Abstract
Objective:
The randomized phase (phase 2) of the Prolonging Remission in Depressed Elderly (PRIDE) study evaluated the efficacy and tolerability of continuation ECT plus medication compared with medication alone in depressed geriatric patients after a successful course of ECT (phase 1).
Method:
PRIDE was a two-phase multisite study. Phase 1 was an acute course of right unilateral ultrabrief pulse ECT, augmented with venlafaxine. Phase 2 compared two randomized treatment arms: a medication only arm (venlafaxine plus lithium, over 24 weeks) and an ECT plus medication arm (four continuation ECT treatments over 1 month, plus additional ECT as needed, using the Symptom-Titrated, Algorithm-Based Longitudinal ECT [STABLE] algorithm, while continuing venlafaxine plus lithium). The intent-to-treat sample comprised 120 remitters from phase 1. The primary efficacy outcome measure was score on the 24-item Hamilton Depression Rating Scale (HAM-D), and the secondary efficacy outcome was score on the Clinical Global Impressions severity scale (CGI-S). Tolerability as measured by neurocognitive performance (reported elsewhere) was assessed using an extensive test battery; global cognitive functioning as assessed by the Mini-Mental State Examination (MMSE) is reported here. Longitudinal mixed-effects repeated-measures modeling was used to compare ECT plus medication and medication alone for efficacy and global cognitive function outcomes.
Results:
At 24 weeks, the ECT plus medication group had statistically significantly lower HAM-D scores than the medication only group. The difference in adjusted mean HAM-D scores at study end was 4.2 (95% CI=1.6, 6.9). Significantly more patients in the ECT plus medication group were rated “not ill at all” on the CGI-S compared with the medication only group. There was no statistically significant difference between groups in MMSE score.
Conclusions:
Additional ECT after remission (here operationalized as four continuation ECT treatments followed by further ECT only as needed) was beneficial in sustaining mood improvement for most patients.
Electroconvulsive therapy (ECT) remains an important treatment for severe depression, particularly for elderly adults. The acute antidepressant efficacy of ECT has repeatedly been shown to be superior to other antidepressant treatment modalities, including pharmacotherapy and psychotherapy. Because of its rapid speed of response, ECT is the treatment of choice for urgently ill patients, including those with psychosis and strong suicidal ideation from depression. Shortcomings of ECT have included a less-than-optimal tolerability profile, with adverse cognitive effects, as well as high relapse rates in the 6-month period after remission. The Prolonging Remission in Depressed Elderly (PRIDE) study was designed to address both of these shortcomings, with the use of right unilateral ultrabrief pulse (1) and randomization to one of two treatment strategies—a novel ECT strategy plus medication compared with medication alone—in the 6-month continuation phase of the study (phase 2). The phase 1 efficacy data are reported separately (1); here, we present the efficacy data from phase 2 of the study.
METHOD
Design Overview
The PRIDE study, funded by the National Institute of Mental Health (NIMH) and begun in 2009, was a randomized, multicenter study contrasting two post-ECT continuation treatment strategies: a medication only arm, based on aggressive standard-of-care pharmacotherapy with a combination of venlafaxine and lithium carbonate; and an ECT plus medication arm, in which medication was enhanced by the addition of four continuation ECT treatments followed by an individualized, flexible, algorithm-based ECT schedule called Symptom-Titrated, Algorithm-Based Longitudinal ECT (STABLE) (2). The trial consisted of two phases. In phase 1, 240 patients age 60 or over who had unipolar major depressive disorder received acute ECT three times per week in combination with open-label venlafaxine. In phase 2, the intent-to-treat sample consisted of 120 patients who remitted in phase 1 and were randomized to receive either medication alone or ECT plus medication. The primary efficacy outcome variable was score on the 24-item Hamilton Depression Rating Scale (HAM-D) (3), measured longitudinally over 6 months; the primary time point for treatment comparison was at the end of the study (week 24). The secondary efficacy outcome was score on the Clinical Global Impressions severity scale (CGI-S) (4). Global cognitive functioning was assessed using the Mini-Mental State Examination (MMSE) (5). The primary neurocognitive performance and functioning outcomes (reported elsewhere) were assessed using an extensive test battery. Patient safety and study progress were monitored by an NIMH data safety and monitoring board.
Participating Centers
The recruiting centers were the Icahn School of Medicine at Mt. Sinai, New York; Columbia University/New York State Psychiatric Institute, New York; Duke University School of Medicine, Durham, N.C.; Zucker Hillside Hospital/Northwell Health System, New York; Mayo Clinic, Rochester, Minn.; University of Texas Southwestern Medical Center, Dallas; Wake Forest University Medical Center, Winston-Salem, N.C.; Augusta University/Medical College of Georgia, Augusta; and New York Presbyterian/Weill Cornell Medical Center, New York and White Plains. The clinical coordinating centers were located at Mt. Sinai and Duke, and the data center was at the Medical University of South Carolina, Charleston. The Columbia site was closed to recruitment in October 2010 in coordination with the opening of the Duke site. The Wake Forest site was closed in August 2012 and was replaced by the Augusta University site. Mayo Clinic discontinued recruitment in May 2012.
