Abstract
Schizophrenia is a disorder with a pronounced developmental component. Accordingly, there is a growing interest in characterizing developmental changes in the period leading up to disease onset, in an effort to develop effective preventative interventions. One of the ongoing neurodevelopmental changes known to occur in the late adolescent period that often overlaps with the prodromal phase and time of onset is white matter development and myelination. In this critical review, a disruption in the normal trajectory of white matter development could potentially play an important role in the onset of psychosis. We seek to summarize the existing state of research on white matter development in prodromal subjects, with a particular focus on diffusion tensor imaging (DTI) measures. First, we describe the physiological basis of developmental white matter changes and myelination. Next, we characterize the pattern of white matter changes associated with typical development across adolescence as measured with DTI. Then, we discuss white matter changes observed in adult patients with schizophrenia and in individuals seen in genetic and clinical high risk states. Finally, we discuss the implications of these findings for future research directions and for potential therapeutic interventions.
Keywords: Schizophrenia, white matter, high-risk, development, adolescence, myelination, diffusion tensor imaging, neuroimaging
INTRODUCTION
There is substantial evidence that schizophrenia has a strong developmental component, in part because onset consistently occurs in late adolescence. The pattern of onset is insidious, and changes are distributed across the lifespan. The interaction of schizophrenia risk genes and environmental factors with normal developmental processes results in a pattern where there are certain periods of special vulnerability across development. The prenatal period is one such window of risk, when maternal stress and infection can have a very deleterious impact on the developing fetal brain, and another such period is in late adolescence, proximal to the period when disease onset often occurs. During adolescence, neurodevelopmental changes in both grey and white matter (WM) are ongoing even in healthy individuals. That the development of WM occurs during the period associated with disease onset is of special interest, as schizophrenia has been hypothesized to be a disorder of disrupted or reduced connectivity [1–4]. Moreover, structural disruptions in WM have been implicated in disease pathogenesis in schizophrenia [5]. Evidence includes neuroimaging studies that report WM volume reductions and structural abnormalities [6–8] as well as myelin-related gene abnormalities [9, 10]. It therefore seems plausible that a disruption in the normal trajectory of WM development could potentially play an important role in the onset of psychosis.
Interest in the changes that occur during this adolescent period, and during the time leading up to onset, has lead to the rapid growth of an area of research focused on individuals putatively in the early ‘prodromal’ period of schizophrenia. Generally, this type of research consists of identifying individuals who are at a heightened risk for developing psychosis, either due to genetic factors, such as having a first relative with a psychotic disorder, or due to clinical changes consistent with the prodromal phases of the illness, such as sub-threshold psychotic symptoms accompanied by a decline in functioning [11]. The strength of performing investigations during this period is that if individuals convert to a psychotic disorder during the course of follow-up there is a unique opportunity to gain traction on what factors lead to their conversion. In addition, these individuals are free of the confounding factors that can come with long term pharmacological treatment and illness chronicity [12].
In recent years there has been a notable increase in the number of studies assessing neural changes in high-risk adolescents. There is a particular growth in the number of studies using neuroimaging techniques to assess grey and WM integrity in these individuals. In this critical review, we seek to summarize the existing state of research on WM integrity in prodromal or high risk subjects, with a particular focus on diffusion tensor imaging (DTI) measures. First, we will describe the structural basis of WM change during adolescence, then characterize the pattern of WM changes associated with typical development across adolescence, and finally to discuss changes observed in adults with schizophrenia and those seen in high risk states. Then, we will address the implications of these findings for intervention and treatment.
NEURAL BASIS OF WHITE MATTER DEVELOPMENT
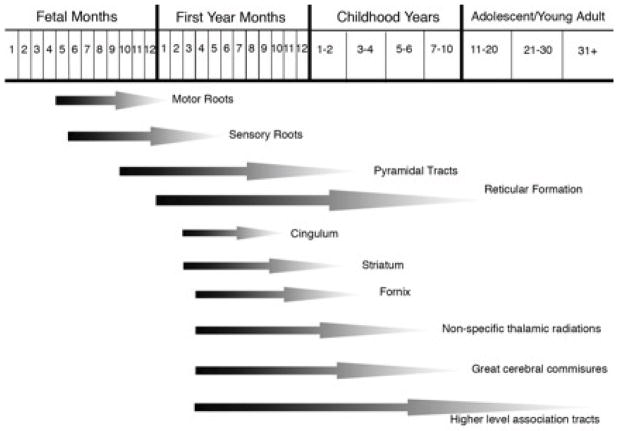
It is clear that while myelination may occur to some extent throughout the lifespan, there are phasic periods in which the process is greatly accelerated. For instance, in the rat, myelin has been found to increase by 500% between 15–30 postnatal days, a period that generally corresponds to late childhood or early adolescence, while during the same period brain volume increases by only 20%. [13]. Some regions, particularly those associated with motor and sensory processes, are myelinated beginning even in utero. The first phase of myelination begins around 10 weeks in the spinal cord; by the time of birth the pons and cerebellar peduncles are myelinated, followed by the internal capsule, splenium of the corpus callosum, anterior limb of the internal capsule, and genu, with parts of the frontal, parietal and temporal lobes myelinating by about a year after birth [14]. In fact, in human fetuses the timing of CNS myelination of different pathways is so specific that age can be determined just based on observing which pathways have been myelinated [15]. However, while the most rapid periods are indeed during these earlier stages, substantial myelination continues to occur up to the second decade of human life, with higher level association areas being the final regions to be fully myelinated [15, 16]. For instance, the hippocampus and frontal lobes undergo the majority of their myelination during adolescence and do not finish until early adulthood [17, 18] (See Fig. 1). These structural changes are also occurring, and potentially lending to, refinement in neuronal signalling where more efficient focal cortical activity is taking place in these regions [19]. In fact, normally during early adolescence, while the grey matter (GM) volume is decreasing, likely due to increased synaptic pruning and decreased neuropil, the WM volume actually undergoes a simultaneous increase [20]. This increase may be related to either increase in the thickness of the myelin sheath, or to increases in axonal diameter (or both).
Fig. (1).
Graphical rendition of timing of myelination in different tracts. Based on data and figure from Yakovlev and Lecours, 1966 [150].
