen D, Yongshun D. Metoclopramide vs isosorbide dinitrate in the treatment of renal colic. Shanghai Med Pharm J. 1997;7:14. [Google Scholar]
- 36.Müller TF, Naesh O, Svare E, Jensen A, Glyngdal P. Metoclopramide (Primperan) in the treatment of ureterolithiasis A prospective double-blind study of metoclopramide compared with morphatropin on ureteral colic. Urol Int. 1990;45(2):112–3. doi: 10.1159/000281681. [DOI] [PubMed] [Google Scholar]
- 37.Kaya FB, Cevik A, Acar N, Kaya S, Zeytin A, Can C, et al. Clinical efficacy of Metoclopramide to treat pain and nausea in renal colic patients: A prospective randomised, double-blind, controlled trial. Hong Kong J Emerg Med. 2015;22(2):93–9. [Google Scholar]
- 38.Qiwei L, Tan Y, Wenguo W, Zhenying W, Chen D, Yongshun D. Comparison of the efficacy of metoclopramide and Xintongding in the treatment of renal colic. Shanghai Med J. 1997;7:23. [Google Scholar]
- 39.Martín Carrasco C, Rodríguez Vázquez M, Palacios Garciá R. A double-blind study of the analgesic efficacy in kidney colic of the combination of dipyrone and spasmolytic with ketorolac trometamol. Arch Esp Urol. 1993;46(9):763–8. [PubMed] [Google Scholar]
- 40.O’Connor A, Schug SA, Cardwell H. A comparison of the efficacy and safety of morphine and pethidine as analgesia for suspected renal colic in the emergency setting. J Accid Emerg Med. 2000;17(4):261–4. doi: 10.1136/emj.17.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safavi M, Honarmand A, Yazdanpanah A. Adding metoclopramide to lidocaine for intravenous regional anesthesia in trauma patients. Adv Biomed Res. 2014;3:45. doi: 10.4103/2277-9175.125753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worster AS, Bhanich Supapol W. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev. 2012:2:CD004926. doi: 10.1002/14651858.CD004926.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Springhart WP, Marguet CG, Sur RL, Norris RD, Delvecchio FC, Young MD, et al. Forced versus minimal intravenous hydration in the management of acute renal colic: a randomized trial. J Endourol. 2006;20(10):713–6. doi: 10.1089/end.2006.20.713. [DOI] [PubMed] [Google Scholar]
- 44.Nation RL, Triggs EJ, Selig M. Lignocaine kinetics in cardiac patients and aged subjects. Br J Clin Pharmacol. 1977;4(4):439–48. doi: 10.1111/j.1365-2125.1977.tb00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orlando R, Piccoli P, De Martin S, Padrini R, Palatini P. Effect of the CYP3A4 inhibitor erythromycin on the pharmacokinetics of lignocaine and its pharmacologically active metabolites in subjects with normal and impaired liver function. Br J Clin Pharmacol. 2003;55(1):86–93. doi: 10.1046/j.1365-2125.2003.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasero C. Intravenous lidocaine for acute pain treatment. J PeriAnesthesia Nurs. 2011;26(3):166–9. doi: 10.1016/j.jopan.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 47.De Martin S, Orlando R, Bertoli M, Pegoraro P, Palatini P. Differential effect of chronic renal failure on the pharmacokinetics of lidocaine in patients receiving and not receiving hemodialysis. Clin Pharmacol Ther. 2006;80(6):597–606. doi: 10.1016/j.clpt.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 48.Abernethy DR, Greenblatt DJ. Lidocaine disposition in obesity. Am J Cardiol. 1984;53(8):1183–6. doi: 10.1016/0002-9149(84)90659-3. [DOI] [PubMed] [Google Scholar]