Patient Sample
Patients enrolled in phase 1 were age 60 and over and were referred for ECT for the treatment of unipolar major depressive disorder, without dementia, with or without psychosis, with a pretreatment HAM-D score ≥21. The study excluded patients with bipolar disorder, schizoaffective disorder, schizophrenia, dementia, delirium, an intellectual disability, a history of substance abuse or dependence in the past 6 months, or a neurological condition or active general medical condition assumed to affect cognition or treatment response. Patients with contraindications to venlafaxine or lithium and patients who had failed to respond to an adequate trial of venlafaxine plus lithium or a trial of ECT in the current episode were also excluded. Inclusion criteria for the randomized phase (phase 2) were achievement of remission in phase 1, defined as 1) having a score ≤10 on the HAM-D on two consecutive ratings and 2) the HAM-D score did not increase >3 points on the second consecutive rating, or it remained ≤6. Informed consent was obtained before entrance to phase 1 and before randomization in phase 2.
Treatments
ECT plus medication arm.
In the ECT plus medication arm, ECT featured an initial fixed, tapered ECT treatment schedule followed by a symptom-driven, flexible component (STABLE), in addition to the same venlafaxine and lithium regimen as in the medication only arm. The initial fixed ECT component consisted of four ECT treatments in 1 month (one treatment in each of the following windows following randomization: 2–5 days, 7–12 days, 14–19 days, and 23—28 days). Treatment frequency in the subsequent flexible component (weeks 5–24) was determined by application of the STABLE algorithm, which prescribed 0–2 ECT treatments in a given week based on the patient’s HAM-D scores (Table 1).
TABLE 1.
Algorithm for Continuation ECT in Phase 2 for the ECT Plus Medication Arm: Symptom-Titrated, Algorithm-Based Longitudinal ECT (STABLE)a
| Weeks 1–4: Fixed ECT schedule | |||
|---|---|---|---|
| One treatment 2–5 days after randomization, one treatment 7–12 days after randomization, one treatment 14–19 days after randomization, and one treatment 23–28 days after randomization (a total of four ECT treatments in 1 month) | |||
| Weeks 5–24: Symptom-Titrated ECT Schedule | |||
| Treatments per Week | Description | Corresponding HAM-D Condition | Relapse Potential |
| 0 | Current symptom level very low or Current symptom level low to moderate, with only small drift from baseline level or Last two HAM-D scores in remitted range with a flat trajectory (remission stable with less than 2-point change from previous) |
Current HAM-D score ≤6 Current HAM-D score 7–12 and is ≤2 points higher than baseline score Current HAM-D score 7–10 and previous score was 5–10 and current score is ≤2 points higher than previous score |
Low Low Low |
| 2 | Current symptom level very high or Current symptom level moderate to high, with trajectory increasing rapidly and large drift from baseline |
Current HAM-D score ≥16 Current HAM-D score 11–15, and current score is ≥3 points higher than previous score, and current score is ≥8 points higher than baseline score |
High High |
| 1 | Patients not requiring 0 or 2 treatments received 1 treatment | Current HAM-D score intermediate between criteria for low or high relapse potential | Moderate |
| Discontinue study | Current and previous HAM-D score ≥21, or the patient is suicidal, or the patient requires psychiatric hospitalization | ||
HAM-D=24-item Hamilton Depression Rating Scale.
The implementation of the STABLE algorithm included a fixed schedule of twice-monthly clinic visits and supplemental HAM-D telephone screenings during intervening (nonclinic) weeks. If the telephone HAM-D score was significantly increased based on the STABLE algorithm criteria for moderate to high relapse potential (Table 1), the patient was scheduled for an interim confirmatory clinic HAM-D evaluation within 48 hours. Treatment determination using the algorithm was based on the confirmatory clinic-administered HAM-D. To ensure consistent application of the STABLE algorithm, the data center implemented a web-based program that received the HAM-D and MMSE scores for both clinic and telephone visits and provided study coordinators at the clinical centers with a description of the appropriate action (e.g., an alert to schedule an interim clinic visit if indicated by telephone HAM-D).
ECT and anesthesia procedures complied with APA guidelines (6) and are described in the article on phase 1 (1). ECT in phase 2 was administered at the same stimulus dose as the last treatment in phase 1. An ECT treatment, if indicated by the algorithm, was postponed for 2 days if the patient’s MMSE score was <21.
Open-label venlafaxine was started in phase 1 at an initial dosage of 37.5 mg/day and was increased by 37.5 mg every 3 days or as tolerated, with a target dosage of 225 mg/day. This dosage was maintained as tolerated through phase 1 and continued after randomization in phase 2. Open-label lithium was started at 300 mg/day on the day of randomization. Lithium was used in moderate dosages as an adjunct to venlafaxine, with a target blood level in the range of 0.4–0.6 mEq/L for most patients, never to exceed 1.0 mEq/L. Thus, full “therapeutic levels” (as used for the treatment of bipolar disorder) were not the goal. Lithium levels were obtained at weeks 2, 3, 4, 5, 6, 8, 12, 16, 20, and 24. Medication changes were made on the basis of blood levels and clinical side effects.
Lithium was held for at least 24 hours before each ECT session, and additional time for clearance of lithium was allowed for patients whose levels were above 0.8 mEq/L.
Medication only arm.
Venlafaxine and lithium dosing and procedures were identical to those of the ECT plus medication arm, except that no lithium doses were withheld because there was no ECT. The schedule of clinic and telephone ratings was identical for both arms. To balance clinical contact between treatment arms, patients in the medication only arm were asked to come in for an unscheduled (alternate week) clinic HAM-D assessment if the telephone HAM-D significantly increased, as described for the ECT plus medication arm.