When considering the possibility of a disruption in the trajectory of WM development as a contributing factor to the onset of psychosis, it is useful to consider the neural underpinnings of the observed changes. This allows us to think about what our histological and imaging measures are truly indexing, and consider what might be appropriate targets for treatment. Myelin in the central nervous system is comprised of the lipid membranes of oligodendrocytes; the cytoplasmic membrane folds out and extends away from the cell body to form long processes that tightly wrap around local axons and provide insulation [15]. This wrapped double layer of lipid membrane is what gives myelin its well-known fatty composition. In fact, myelin consists of 70% lipids, and membrane lipids comprise 50–60% of solid matter in the brain [21]. Membrane lipids in all mammalian species include glycolipids, phosopholipids and cholesterol, with phosopholipids and cholesterol accounting for the largest proportion of membrane lipids [15, 22]. Polyunsatureated fatty acids (often known as PUFAs) are structural components of these membrane phospholipids. EPUFAs are the essential PUFAs, which cannot by synthesized and must be provided through the diet. Two of these are arachidonic acid (AA) and docosahexaenoic acid (DHA), and together AA and DHA comprise more than 90% of EPUFAs [23]. Since they must be acquired dietarily, the amount of EPUFA intake determines the rate of phosopholipid synthesis, which in turn influences the quantity and quality of membrane phospholipids found in both the peripheral and central nervous systems [23]. Presumably because of this link, dietary lipids have been associated with biogenesis of myelin [24]. Accordingly, if these lipids are unavailable during myelin synthesis, disruptions in the form of amyelination or dysmyelination have been found to occur [25]. The findings that fatty acid intake may be a limiting factor for myelination has intriguing implications for disorders with disrupted WM development. However, the relevance of these data to schizophrenia must be interpreted with caution, as it appears that the degree of the effects are related to stage of myelination [26–29], and while there translational studies showing positive effects of EPUFA supplementation in animal models, the majority of these have been carried out in utero or in very early post-natal stages [13, 30], not in adults or adolescents.
It is important to consider what impact a deficit in myelination might have on other aspects of neuronal integrity. Interestingly, there is evidence for a great deal of interaction between the neurons being myelinated and the oligodendrocytes that myelinate them. First, electrical activity of cells can influence the amount of oligodendrocyte precursor cells that accumulate nearby [15, 31]. Secondly, features of the axon may influence how thick the myelin sheath is [32]. However, the interaction also goes in the other direction. Signals from a myelinating glial cell can influence the degree of axon growth and girth [32], and play a role in axonal survival and maintenance [15]. In addition, in axons that have been myelinated, oligodendrocytes may inhibit neurite growth, something that would have a bigger effect on the late myelinating tracts that are of particular interest during adolescence. It is possible that the reason for this in healthy individuals is so that oligodendrocytes can serve as a “guard rail” to keep those later myelinating tracts inside appropriate boundaries [34]. Thus, it is possible to imagine that in a disorder in which myelination was delayed, or the trajectory was abnormal, there could be profound implications of these interactions on not just the WM, but on the GM as well. This interactive relationship is particularly important for a disorder such as schizophrenia, which may be associated with disruptions in both grey and WM development-these two phenomena are usually discussed as independent entities, but it is possible that they are more highly intertwined (for an excellent review of oligodendrocyte biology and development, see Baumann, 2001).
NEUROIMAGING MEASURES OF WHITE MATTER DEVELOPMENT
Early attempts to quantify WM changes were based on anatomical dissection and standard structural MRI. Historically, neuro-imaging techniques have not had the fidelity to allow detailed analysis of WM microstructure in vivo, and so investigations have been predominately post-mortem. These studies carry the benefit of being able to identify specific changes at the cellular level, and thus provide invaluable insight into the mechanisms of development. However, there are a variety of methodological issues associated with post-mortem studies that can seriously complicate interpretation of the data collected, these issues include the influence of postmortem interval and cause of death on the tissue, as well as the necessity of relying on registry data or medical records for patient diagnoses [35]. As an alternative to histological and dissection studies, there has been a shift towards using neuroimaging techniques for the investigation of WM in living subjects as these technologies have become more widely available. However, while traditional MRI does allow a general overview of the WM structure, it is largely limited to measures such as gross WM volume, or the presence of WM hyperintensities. Traditional MRI cannot assess whether cells are in place but improperly connected, whether axonal tracts are disorganized, or whether cells are functioning normally; in each case volumetric WM measures may remain unchanged while functional connectivity may be fundamentally altered.
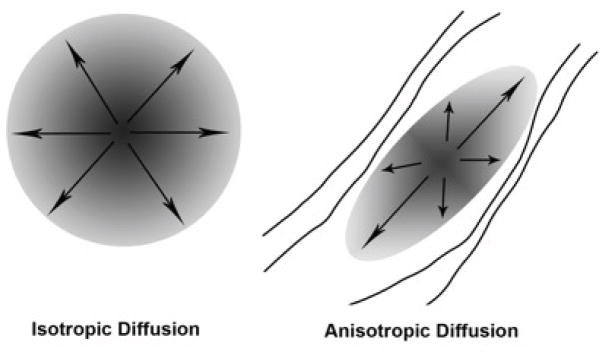
Diffusion tensor imaging (DTI) is a relatively newer technique that can be performed on a standard MRI scanner, and that allows for a more detailed analysis of the integrity of the WM tracts. The data collected during DTI are based on the idea that it is possible to measure the pattern of Brownian motion (the natural motion driven by thermal energy) of water molecules and use measures about the ways in which diffusion is restricted to glean information about the environment in which the water is moving [36]. If unobstructed, as in cerebral spinal fluid, that motion has an equal likelihood of going in any direction, a pattern known as isotropic diffusion. Water molecules in the brain, however, are moving in an environment full of axons, cell membranes, fibers, and other tissue components that limit their motion. In WM tracts, the motion of the molecules is restricted by the myelinated axons and tends to move more easily in the direction along the axon than perpendicular to it, creating motion in an ellipsoid shape rather than a spherical one (a pattern known as anisotropic diffusion; see Fig. 2). The shape of this ellipsoid can give information such as the degree of myelination, the number of axons, the average fiber diameter, fiber packing/axon density, and the similarity in the direction of the fibers within the tract [37, 38]. The most widely reported index of diffusivity is fractional anisotropy (FA) which characterizes the eccentricity of the ellipsoid, and is commonly interpreted as a measure of WM integrity. The mean diffusivity (MD) index measures the overall diffusion area, it may index changes in intra and extracellular space, and in GM may index decreases in neuropil. Radial and axial diffusion measures of the length of the longest and shortest axes of the ellipse and are thought to index tract organization or axonal integrity and myelination, respectively [39–41]. The direction of the ellipsoid can give information about the orientation of fibers in that region. Using fiber orientation data it is possible to use a technique known as tractography to trace fiber tracts through adjacent voxels with a common principle direction [42,43]. This procedure allows the tracing of anatomical connections between regions of interest, or the determination of whether the tracts themselves are appropriately placed and connected. With these capabilities, coupled with the ability to perform the technique in a standard MRI scanner over relatively short scan periods, DTI has become a very practical way to investigate structural integrity of fiber tracts in living subjects.
Fig. (2).
The basis of the diffusion tensor imaging (DTI) signal is the pattern of diffusion. Isotropic diffusion would occur when the water molecules are unobstructed, for instance in the cerebral spinal fluid filled ventricles. Anisotropic diffusion, where the ellipse is narrowed and the eccentricity of the ellipse is increased, would occur in white matter tracts where diffusion is obstructed in a particular direction.