Assessments
The clinical pre-ECT medical and anesthesia evaluations (physical examination, blood and urine analyses, electrocardiogram, and chest X-ray) followed published guidelines (6) and local ECT policies. Diagnosis of unipolar major depressive disorder was made by study psychiatrists using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (7) or the Mini International Neuropsychiatric Interview (8). Diagnosis of dementia was made using DSM-IV criteria. Suicidal ideation was assessed at each twice-monthly clinic visit using the Beck Scale for Suicide Ideation (9, 10). Hallucinations were assessed at each visit using item 10 of the Brief Psychiatric Rating Scale (11).
Depressive symptom severity (the primary efficacy outcome) was assessed at baseline and at twice-monthly clinic visits using the HAM-D. For the alternating twice-monthly telephone screening assessment, the HAM-D with minor modifications as described by Mundt et al. was used (12). The CGI-S was also assessed by the study psychiatrists at each clinic visit (13).
The MMSE, a measure of global cognitive impairment, was administered at each twice-monthly clinic visit. The impacts of ECT plus medication and medication alone on neurocognitive performance and health status/functioning were assessed using an extensive test battery measured monthly (partial battery) and at the midpoint and end (full battery) of the study period. The full results of the neuropsychological and health status/functioning battery will be reported elsewhere.
Safety measures were adverse events and serious adverse events, recorded as per Office for Human Research Protections guidelines. Adverse events and serious adverse events were monitored externally by an NIMH data and safety monitoring board.
Raters
Raters received training in the administration and scoring of all rating scale instruments during 8-hour training sessions at the initial startup meeting and at yearly investigators’ meetings. Ongoing consistency of HAM-D ratings across sites was addressed by having each rater score a new set of videos for comparison with the consensus ratings of the study co–principal investigator and project coordinator. A more detailed description of the standardization of ratings is provided in the data supplement that accompanies the online edition of this article.
Randomization and Masking of Treatment Assignment
The stratified permuted block method of randomization was used. Block size was varied to minimize the likelihood of unmasking. Randomization was stratified by clinical center and was delivered using a web-based system developed by the PRIDE data center. Clinical raters and neuropsychological technicians were blind to treatment assignment. Patient ratings were administered in a neutral location to protect the blinding of treatment assignment.
Data Management and Quality Control
Data were managed using a web-based clinical trials management system that incorporated secure direct data entry at the site level, central randomization, and real-time reporting capabilities (14). The system was designed to monitor recruitment, safety reporting, protocol violations, and data collection and data entry progress. The database processed all HAM-D ratings (entered within 24 hours of administration) and alerted sites when a treatment in the ECT plus medication arm was indicated by the algorithm or when an intervening clinic visit should be scheduled for patients in either treatment arm based on elevated HAM-D ratings on telephone screenings.
Statistical Analysis
Intent-to-treat sample and dropouts.
Randomized patients who had at least one postbaseline assessment were included in the intent-to-treat sample. Patients in the intent-to-treat sample were classified as dropouts if they withdrew consent or were discontinued by the study psychiatrist before the end of the 24-week treatment period. Protocol-defined reasons for physician-directed patient discontinuations were two consecutive HAM-D scores ≥21; psychiatric hospitalization; suicidality, based on clinical judgment; or adverse events that raised safety concerns. Patients who were discontinued from the randomized treatment phase were treated as clinically appropriate.
Missing data.
The primary analysis used a mixed-effects modeling approach, which accommodates missing data under the assumption that the missing data mechanism is missing at random.
Efficacy analyses.
The primary efficacy outcome was HAM-D score, measured at twice-monthly clinic visits over the 6-month study period, with the end-of-study assessment (week 24) as the primary time point for treatment comparisons. Secondary analyses of HAM-D score focused on comparison of the mean trajectory over the study period. A mixed-effects repeated-measures modeling approach was used to compare HAM-D score for the ECT plus medication and medication only groups at study end (15, 16). A more detailed description of the modeling process is provided in the online data supplement.
The secondary seven-category ordinal-level efficacy outcome, CGI-S, had sparse cell counts for categories 3–7, which necessitated the collapsing of categories into the dichotomous response “normal” (category 1) versus “not normal” (categories 2–7) to avoid convergence problems with analyses. A generalized linear mixed-models approach was used to model the longitudinal profile using a logit link function for the binary outcome. The odds ratio (and 95% confidence interval) for receiving a “normal” rating for patients in the ECT plus medication group compared with those in the medication only group at study end was estimated from the model. The mean CGI-S score at end of study, using the full scale, was estimated for the two treatment groups using a multiple imputation procedure.
In addition, efficacy was described in terms of relapse status and time to relapse. Patients were categorized as having relapsed if they had two consecutive HAM-D scores ≥21, required psychiatric hospitalization, or became suicidal. Relapse status was described using the odds ratio (and 95% confidence interval) and the percentage of patients in each group who relapsed. Time to relapse was described using median and mean numbers of weeks from randomization to study exit due to relapse. Kaplan-Meier survival curves for time to relapse were also determined for the groups. Relapse analyses are considered descriptive only (and no p values are reported) because relapse was not an a priori protocol-specified outcome variable and the study was not powered to allow valid inferential results for relapse comparisons.
Global cognitive functioning and safety analyses.
MMSE scores for the ECT plus medication and medication only groups were compared using the mixed-effects repeated-measures methodology as described above for HAM-D scores. Safety was evaluated, per Office for Human Research Protections guidelines, using adverse events and serious adverse events summarized by number of subjects experiencing the event and relatedness to the study interventions.