WHITE MATTER DEVELOPMENT IN TYPICAL ADOLESCENCE AS REVEALED BY DTI
Advances in the rapidly developing field of diffusion weighted MR imaging have resulted in a series of informative studies which have enabled researchers to track WM development across the lifespan (see [44–46]). To date, there are over 30 published studies of WM development using DTI measures which have included adolescents as a part of their examined age cohort (for a recent review see [47]). However, only a handful of studies have specifically examined changes over adolescence rather than across the lifespan. Age-related changes in WM volume and diffusivity measure rarely occur in a linear fashion, nor do they mirror contemporaneous GM volume changes. Findings from the large NIH longitudinal study of healthy brain development [48] indicate that volumetric WM development across the lifespan is best described by a combination of linear and cubic models depending on which regions are the focus of interest. More recent studies of corresponding developmental changes in DTI diffusivity measures (FA, MD radial and axial) are consistent with models of WM change [45], showing corticospinal, external capsule, corona radiata, and internal capsule to be early maturing tracts, with regions associated with higher level functions, such as corpus callosum, cingulum bundle, sagittal stratum, superior longitudinal fasiculus (SLF) and the fronto-occipital fasciculi to be later maturing [49]. Taken together the existing studies suggest a pattern of increasing FA with corresponding decreases in MD and radial diffusivity in frontal and temporal regions and corpus callosum across early development and into adolescence. Such diffusivity changes are consistent with previously observed concurrent changes in WM volume and density [49]. The neurobiological basis for the observed changes in diffusivity has been recently reviewed by Paus [50]. On the basis of existing neuropathological evidence he concludes that developmental changes in both axon diameter and myelination are driving the changes in MRI diffusion signal. Interestingly, the most consistent finding across studies is gender effects, different developmental trajectories for genders, males increasing WM volume but girls demonstrate increased maturity of white mater microstructure as measured by DTI [51–53].
Given the growing evidence that there are ongoing maturational changes in areas associated with higher level function, even up into young adulthood, there has been growing interest in the relationship between the acquisition of cognitive abilities and newly acquired skills and diffusivity measures in healthy adults [54]. With respect to developmental trajectory, there is some evidence that, independent of age and gender, maturation of WM in the left hemisphere is directly associated with verbal cognitive abilities [55]. While only a handful of studies have specifically examined the relationship between cognitive function and WM development as measured by DTI (reviewed [47]) there is growing evidence that maturation of these two domains are robustly related. Further, Olson et al [56] found that increased impulsive behavior in children and adolescents was associated with relatively decreased FA in bilateral frontal and temporal tracts suggesting delayed or interrupted maturation in this regions. These findings support the notion that honing of the white matter that occurs in adolescence has measurable functional relevance, and further highlight the importance of understanding not only the cognitive changes that can be expected when this process is normal, but also when the trajectory is disrupted.
There are a few key issues that make integration of existing findings problematic. Often, there are as many reported discrepancies between studies as common findings, most likely due to methodological differences. Acquisition parameters (for instance, number of diffusion directions, slice thickness) vary widely by site, and often age ranges are non-overlapping between studies, with some focusing on younger and some on older subjects. In addition, the analytical approach adopted can have a profound effect on how easily data can be compared between studies. In particular, while both region of interest (ROI) based approaches and whole brain voxel-wise analyses have their own costs and benefits, the results can sometimes be hard to reconcile. In addition, a voxel-based morphometry (VBM) approach that takes into account all tissue types and is very vulnerable to registration issues can be difficult to compare with output from the recently developed Tract Based Spatial Statistics (TBSS; FMRIB Software Library) program, which focuses the statistical comparisons on a WM “skeleton” that represents areas in which all subjects have data. Significantly, we are aware of only one longitudinal investigation of WM microstructure change in adolescence [57]. Overall, the findings of this study support prior cross-sectional investigations showing relative increases in diffusivity in fronto-temporal tracts as well as thalamic radiations and portions of the internal capsule. It is critical to continue to explore these changes longitudinally, to gain a deeper understanding of both within subject and between subject changes, and to provide a comprehensive backdrop against which to interpret disorders with alterations in the trajectory of WM development.
WHITE MATTER ALTERATIONS IN PATIENTS WITH SCHIZOPHRENIA AND THOSE AT RISK
Disconnectivity in Schizophrenia
Observations that schizophrenia has its onset in late adolescence during the period when higher level association areas are still myelinating have prompted the notion that schizophrenia is a disconnection syndrome adversely affecting the structure of neurocognitive networks in the brain [1, 4, 57]. Here, we propose that this disconnection syndrome may arise from the disruption of the normal neurodevelopmental trajectory during adolescence.
The disconnection hypothesis has received copious amounts of empirical support from functional brain imaging studies. These findings point to potential mechanisms at the synaptic or molecular level, however functional studies show a lack of consensus on which brain networks are the site of the primary abnormality. Some investigators propose that the core abnormality is a disruption of frontal-temporal integration [1] while others propose a disruption of cortico-cerebellar-thalamo-cortical circuitry (CCTCC) integration [59]. Another model suggests that fronto-temporo-limbic interactions with the ventral striatum are impaired [60, 61], which could partially be explained by the dopamine hypothesis of schizophrenia [62]. Aberrant parietal activity has been less emphasized in schizophrenia research but there is emerging evidence that fronto-parietal integration may be abnormal [63, 64]. Yoon et al. [65] found a pattern of connectivity in controls, between dorsal lateral prefrontal cortex (DLPFC) and right inferior parietal lobule during context processing when performing a continuous performance test (CPT), the AX-CPT, however schizophrenia patients did not show any regions with enhanced functional connectivity to the DLPFC.
With the growing interest in the prodromal phase and timing of disease onset, functional connectivity investigations in clinical high-risk (CHR) youth are of special interest. Recently, Benetti et al. [66] explored effective connectivity during a working memory task in first-episode, at-risk and healthy control subjects. They set out to assess bi-directional connectivity between the PFC and hippocampus, particularly between the IFG which has prominent projects to the posterior subdivision of the hippocampus. They examined correct recognitions to previously presented stimuli and found reduced effective connectivity from the right posterior hippocampus to the right inferior frontal gyrus in the first-episode and at-risk subjects, compared to controls. Findings of decreased functional connectivity in subjects at high-risk is consistent with reports of abnormal PFC, posterior, and also sub-cortical brain regions functional connectivity [67]. Recent reports echoing the functional findings in CHR have discovered volumetric reductions in PFC and medial temporal cortices [68–70]. Disruptions in fronto-hippocampal connectivity are rapidly becoming a compelling vulnerability marker in individuals who are at-risk.
DTI and Schizophrenia
Post-mortem evidence has revealed disturbed myelin pathology in patients with schizophrenia. Changes include alterations in the distribution of the interstitial cells of the WM [71–73] as well as significant reductions in the number of oligodendroglial cells and ultrastructural alterations of myelin sheaths, mainly in the PFC and caudate nucleus, which may underlie other structural abnormalities in schizophrenia [74]. DTI evidence supporting disturbed connectivity in schizophrenia has been evidenced by decreased FA in widespread brain regions. These include prefrontal, temporo-parietal, and parieto-occipital regions [75], with meta-analytic reviews showing particular deficits in left frontal WM [76] and along the cingulum, arcuate, and uncinate fasciculus [77]. Sophisticated network analyses have revealed impaired connectivity between medial frontal, parietal/occipital and left temporal nodes, supporting the idea of widespread disconnectivity across key regions [78]. Interestingly, in a study examining FA, axial, and radial diffusion, Seal and colleagues [79] found that FA changes in the external capsule could be attributed to increases in radial but not axial diffusivity, consistent with the idea that the observed changes in schizophrenia are due to decreased or abnormal myelination rather than to axonal damage or profound tract disorganization. This confirmation of a specific myelin disruption highlights the importance of understanding the trajectory of myelination in young and at risk subjects.