Analyses were carried out using SAS, versions 9.3 and 9.4. All statistical tests were two-tailed using an alpha of 0.05.
RESULTS
Sample
Of the 240 patients who began phase 1,148 (62%) remitted and were eligible for randomization; 120 patients who consented for phase 2 and received at least one randomized treatment were included in the intent-to-treat sample (for the study CONSORT chart, see Figure S1 in the online data supplement). Demographic and baseline clinical characteristics (Table 2) were similar for the groups, except for presence of psychotic features at baseline (8.2% for the ECT plus medication group and 20.3% for the medication only group). The patterns for dropouts across demographic and clinical indicators were not significantly different for the two treatment arms (Table 3).
TABLE 2.
Patient Characteristics for the Intent-to-Treat Sample and the Treatment Groups in a Study of Continuation ECT in Geriatric Depressiona
| Characteristic | Total Sample (N=120) | ECT Plus Medication (N=61) | Medication Only (N=59) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 70.5 | 7.2 | 70.8 | 7.2 | 70.3 | 7.3 |
| Education (years) | 14.5 | 3.3 | 14.4 | 3.3 | 14.5 | 3.4 |
| Hamilton Depression Rating Scale (24 item) | ||||||
| Baseline, phase 1 | 30.3 | 7.4 | 29.6 | 6.8 | 31.1 | 7.9 |
| Baseline, phase 2 | 6.1 | 2.5 | 6.0 | 2.5 | 6.1 | 2.5 |
| Mini-Mental State Examination | ||||||
| Baseline, phase 1 | 27.5 | 2.2 | 27.6 | 2.2 | 27.4 | 2.3 |
| Baseline, phase 2 | 27.9 | 2.4 | 27.9 | 2.5 | 27.8 | 2.4 |
| Clinical Global Impressions severity scale | ||||||
| Baseline, phase 1 | 5.2b | 0.9 | 5.1b | 0.8 | 5.3 | 0.9 |
| Baseline, phase 2 | 1.9 | 0.9 | 1.9 | 0.9 | 1.8 | 0.8 |
| Seizure threshold (mC) (baseline, phase 1) | 29.8b | 12.8 | 29.4b | 12.7 | 30.1 | 13.0 |
| Prior antidepressants (baseline, phase 1) | 2.3c | 1.5 | 2.3d | 1.6 | 2.4e | 1.5 |
| N | % | N | % | N | % | |
| Female | 74 | 61.7 | 37 | 60.7 | 37 | 62.7 |
| White | 114 | 95 | 58 | 95.1 | 56 | 94.9 |
| Psychosis (at baseline) | 17 | 14.2 | 5 | 8.2 | 12 | 20.3 |
| Age group (years) | ||||||
| 60–69 | 57 | 47.5 | 29 | 47.5 | 28 | 47.5 |
| 70–79 | 49 | 40.8 | 24 | 39.3 | 25 | 42.4 |
| 80–89 | 14 | 11.7 | 8 | 13.1 | 6 | 10.2 |
No significant difference between groups on any variable; baseline psychosis approached significance, at p=0.06.
Missing data for one subject.
Missing data for 15 subjects.
Missing data for 10 subjects.
Missing data for five subjects.
TABLE 3.
Comparison of Characteristics for Dropouts in the ECT Plus Medication and Medication Only Treatment Arms in a Study of Continuation ECT in Geriatric Depressiona
| Characteristic | ECT Plus Medication (N=22) | Medication Only (N=26) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 73.3 | 8.1 | 70.4 | 7.3 |
| Education (years) | 13.9 | 3.6 | 13.9 | 3.0 |
| Hamilton Depression Rating Scale (24 item) | ||||
| Baseline, phase 2 | 6.1 | 2.7 | 6.0 | 2.4 |
| Last assessment, phase 2 | 15.9b | 8.5 | 15.7 | 8.2 |
| Mini-Mental State Examination | ||||
| Baseline, phase 2 | 27.3 | 2.7 | 28.3 | 2.0 |
| Last assessment, phase 2 | 27.9c | 2.3 | 27.7 | 3.0 |
| Clinical Global Impressions severity scale | ||||
| Baseline, phase 2 | 1.9 | 0.8 | 1.8 | 0.8 |
| Last assessment, phase 2 | 3.3b | 1.3 | 3.4c | 1.2 |
| ECT treatments in phase 1 | 6.9 | 2.6 | 7.9 | 3.2 |
| N | % | N | % | |
| Female | 13 | 59.1 | 18 | 69.2 |
| White | 21 | 95.5 | 26 | 100.0 |
| Psychosis (at baseline) | 1 | 4.6 | 4 | 15.4 |
No significant difference between groups on any variable.
Missing data for two subjects.
Missing data for one subject.
The overall average venlafaxine dosage in phase 2 was 191.6 mg/day (SD=55.8), with no significant difference between treatment arms. The mean lithium blood level was 0.53 mEq/L (SD=0.27) for the medication only group and 0.36 mEq/L for the ECT plus medication group (SD=0.23). The lower lithium value in the ECT plus medication group was anticipated because of holding doses prior to ECT.