The superior longitudinal fasciculus (SLF) is one fiber tract of particular interest in schizophrenia, serving as the primary connection between the frontal and parietal lobes and its role in supporting working memory functions [80]. FA reductions along the SLF have been found in schizophrenia patients [81]. In addition, a recent network analysis indicated that a fronto-parietal-occipital network was specifically impaired in schizophrenia patients [78]. FA reductions have been found in first-episode, young adult patients and are associated with impaired verbal working memory [82] and executive functioning [83]. Similar WM alterations along the SLF and uncinate fasciculus have also been found in a separate first episode psychosis sample and were more prominent in patients with a poorer functional outcome [84].
The cingulum fiber bundle is another structural region area of interest in schizophrenia because of functional deficits found in the connected regions [85, 86]. The cingulum connects the PFC, cingulate gyrus, medial temporal lobe, including the hippocampus and parahippocampus, and precuneus [87]. There have been a number of findings of reduced FA in schizophrenia patients in this tract [88–91], specifically falling in PFC [92], cingulate gyrus [90, 93], and hippocampus in children and adolescents with schizophrenia [94]. Structural abnormalities in the hippocampus are believed to be a result of aberrant neurodevelopment, effecting synaptic organization and neuronal connectivity, rather than neurodegenerative tissue damage, since it is observed in first-episode patients (Harrison, 2004). A combined fMRI and DTI study of working memory revealed hypoactivity in the PFC, superior parietal lobe, and occipital lobe along with FA reductions in the right parahippocampal gyrus and right frontal lobe of schizophrenia patients [95]. At this stage the cingulum is serving as an intriguing area of reduced microstructural integrity which could serve as an early indicator of the risk for the illness which persists throughout the various stages of the disease progression.
White Matter Neurodevelopment and Schizophrenia
During normal cortical development the brain undergoes progressive neuronal pruning moving from posterior (in the parietal lobe), to anterior regions (in the prefrontal and temporal cortices) [96, 97]. In early-onset schizophrenia GM tissue loss in these regions follows the same pattern, beginning in the parietal cortex, but is accelerated [98] particularly in the superior medial frontal and cingulate gyrus [99]. Although there is evidence to suggest that accelerated GM loss is apparent in early and adult onset-schizophrenia few studies have explored if WM abnormalities found in the disease-state are related to the age of onset. In a cross-sectional DTI study comparing FA in adolescent-onset and adult-onset schizophrenia differential effects of age were found to interact with the pattern of FA reductions [100]. Adolescent-onset patients showed reduced FA within parietal WM, while adult-onset revealed reductions in frontal and temporal lobes compared to their age-matched controls. In the direct comparison of age of onset between the adolescent and adult patient groups there were two bilateral regions in the medial frontal WM showing group differences. In these frontal regions higher FA was found in the younger adolescent-onset patients than younger adult-onset patients and younger controls; however, the older adolescent-onset patients failed to show normal WM increases with age and instead had drastically reduced FA than the older adult-onset group and older controls. This study provides strong evidence to account for neurodevelopment stage and age when exploring WM changes related to the disease.
Longitudinal changes in WM in adolescent-onset schizophrenia patients has been explored [101] Baseline adolescent patient data revealed reduced FA along the pyramidal fiber tract, which facilitates motor control, and in the arcuate fasciculus, which in an integral tract for connecting brain regions involved in language production and comprehension. However, after longitudinal follow-up, roughly 2.5 years, the differences in WM disappeared and patients had similar increases in WM to their adolescent control subjects. The initial reductions in FA along the pyramidal fiber tract are important to understanding the neuromotor abnormalities which are found in schizophrenia patients in those at-risk, which have been previously found. The tract is associated with the corticospinal tract and the sensori-motor regions falling along it are still developing during adolescence [102, 103]. FA reductions in this region could reflect a marker of motor skill delays related to WM maturational delays which occur in adolescent-onset schizophrenia, due to the coinciding refinement of motor capacities which occur during childhood and adolescence [104].
DTI in High-Risk Schizophrenia Studies
Genetic High-Risk
Investigations in individuals who are at risk for, but are not yet diagnosed with, schizophrenia or psychosis represent a powerful approach to understanding the factors that contribute to disease onset, and can also help identify points of potential intervention. Two common ways of identifying at-risk individuals are through familial loading for schizophrenia (siblings, children, or twins of probands) and through observation of clinical features consistent with the prodromal period of schizophrenia. Studies exploring WM FA in individuals at-risk have predominately used a genetic high-risk approach. This strategy is powerful in that all subjects assessed do share risk genes for schizophrenia, whether or not they ultimately convert to the disorder themselves, and this allows us to observe the effects of these genes in the absence of confounds such as treatment or frank psychosis. Genetic high-risk studies have yielded a rich literature describing cognitive and anatomical endo-phenotypes for schizophrenia. For instance, unaffected twins of schizophrenia patients show a similar magnitude of reduced FA in the left PFC and the relative to healthy controls [105]. However, the schizophrenia patients exhibited reduced WM FA in the left ACC, which was not found in either the relative or control groups. Therefore, the left PFC and the hippocampus may be related to higher genetically-mediated risk for developing schizophrenia while WM disruptions in the left ACC may be specific to the presence of the disease. Muñoz Maniega et al. [106] examined FA in unaffected relatives and schizophrenia probands and healthy controls. Lower FA was found in both patients and unaffected relatives in the anterior limb of the internal capsule. This revealed lower FA in fronto-temporal WM which may be a possible genetically-mediated indicator of a higher vulnerability to develop schizophrenia. DeLisi and colleagues [107] utilized diffusion weighted imaging to investigate the appearance of cortical atrophy in individuals at a high genetic-risk (aged 12–30) along with schizophrenia patients (aged 20–55 years old) and controls. They found an increase in the amount of cerebral spinal fluid occupying the interstitial brain space in the region of the left parahippocampal gyrus in both patients and risk subjects. This increase indicates that tissue atrophy may be occurring in this region. Additional FA reductions have also been observed along the SLF at baseline examination in individuals at a high genetic-risk [108]. Another study of young adults at high genetic-risk found focal FA reductions within the parietal lobe, angular gyrus and right precuneus, in the at-risk group compared to healthy controls [109]. These findings suggest that the FA reduction in the parietal region of the SLF may be apparent in the risk stage of the disease. Additionally, these reductions have been correlated with verbal working memory performance in first-episode, young adult patients [82], suggesting a disruption along this fiber track has implications on a core cognitive dysfunction which is observed through all stages of the disease.
Another approach used in identifying risk biomarkers for psychosis is to examine genetic variations which may lead to the disruptions in WM in schizophrenia. One example of a gene of interest in this regard is the schizophrenia risk gene neuregulin-1 (NRG1), which is involved in neuronal migration, axon guidance and myelination [110]. A haplotype in the 5′ end region of the gene, which is a genetic variation found to double the risk for schizophrenia in an Icelandic population [111], with reduced medial frontal FA significantly associated with the NRG1 gene variation. This finding suggests that a single nucleotide polymorphism in a specific region along the NRG1 gene may contribute to the risk for schizophrenia via its impact on myelination in frontal lobe WM. McIntosh et al. [112] also explored NRG1 and WM, but in unaffected subjects who were genotyped at the NRG1 single nucleotide polymorphism locus. Subjects with the risk-associated genotype had reduced WM volume and FA in the anterior limb of the internal capsule. They therefore provide the first imaging evidence that genetic variation in NRG1 is associated with reduced WM density and integrity in human subjects. In addition to NRG1, its receptor, ErbB4 has also been investigated. One such study focused on a SNP (rs4673628), found that variation at this site was related to white matter reductions in bilateral regions of the anterior limb of the internal capsule [113]. Another candidate gene (ZNF804A) has become a point of interest, as the most commonly implicated SNP (rs1344706) potentially serves as a binding site for myelin transcription factor [114], although existing DTI studies have not revealed significant differences.
Clinical High-Risk
To complement genetic-risk studies, a second approach to identifying high-risk individuals is through use of clinical characteristics. Typically, these samples consist of individuals in the age-range associated with onset who are experiencing a decline in functioning accompanied by below-threshold psychotic symptoms. The strength of these studies lies in the possibility of assessing the same individual before, during, and after onset, which offers an unprecedented opportunity to fully characterize the factors contributing to or associated with transition to psychosis. However, such studies can be methodologically challenging, as only a portion of recruited individuals will develop full-blown schizophrenia. To date, only a few studies have examined WM integrity in CHR individuals. Karlsgodt and colleagues [108] were the first to explore WM alterations in a CHR sample. They found lower baseline WM FA in CHR compared to healthy controls, and furthermore this deficit was predictive of a steep decline in psychosocial functioning in the CHR group. Additionally the CHR group failed to show the normal age-related increase in medial temporal lobe WM. In the first DTI study to examine conversion to psychosis they compared baseline WM FA within regions of interest along a priori WM tracts in CHR subjects who converted to psychosis and those who did not [115]. In this study they did not find any baseline differences between the psychosis groups and the healthy subjects or between the converters and non-converters. Bloemen and colleagues [116] carried out a prospective study of risk for conversion to psychosis in which they investigated whole-brain FA maps for high-risk individuals who later converted to psychosis compared to those who did not. They found reduced FA in the converters compared to the controls in the left anterior thalamic radiation and along the inferior fronto-occipital fasciculus. They also found reduced FA along the SLF and inferior longitudinal fasciculus in the superior temporal gyrus and inferior fronto-occipital fasciculus in high-risk individuals who converted compared to those who did not. In a study of children aged 11–13 who were experiencing sub-threshold psychotic-like symptoms whole-brain derived FA differences were explored between this symptomatic high-risk group and healthy control children [117]. They found decreased FA in the high-risk group along the inferior fronto-occipital fasciculus within the visual cortex, along the cingulum within the parahippocampal gyrus and along the inferior longitudinal fasciculus in the superior temporal gyrus. This study was the first to identify WM deficits in a non-treatment seeking sample of at-risk children and gives rise to the notion that these subtle microstructural differences in WM integrity may be apparent not only in the risk state but are identifiable at a younger age than previously suspected. Reduced WM volume within the superior temporal lobe has also been found in high-risk subjects compared to healthy controls [118, 119]. Increased corticial thinning over time within the superior temporal gyrus has also been found to be predictive of CHR who convert to psychosis compared to those who did not [120]. A multimodal imaging study has uncovered a direct association between the WM volume underlying the right supramarginal gyrus extending into the right arcuate fasciculus and the electrophysiological alterations (as measured with P300 amplitude) of CHR subjects [121] Such correlation was pathological as it was observed in the CHR but not in the control group. Previous MRI-EEG studies showed that superior temporal gyrus volume is associated with smaller P300 in chronic [122] and first episode [123] schizophrenia. These findings are in line with recent theories suggesting that electrophysiolgical alterations in schizophrenia are associated with early temporo-parietal maturation abnormalities followed by further functional impairments later in life [124].
One of the key goals of high risk studies is to assess individuals across the transition to psychosis. However, this is technically difficult due to time limitations of follow-up, impact of treatments offered during the course of the study, and subject retention. However, in the first DTI study to examine conversion to psychosis baseline WM FA within regions of interest along a priori WM tracts was compared between in CHR subjects who converted to psychosis and those who did not [125, 126]. In this study they did not find any baseline differences between the high risk group as a whole and the healthy subjects or between the converters and non-converters. However, at follow-up, when using an uncorrected analysis, they found that the CHR converters and non-converters had increased FA in the right anterior cingulate. While this study cannot be conclusive, due to a very small sample size it is an important first step to further studies focused on the important issue of conversion.
IMPLICATIONS AND FUTURE DIRECTIONS
As we have highlighted above an important component of investigations focused on risk and onset are longitudinal studies. Understanding which aspects of WM abnormalities are present before the onset of the illness and are specifically disrupted in those who progress to the disease state will help researchers to converge on WM tracts of interest in targeting for disease prevention. In pursuit of this goal, a number of multi-site studies across the globe are ongoing, including the North American Prodrome Longitudinal Study (NAPLS), which is collecting structural data, including DTI and fMRI in a sample of ~720 adolescent and young adult CHR patients. In addition to understanding the process of onset, another important goal of studies in CHR subjects is to develop algorithms that can predict which subjects will progress to disease onset, or will need additional intervention. We have shown evidence that baseline levels of WM integrity may be predictive of both later functional outcome [108] or even conversion to psychosis [116]. Further work, and larger longitudinal samples, are necessary to determine whether the WM deficits can be specifically defined enough to be used (either alone or as part of a more complex combination of measures) as a disease or risk marker.
The idea that dysfunctional WM development may be a central component of disease onset begs the question of whether it would be possible to intervene and repair, halt, or blunt, the disrupted process. The hope would be that for patients with a WM disruption, development of an intervention that can help normalize this pattern may play a critical role in rescuing their function as adults. One area of study that has grown out of the findings is the absence of sufficient fatty acid intake results in deficits in myelination. There is an interest in whether in the case of injury or pathology, the long chain EPUFAs of the omega-3 group (DHA) might have potential as a therapeutic agents. For instance, omega-3 EPUFAs may offer neuroprotection after ischemic and traumatic spinal cord injury, both after administration as an acute post-injury injection and as the result of a longer term enhanced diet [127–129]. In part because of such findings, this treatment has been repeatedly been proposed across a wide range of psychiatric disorders [127, 130–133]. However, clinical trials in which EPUFAs have been administered have had mixed results [134]. A recent meta-analysis has comprehensively assessed the antipsychotic efficacy of EPUFA in about 160 schizophrenic subjects randomly allocated to standard antipsychotic treatment with or without EPA augmentation [135]. Meta-analysis of RCTs on symptomatic outcome revealed no beneficial effect of EPA-augmentation in established schizophrenia. However, no conclusion can be made for medium to long-term effects of EPA in schizophrenia, in particular on relapse prevention in the early course of psychotic disorders.