Efficacy Analyses
The baseline-adjusted mean HAM-D score for the ECT plus medication group at the primary 24-week study endpoint (mean=5.5, 95% CI=3.7, 7.3) was significantly lower than that of the medication only group (mean=9.4, 95% CI=7.5, 11.3) with a difference in mean HAM-D scores (effect size) of 3.9 points (95% CI=1.3, 6.5, p=0.004 from mixed-effects repeated-measures model) (Figure 1). After additional adjustment for site and psychosis, the effect size increased to 4.2 points (95% CI=1.6, 6.9, p=0.002). There was no evidence of a differential effect of treatment on depression severity by site or psychosis. The ECT plus medication group had a significantly steeper decline in mean HAM-D scores over time than did the medication only group (main effect of time for the ECT plus medication group, p<0.001; main effect of time for the medication only group, p=0.398; see the footnote to Figure 1).
FIGURE 1. Longitudinal Trajectory of Modeled Hamilton Depression Rating Scale (HAM-D) Score Least Squares Means in a Study of Continuation ECT in Geriatric Depressiona.
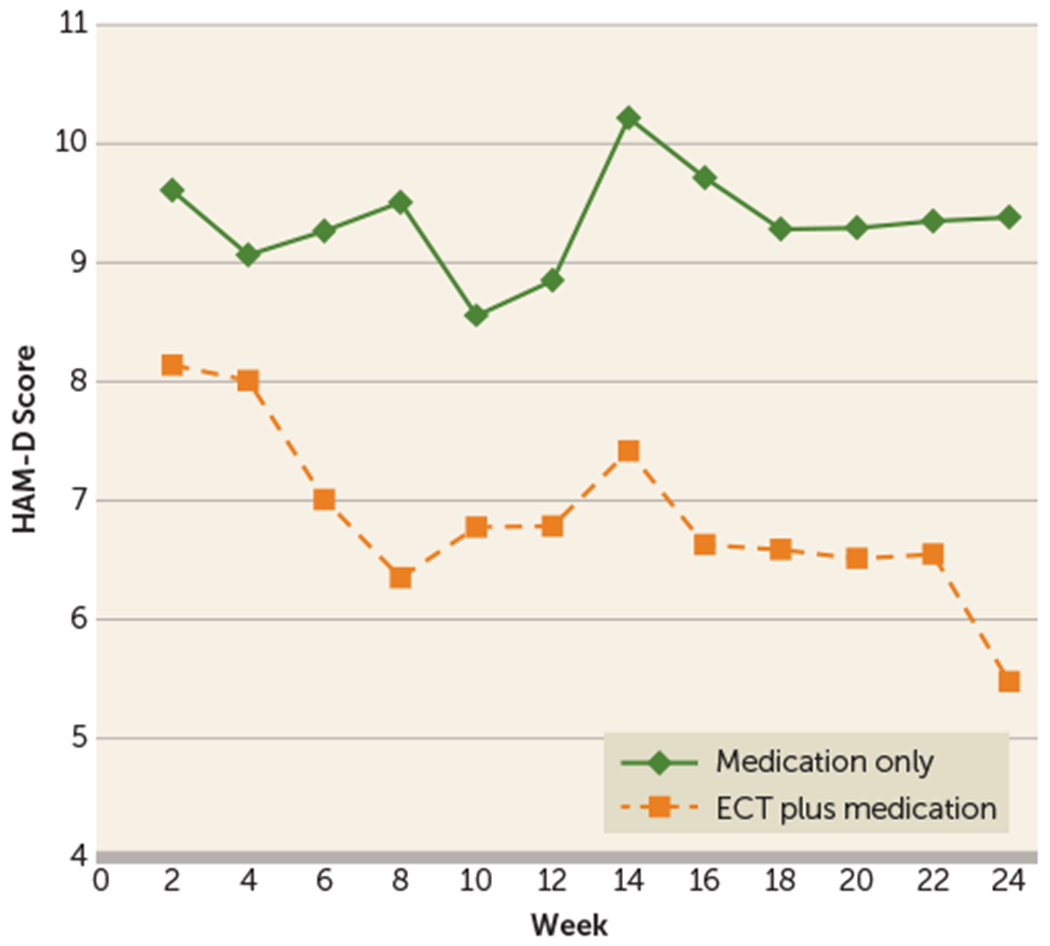
a The graph shows baseline-adjusted least squares 24-item HAM-D mean scores from a basic mixed-effects repeated-measures model (unstructured covariance), with difference in baseline-adjusted least squares treatment means at study end (week 24) (Δ)=3.9 (95% CI=1.3, 6.5, p=0.004). For the model containing baseline HAM-D score, site, and psychosis, Δ=4.2 (95% CI=1.6, 6.9, p=0.002, unstructured covariance). Treatment-by-site, treatment-by-psychosis, and treatment-by-baseline HAM-D score interaction terms were not significant. For the comparison of the trajectories of HAM-D mean scores over time (time as continuous) in a mixed-effects model (baseline-adjusted model, unstructured covariance): treatment-by time interaction, p=0.084; main effect of time, p=0.001; main effect of treatment, p=0.12; and baseline HAM-D score, p<0.001. For analyses by treatment: main effect of time for the ECT plus medication group, p<0.001; main effect of time for the medication only group, p=0.398.
For CGI-S score, the odds of patients in the ECT plus medication group being rated as “not at all ill” at study end were 5.2 times those of patients in the medication only group (odds ratio=5.2, 95% CI=1.5,17.7, p=0.009, from generalized linear mixed model with baseline CGI-S, site, and psychosis as covariables). The mean CGI-S score at week 24 was 2.2 for the medication only group and 1.7 for the ECT plus medication group (from multiple imputation).