Although there is only limited evidence supporting that EPA augmentation of antipsychotic medication in established psychosis improves symptomatic outcome, they may exert positive effects as a preventive agents, in particular during the pre-psychotic phases. A recent study has confirmed a mixture of EPA and DHA significantly reduces transition rates in subjects at ultra high risk for psychosis [136] pointing to a potential neuroprotective effect of omega-3 fatty acids supplementation in at risk mental states. There is growing evidence suggesting that the emergence of psychosis is associated with subtle structural (for a review see [137]), functional (for reviews see [138, 139]) and neurochemical (mostly involving dopamine and glutamate, for reviews see [140, 141]) alterations which may ultimately lead to the onset of frank psychosis. The potential underlying mechanism of the therapeutic action of omega-3 fatty acids during the pre-psychotic phases may be neuroprotective, e.g. via modulation of the antioxidative intracellular defense [142], antiapoptotic actions [143] or be the result of a direct interaction between EPA and glutamatergic neurotransmission (for a review on omega-3 fatty acid interventions in early psychosis see [144]). In particular, glutamate alterations have been shown to predate the onset of psychosis and to be directly related to the GM abnormalities [145], neurofunctional alterations [146] and dopaminergic abnormalites [147] observed in subjects at risk for psychosis. It is thus possible to speculate the EPA neuroprotective effects during this phase may be associated with a modulation of glutamatergic cycle between the neuronal and glial compartment [148]. In line with such a hypothesis, a recent magnetic resonance spectroscopy study in first episode subjects has confirmed that EPA augmentation modulates glutathione and the glutamine/glutamate cycle in early psychosis, with some of the metabolic brain changes being correlated with negative symptom improvement [142]. The EPA neuroprotective interventions may therefore be particularly effective in indicated or secondary prevention of psychotic disorders, but not necessarily in treatment of established schizophrenia where the underlying neurobiological changes may have progressed too far and we are dealing with an end stage of an illness. However, while the idea of a fatty acid based intervention for developmental neuropsychiatric disorders is highly intriguing, basic research is still needed so that we can understand whether or not we can expect supplementation with fatty acids to have a significant effect on typical outcome measures. Importantly, it is necessary to determine if there is an optimal age range in which to target these treatments. If this intervention does impact myelination in younger subjects, then it may be a reasonable first line of intervention in disorders association with dysregulated myelination. If it fails to show an effect in older subjects, this may mean that for those individuals, further treatments need to be developed.
In general, it appears that as the symptoms of schizophrenia emerge in late adolescence or early adulthood, a period associated with continuing brain development in healthy individuals [98, 149], understanding how Đand when-brain development in schizophrenia may diverge from that in typical development is critical for conceptualizing the emergence of the disorder and its associated functional deficits. The proximity of these changes to the age of onset of schizophrenia raises the question of whether an alteration in development of the WM connections that facilitate neural connectivity may have a causal influence on the emergence of schizophrenia symptomatology and cognitive deficits. Understanding the relationship of these structural changes to the emergence of cognitive deficits and symptomatology will give us a unique window into the process of disease onset, and to potential windows for intervention.
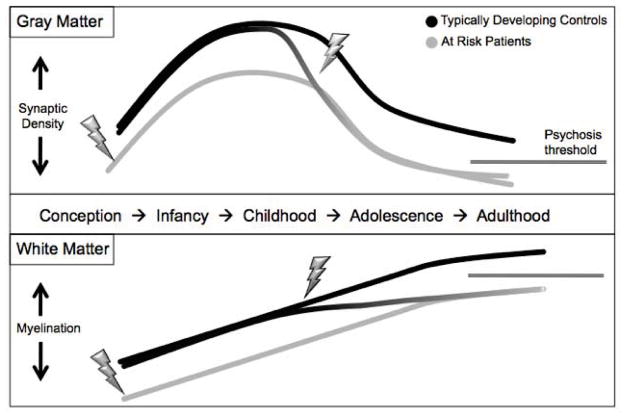
Fig. (3).
Potential trajectories of grey and white matter development in schizophrenia. Patients show decreased cortical thickness and myelination in adulthood, as the result of either an early insult, a later insult, a disrupted developmental trajectory, or a combination of these factors.
Table 1.
| Study | High-Risk Subtype | Sample | Diagnostic Criteria | Age (years±stdev) | Gender (M,F) | Method | Medication | ROIs | Main Finding |
|---|---|---|---|---|---|---|---|---|---|
| Fusar-Poli et al, 2011 | Clinical | 39 HR 41 HC |
PACE criteria | 24.49±4.55 25.88±5.24 |
24,15 33,8 |
sMRI, EEG | 32/39 CBT (CBT=Cognitive Behavioral Therapy) 18/39 atypical |
Whole brain and P300 amplitude | HR vs HC: HR ↓L STG and ↓L SFG HR: Pos. corr. WM vol in R SMG and ↑P300; Neg. corr. WM vol in R MiFG and P300 |
| McIntosh et al, 2008 | Genetic | 11 TT 30 CT 25 CC |
SCID NRG1 haplo-type (SNP: rs6994992) |
35.8±11.1 35.5±10.9 37.1±12.3 |
5,6 8,22 10,15 |
DTI (FA) | n/a | Bilateral ALIC | ↓ FA in R ALIC in TT vs C allele carriers at SNP |
| Winterer et al, 2008 | Genetic | 19 TT 24 C-Carriers |
SCID NRG1 haplo-type (SNP: rs6994992) |
22.7±1.7* | 27,23* | DTI (FA) | n/a | whole brain | ↓ FA L SMG in subjects with a C allele at SNP |
| Bloemen et al, 2010 | Clinical | 10 HR-converted 27 HR 10 HC |
SIPS, SCID | 20.7±4.3 18.9± 4.0 22.7±3.9 |
8.2 18,9 8,2 |
DTI (FA), sMRI | 26/37 CHR | whole brain | ↓ FA in MTL, putamen, STG in HR converters |
| Jacobson et al, 2010 | Clinical | 11 HR 14 HC |
SIPS | 12.2±.6 12.5 ±.4 |
3,11 | DTI (FA) | none | whole brain | ↓ FA in IFOF (lingual gyrus), L ILF (STG), L CB (PHG) in HR |
| Karlsgodt et al, 2009 | Clinical | 36 HR 25 HC |
SIPS | 17.02±3.37 17.96±3.40 |
27,9 12,13 |
DTI (FA) | 21/36 HR (9 atypical, 12 SSRI) | ATR, CB, ILF, MTL, SLF, UNC | HR vs HC: ↓ FA LSLF HR: correlation of ILF with outcome |
| Peters et al, 2008 | Clinical | 10 HR 10 HC 10 FE SZ |
SIPS, SCID, BSABS | 21.6 ± 2.8 21.1 ± 2.8 21.2 ± 3 |
Not reported | DTI: FA, trace (fiber tracking) | Duration: 14 weeks (10–20) Dosage: 1.1 mg/day (.7–1.3) Duration: 33.5 weeks (7.1–97) Dosage: 2.4 mg/day (1.3–5) |
Dorsal CB, anterior CB, UF, AF, genu and spelnium of the callosum | No group differences in FA. |
| Peters et al, 2010 (Follow-up from Peters et al., 2008) | Clinical | 7 HR-converted 10 HR 10 HC |
SIPS, SCID, BSABS | 22.6±3.9 21.2±3.2 21.1±2.8 |
Not reported | DTI: FA, trace (fiber tracking) | N=3 (10, 20, 38 weeks N=1 (12 weeks) N=0 |
Dorsal CB, anterior CB, UF, AF, Corpus callosum (4 sub-regions) | ↑ FA in HR-convert and HR vs. HC in R anterior cingulate (Note: only significant when voxel size was not added as a covariate) |
| Witthaus et al, 2008 | Clinical | 30 HR 29 HC 23 FE SZ |
SIPS, PANSS | 25.1±4.3 25.7±5.2 26.4±6.1 |
20,10 17,12 16,7 |
T-1 MPRAGE: VBM | 12 Yes, 18 No 0 Yes, 29 No 6 Yes, 17 No |