Overall, 16.7% (20/120) of patients relapsed; 20.3% of patients in the medication only group (12/59) relapsed, and 13.1% of those in the ECT plus medication group (8/61) relapsed. The odds of relapsing were 1.7 times higher for patients in the medication only group (odds ratio=1.7, 95% CI=0.6,4.5). There was a tendency toward a shorter time to relapse for the medication only group compared with the ECT plus medication group (Figure 2). The median time to relapse for patients in the ECT plus medication group was 7.5 weeks (mean=8.3, SD=5.0), compared with 6.0 weeks for the medication only group (mean=7.6, SD=6.1). Overall, 60% (72/120) of patients had neither relapsed nor dropped out at study end (64% of the ECT plus medication group [39/61] and 56% of the medication only group [33/59]). The mean last observed HAM-D score (week 24 for completers and time of exit for dropouts) was 7.7 (SD=5.5) for the ECT plus medication group and 10.5 (SD=8.2) for the medication only group (effect size=2.8 points, 95% CI=−0.2, 5.8, p=0.065, pooled t test).
FIGURE 2. Time to Relapse for Patients in the ECT Plus Medication and Medication Only Treatment Arms in a Study of Continuation ECT in Geriatric Depressiona.
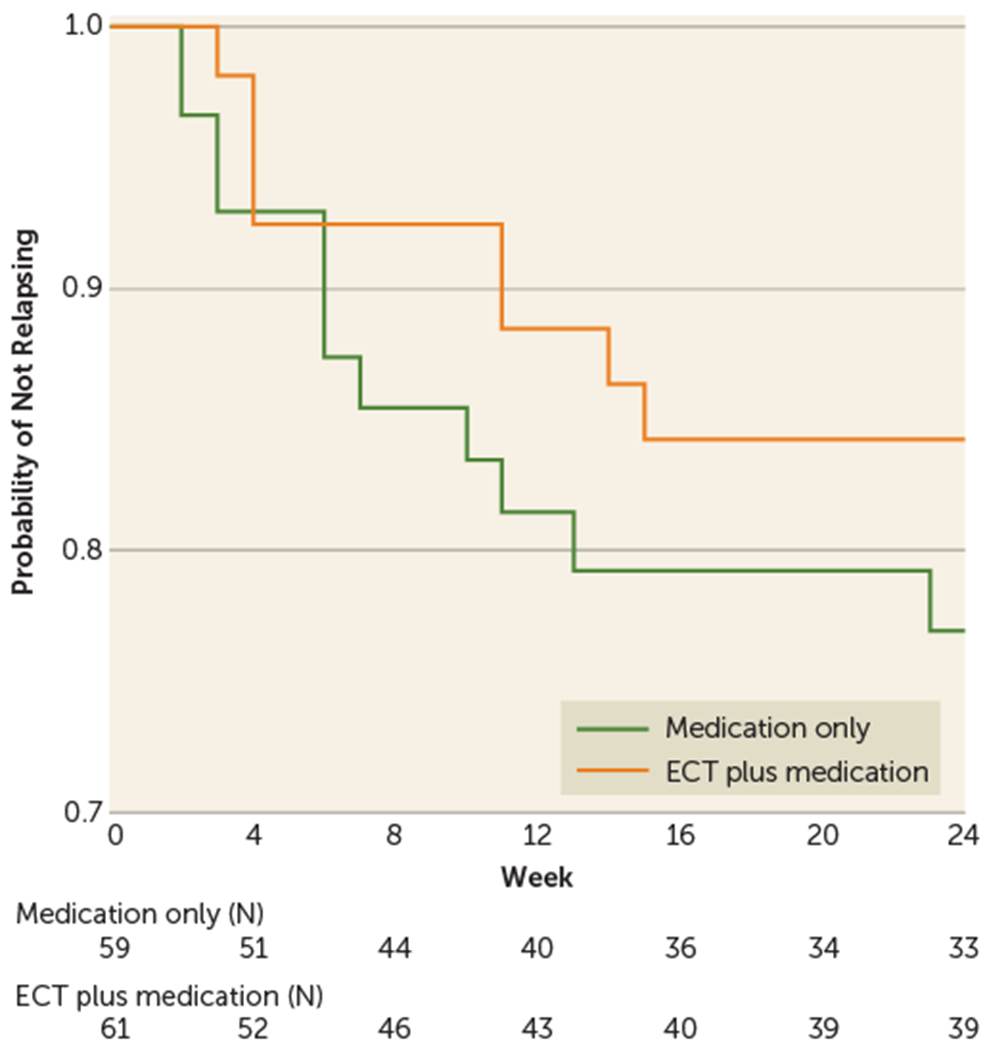
a The graph shows a Kaplan-Meier survival curve with probability of not relapsing over time. The “event” is relapse, with eight patients in the ECT plus medication arm relapsing compared with 12 in the medication only arm. The numbers in the box below the figure are the number of patients “at risk” of relapsing at the beginning of each time interval. Patients who have dropped out or relapsed in prior intervals are no longer “at risk” and are removed from the denominator when determining probability of not relapsing in that interval. Note that “not relapsing” is not equivalent to “remaining remitted” because not all patients who have not relapsed have HAM-D scores that meet the criteria for remission.