whole brain | HR vs HC: HR ↓ R STG; FE SZ vs HR: FE SZ ↓R SFG, L MFG, R MTG, Bilateral Occipital, R Fornix, L MCP; FE SZ vs HC: FE SZ ↓ L CB, Bilateral SFG, MTG, Precentral, L MFG, L MiFG, R Thalamus, L Postcentral, L Inferior Occipital |
| DeLisi et al, 2006 | Genetic | 15 HR 25 HC 15 SZ |
n/a | 19.27±4.64 23.72±3.73 34.27±10.67 |
6,9 9,16 12,3 |
DTI (ADC), sMRI | none | whole brain | ↓ ADC in combined SZ and HR in left parahip-pocampal gyrus, lingul gyrus, SFG, MFG |
| Hao et al, 2009 | Genetic | 34 HR 32 HC 34 SZ |
SCID | 25.77±7.11 26.59±5.96 25.44±5.90 |
20,14 19,13 20,14 |
DTI (FA) | SZ patients, 451.79 ± 107.57 chlorpromazine equivalent (mg) | whole brain | ↓ FA in HR and SZ in left PFC, L hipp. ↓ FA in ACC in SZ only. |
| Hoptman et al, 2008 | Genetic | 22 HR 37 HC 23 SZ |
DIGS, SIPS, SOPS | 20.05 ±4.08 23.08 ±4.04 36.83 ±10.95 |
7,15 17.20 16,7 |
DTI (FA) | neuroleptics in all SZ | whole brain | ↓ FA in ACC and angular gyri in HR; |
| Munoz Maniega et al, 2008 | Genetic | 22 HR 31 SZ 51 HC |
SCID | 30±3 37±10 35±11 |
13,9 16,15 24,24 |
DTI (FA) | Not reported | UF, AF, CB, ALIC | ↓ FA in HR in ALIC ↓ FA in SZ in Bilateral UF and ALIC, L AF |
| Voineskos et al, 2011 | Genetic | 39 C-carriers 23 AA |
SCID, ZNF804A genotype (SNP: rs1344706) | 37 ± 13 38 ± 12 |
24, 15 16, 7 |
DTI (FA, radial diffusivity) | n/a | UF, AF, CB, genu of corpus collosum | No group differences in FA or radial diffusivity. |
| Zuliani et al, 2011 | Genetic | 10 GG 14 GA 12 AA |
SCID or PSE ERbB4 genotype (SNP: rs4673628) |
34.4 ± 10.7 37.3 ± 11.7 40.9 ± 13.7 |
5, 5 8, 6 6, 6 |
DTI(FA) | n/a | Bilateral ALIC | Subjects with a G allele at SNP: ↓ FA bilateral ALIC |
HR, High-Risk; HC, Healthy Controls; SIPS, Structured Interview for Prodromal Syndromes; BSABS, Bonn Scale for the Assessment of Basic Symptoms; SCID, Structured Clinical Interview for DSM-IV; PSE, Present State Examination; L, Left; R, Right; DTI, Diffusion Tensor Imaging; FA, Fractional Anisotropy; IFOF, Inferior fronto-occipital fascicles; ILF, Inferior longitudinal fasciculus; STG, Superior temporal gyrus; PHG, Parahippocampal gyrus; CB, Cingulum bundle; UF, uncinate fasciculus; AF, arcuate fasciculus; ALIC, Anterior limb of the internal capsule; SMG, supramarginal gyrus; VBM, Voxel-based morphometry; SFG, Superior frontal Gyrus, MFG, Medial frontal gyrus; MTG, Middle temporal gyrus; MCP, Middle cerebellar peduncle; MiFG, Middle frontal gyrus; UF, uncinate fasciculus; AF, arcuate fasciculus
Winterer et al., 2008 reported mean data for age and or gender for whole sample not just the DTI subjects or separate genotyped groups (N=50)
References
- 1.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- 2.Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149(7):890–7. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DR, Aloia MS, Goldberg TE, Berman KF. The frontal lobes and schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6(4):419–27. doi: 10.1176/jnp.6.4.419. [DOI] [PubMed] [Google Scholar]
- 4.Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28(2–3):143–56. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- 5.Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60(5):443–56. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, van Erp TG, Huttunen M, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55(12):1084–91. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- 7.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 8.Sachdev P, Brodaty H. Quantitative study of signal hyperintensities on T2-weighted magnetic resonance imaging in late-onset schizophrenia. Am J Psychiatry. 1999;156(12):1958–67. doi: 10.1176/ajp.156.12.1958. [DOI] [PubMed] [Google Scholar]
- 9.Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27(10):1193–200. doi: 10.1023/a:1020981510759. [DOI] [PubMed] [Google Scholar]
- 10.Hakak Y, Walker JR, Li C, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98(8):4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353–70. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 12.Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, Huttunen MO, Keshavan MS, Seidman LJ, Tsuang MT. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schiz Res. 2003;29(4):653–69. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- 13.Salvati S, Attorri L, Avellino C, Di Biase A, Sanchez M. Diet, lipids and brain development. Dev Neurosci. 2000;22(5–6):481–7. doi: 10.1159/000017479. [DOI] [PubMed] [Google Scholar]
- 14.Paus T, Zijdenbos A, Worsley K, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283(5409):1908–11. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 15.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 16.Yakovlev P, Lecours A. Regional development of the brain in early life. Boston: Blackwell Scientific Publications; 1967. [Google Scholar]
- 17.Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15(4):585–93. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SE, Rioux L. Challenges, status, and opportunities for studying developmental neuropathology in adult schizophrenia. Schizophr Bull. 2001;27(3):395–416. doi: 10.1093/oxfordjournals.schbul.a006883. [DOI] [PubMed] [Google Scholar]
- 19.Durston S, Davidson MC, Tottenham N, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 20.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–66. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien JS. Stability of the Myelin Membrane. Science. 1965;147:1099–107. doi: 10.1126/science.147.3662.1099. [DOI] [PubMed] [Google Scholar]
- 22.Crawford MA, Sinclair AJ. The limitations of whole tissue analysis to define linolenic acid deficiency. J Nutr. 1972;102(10):1315–21. doi: 10.1093/jn/102.10.1315. [DOI] [PubMed] [Google Scholar]
- 23.Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res. 2003;62(3):195–204. doi: 10.1016/s0920-9964(02)00284-0. [DOI] [PubMed] [Google Scholar]
- 24.Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24(2):69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Shinnoh N, Kondo A, Yamada T. Adrenoleuko-dystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochem Biophys Res Commun. 1997;232(3):631–6. doi: 10.1006/bbrc.1997.6340. [DOI] [PubMed] [Google Scholar]
- 26.Wiggins RC. Myelin development and nutritional insufficiency. Brain Res. 1982;257(2):151–75. doi: 10.1016/0165-0173(82)90016-9. [DOI] [PubMed] [Google Scholar]
- 27.McKenna MC, Campagnoni AT. Effect of pre- and postnatal essential fatty acid deficiency on brain development and myelination. J Nutr. 1979;109(7):1195–204. doi: 10.1093/jn/109.7.1195. [DOI] [PubMed] [Google Scholar]
- 28.Berkow SE, Campagnoni AT. Essential fatty acid deficiency: effects of cross-fostering mice at birth on brain growth and myelination. J Nutr. 1981;111(5):886–94. doi: 10.1093/jn/111.5.886. [DOI] [PubMed] [Google Scholar]