During the flexible phase (weeks 5–24), 34.4% of patients in the ECT plus medication group (21/61) received at least one additional ECT treatment; seven of them received only one additional treatment (beyond the four fixed sessions). In the flexible phase, only 12 patients ever received two ECT treatments in a single week; nine of these received two treatments per week only once; the remaining three patients received two treatments per week during two or more weeks (one received two treatments in weeks 7 and 15; one received two treatments in weeks 10, 13, 17; and one received two treatments in weeks 10, 11, 12, and 14). The highest density of ECT treatments in the flexible phase occurred in weeks 5–15 (Figure 3). Among the 21 patients who received additional ECT, only three relapsed despite treatment, 14 had HAM-D scores ≤10 at study endpoint, and four had HAM-D scores of 11–15 (three of whom would still have met the traditional response criterion of a reduction of at least 50% from phase 1 baseline). Rescue ECT was associated with immediate large decreases in HAM-D ratings (Figure 3).
FIGURE 3. Trajectories of 24-item Hamilton Depression Rating Scale (HAM-D) Scores for Individual Patients in the ECT Plus Medication Arm Who Had ECT During the Flexible Phase (Weeks 5–24) (N=21) in a Study of Continuation ECT in Geriatric Depressiona.
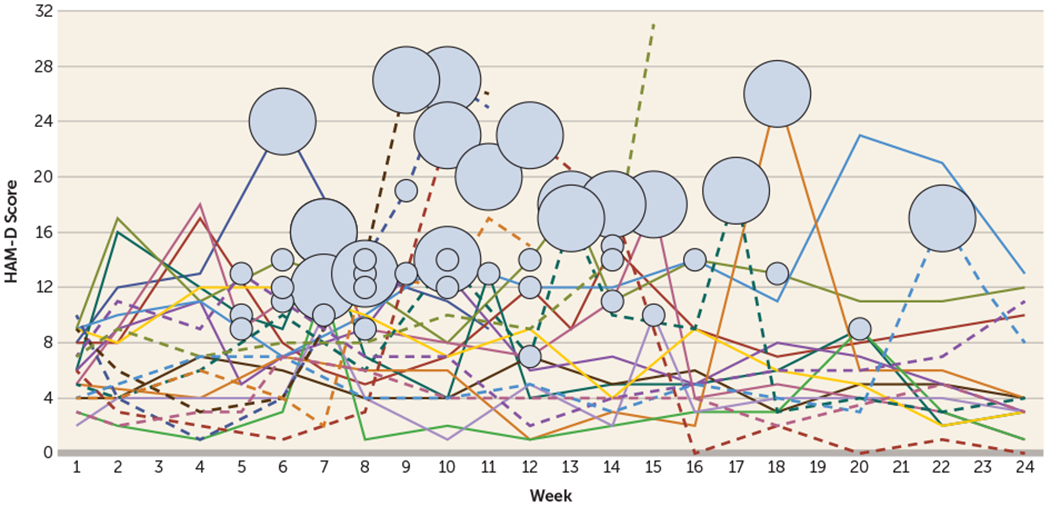
a The graph shows the number and timing of rescue ECT treatments given in the ECT plus medication arm for those requiring an ECT treatments beyond the four ECT treatments received in the fixed phase. Each line represents a patient who received rescue ECT treatments (N=21). Small circles indicate that the patient received one ECT treatment in a given week, and large circles indicate that a patient received two treatments in a given week. Discontinuation of a line indicates patient dropout. Note the steep declines in individual trajectories immediately following the small and large circles.
Global Cognitive Functioning
No statistically significant difference was observed in mean MMSE scores between the ECT plus medication and medication only arms at the primary 24-week endpoint (effect size=−0.38 points, 95% CI=−1.0, 0.2), taking into account the effect of depression symptom severity changes over time, baseline MMSE score, site, psychosis, and baseline MMSE score-by-time interaction.
Safety Analyses
The frequencies of adverse events and serious adverse events are listed in Tables S3 and S4 in the online data supplement. Among the adverse events, two events were rated as probably related to ECT: decreased heart rate (one patient) and sinus pause (one patient). Overall, there were no remarkable treatment differences in occurrence or type of adverse events (see Table S3). The serious adverse event that occurred with greatest frequency was suicidal ideation, which occurred in three patients in the ECT plus medication arm (see Table S4). These events were assessed as being unrelated to either ECT or lithium for all three patients; in one patient, the event was assessed as possibly related to venlafaxine. One patient in the ECT plus medication arm developed lithium toxicity and was discontinued from study. There were no serious adverse events judged to be definitely, probably, or possibly related to ECT. No deaths occurred in phase 2.
DISCUSSION
Our results demonstrate a beneficial effect of right unilateral ultrabrief pulse ECT combined with medication compared with medication alone in maintaining low depression symptom severity for 6 months after achieving remission. Rates of adverse events were low for both treatment arms, and no serious adverse events were attributable to ECT. There was no group difference in global cognitive functioning. Taken together, these results demonstrate that additional ECT beyond the traditional endpoint of an acute course, plus rescue ECT as needed, is valuable and feasible in maintaining the long-term antidepressant benefits of ECT in a vulnerable geriatric population.
Limitations of this study include potential non-generalizability of the findings because of the acknowledged bias of a sample willing to consent to a complex research study (for example, many patients with severe depression are too ill to participate even in an ECT trial of this nature) and sample size constraints for feasibility that necessitated the use of depression symptom severity rather than relapse as the primary outcome variable. While our descriptive data on relapse as a dichotomous outcome are informative, our study was not powered to apply stronger inferential statistical procedures regarding relapse. In addition, the study was not designed to distinguish the relative contributions of the components of the ECT plus medication and medication only arms. Specifically, the study design does not allow separation of the relative contributions of each of the two components of the ECT algorithm (four continuation ECT treatments, additional flexible ECT treatments as needed) or of the two components of the medication only arm (venlafaxine and lithium). The STABLE algorithm for determining when to provide additional ECT, while complex, was necessary for tight standardization of procedures in this multicenter trial. In clinical practice, it should be possible to implement a much simpler process, using a clinician- or patient-administered rating scale, along with clinical judgment.
The clinical implications of these findings are that continuing ECT after remission, rather than abruptly stopping a course of ECT, is likely to be beneficial in sustaining mood improvement for most patients, and that clinicians should be willing to prescribe additional ECT if patients begin to show symptom reemergence. The newer right unilateral ultrabrief pulse ECT technique allows practitioners to be more liberal in prescribing additional ECT when needed. Such practice is further facilitated by the fact that much of the ECT prescribed in the United States is now delivered in the outpatient setting. Moreover, the finding in both phases of our study of variability in number of ECT treatments needed should put to rest the long-held clinical notion of a rigidly fixed ECT course of six treatments (17). The provision of rescue ECT could be easily implemented in clinical practice. Our data suggest that tapering a course of ECT and early intervention with additional ECT if symptoms worsen can prevent full syndromic relapse and its potentially catastrophic consequences.
Supplementary Material
Acknowledgments
Supported by NIMH grants U01MH055495, U01MH081362, U01MH086127, U01MH086127, U01MH086130, U01MH08612005, U01MH084241, and U01MH086122.
Dr. Kellner receives honoraria from UpToDate, Psychiatric Times, and Northwell Health and royalties from Cambridge University Press. Dr. Husain has received grant support from Alkermes, Assurex, Avanir, Cyberonics, Brainsway, MagStim, NARSAD, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, NeoSync, Neuronetics, NIDA, NIMH, the Stanley Foundation, and St. Jude Medical (Advanced Neuromodulation Systems). Dr. McCall has served as a scientific adviser for Multiple Energy Technologies, and he has received research support from the American Foundation for the Prevention of Suicide and NIMH, royalties from Wolters Kluwer, and honoraria from Anthem, Inc., CME Outfitters, and Global Medical Education. Dr. Petrides has received research support from Amgen, AstraZeneca, Corcept, Eli Lilly, Proteus, St. Jude Medical, and Sunovion, and he has served on an advisory panel for Corcept. Dr. Young is a consultant to NIH and has received research support from NIMH. Dr. McClintock has received research support from NIMH and a teaching honorarium from TMS Education Solutions; he is a member of the editorial board of the Journal of ECT. Dr. Lisanby has received grant support from the Brain and Behavior Research Foundation, the Stanley Medical Research Foundation, Neosync, Nexstim, NIH, and Brainsway; this work was performed while Dr. Lisanby was at Duke University School of Medicine. The other authors report no financial relationships with commercial interests.
Footnotes
ClinicalTrials.gov identifier: NCT01028508.
REFERENCES
- 1.Kellner CH, Husain MM, Knapp RG, et al. Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am J Psychiatry 2016; 173:1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisanby SH, Sampson S, Husain MM, et al. Toward individualized post-electroconvulsive therapy care: piloting the Symptom-Titrated, Algorithm-Based Longitudinal ECT (STABLE) intervention. J ECT 2008; 24:179–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222 [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198 [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association, Task Force on Electroconvulsive Therapy: The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging, 2nd ed. Washington, DC, American Psychiatric Association, 2001 [Google Scholar]
- 7.Spitzer RL, Williams JB, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629 [DOI] [PubMed] [Google Scholar]
- 8.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59 (suppl 20):22–33 [PubMed] [Google Scholar]
- 9.Beck AT, Kovacs M, Weissman A: Assessment of suicidal intention: the scale for suicide ideation. J Consult Clin Psychol 1979; 47: 343–352 [DOI] [PubMed] [Google Scholar]
- 10.Beck A, Kovacs M, Weissman A: Beck Scale for Suicide Ideation (BSS), in Handbook of Psychiatric Measures. Washington, DC, American Psychiatric Association, 2000, pp 264–266 [Google Scholar]
- 11.Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812 [Google Scholar]
- 12.Mundt JC, Kobak KA, Taylor LV, et al. Administration of the Hamilton Depression Rating Scale using interactive voice response technology. MD Comput 1998; 15:31–39 [PubMed] [Google Scholar]
- 13.Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222 [Google Scholar]
- 14.Mueller M, Zhao W, Kellner CH, et al. Enhanced protocol compliance in a complex clinical trial using web-based response adaptive treatment instructions [abstract]. 34th Annual Meeting of the Society for Clinical Trials, Boston, May 2013 [Google Scholar]
- 15.Fitzmaurice GM, Laird NM, Ware JH: Applied Longitudinal Analysis, 2nd ed. Hoboken, NJ, John Wiley & Sons, 2011 [Google Scholar]
- 16.O’Kelly M, Ratitch B: Clinical Trials With Missing Data. Chichester, UK, John Wiley & Sons, 2014 [Google Scholar]
- 17.Shapira B, Calev A, Lerer B: Optimal use of electroconvulsive therapy: choosing a treatment schedule. Psychiatr Clin North Am 1991; 14:935–946 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