- 29.DeWille JW, Farmer SJ. Postnatal dietary fat influences mRNAS involved in myelination. Dev Neurosci. 1992;14(1):61–8. doi: 10.1159/000111648. [DOI] [PubMed] [Google Scholar]
- 30.Connor JR, Menzies SL. Altered cellular distribution of iron in the central nervous system of myelin deficient rats. Neuroscience. 1990;34(1):265–71. doi: 10.1016/0306-4522(90)90320-4. [DOI] [PubMed] [Google Scholar]
- 31.Barres BA, Raff MC. Axonal control of oligodendrocyte development. J Cell Biol. 1999;147(6):1123–8. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waxman SG, Sims TJ. Specificity in central myelination: evidence for local regulation of myelin thickness. Brain Res. 1984;292(1):179–85. doi: 10.1016/0006-8993(84)90905-3. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J Neurosci. 1996;16(16):5095–105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab M, Schnell L. Region-specific appearance of myelin constitutents in the developing rat spinal cord. J Neurocytol. 1991;18:709–21. doi: 10.1007/BF01206659. [DOI] [PubMed] [Google Scholar]
- 35.Lewis DA. The human brain revisited: opportunities and challenges in postmortem studies of psychiatric disorders. Neuropsychopharmacology. 2002;26(2):143–54. doi: 10.1016/S0893-133X(01)00393-1. [DOI] [PubMed] [Google Scholar]
- 36.Peled S, Gudbjartsson H, Westin C, Kikinis R, Jolesz F. Magnetic resonance imaging shows orientation and asymmetry of white matter fiber tracts. Brain Res. 1998:27–33. doi: 10.1016/s0006-8993(97)00635-5. [DOI] [PubMed] [Google Scholar]
- 37.Westin CFMSEKBEPJFA, Kikinis R. Image Processing for Diffusion Tensor Magnetic Resonance Imaging. 1999 [Google Scholar]
- 38.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13(6 Pt 1):1174–85. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 39.Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev. 2006;30(6):762–74. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song AW, Harshbarger T, Li T, et al. Functional activation using apparent diffusion coefficient-dependent contrast allows better spatial localization to the neuronal activity: Evidence using diffusion tensor imaging and fiber tracking. Neuroimage. 2003 doi: 10.1016/S1053-8119(03)00292-1. [DOI] [PubMed] [Google Scholar]
- 41.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 42.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 43.Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10(13):2817–21. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 44.Giorgio A, Watkins KE, Chadwick M, et al. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49(1):94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Kochunov P, Glahn DC, Lancaster J, et al. Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage. 2011;58(1):41–9. doi: 10.1016/j.neuroimage.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westlye LT, Walhovd KB, Dale AM, et al. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055–68. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- 47.Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72(1):16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Lenroot RK, Schmitt JE, Ordaz SJ, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2009;30(1):163–74. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kochunov P, Williamson DE, Lancaster J, et al. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2010 Jan 30; doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 51.De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11(6):552–7. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 52.Asato MR, Terwilliger R, Woo J, Luna B. White Matter Development in Adolescence: A DTI Study. Cereb Cortex. 2010 Jan 5; doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bava S, Boucquey V, Goldenberg D, et al. Sex differences in adolescent white matter architecture. Brain Res. 2011;1375:41–8. doi: 10.1016/j.brainres.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johansen-Berg H. Behavioural relevance of variation in white matter microstructure. Curr Opin Neurol. 2010;23(4):351–8. doi: 10.1097/WCO.0b013e32833b7631. [DOI] [PubMed] [Google Scholar]
- 55.Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-T⊘nnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20(3):534–48. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 56.Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: A diffusion tensor imaging study. J Cogn Neurosci. 2009;21(7):1406–21. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30(2):115–25. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 59.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56(9):781–7. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 60.Buchsbaum MS. The frontal lobes, basal ganglia, and temporal lobes as sites for schizophrenia. Schizophr Bull. 1990;16(3):379–89. doi: 10.1093/schbul/16.3.379. [DOI] [PubMed] [Google Scholar]
- 61.Csernansky JG, Bardgett ME. Limbic-cortical neuronal damage and the pathophysiology of schizophrenia. Schizophr Bull. 1998;24(2):231–48. doi: 10.1093/oxfordjournals.schbul.a033323. [DOI] [PubMed] [Google Scholar]
- 62.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1081–90. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Hugdahl K, Rund BR, Lund A, Asbjornsen A, Egeland J, Ersland L, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004;161(2):286–93. doi: 10.1176/appi.ajp.161.2.286. [DOI] [PubMed] [Google Scholar]
- 64.Quintana J, Wong T, Ortiz-Portillo E, et al. Prefrontal-posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biol Psychiatry. 2003;53(1):12–24. doi: 10.1016/s0006-3223(02)01435-x. [DOI] [PubMed] [Google Scholar]
- 65.Kim H, Samsonenko DG, Yoon M, et al. Temperature-triggered gate opening for gas adsorption in microporous manganese formate. Chem Commun (Camb) 2008;21(39):4697–9. doi: 10.1039/b811087e. [DOI] [PubMed] [Google Scholar]
- 66.Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132(Pt 9):2426–36. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- 67.Whalley HC, Simonotto E, Marshall I, et al. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128(Pt 9):2097–108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- 68.Borgwardt SJ, Riecher-Rossler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61(10):1148–56. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Broome MR, Matthiasson P, Fusar-Poli P, et al. Neural correlates of movement generation in the ‘at-risk mental state’. Acta Psychiatr Scand. 2010;122(4):295–301. doi: 10.1111/j.1600-0447.2009.01524.x. [DOI] [PubMed] [Google Scholar]
- 70.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–8. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 71.Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr, Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53(5):425–36. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- 72.Kirkpatrick B, Conley RC, Kakoyannis A, Reep RL, Roberts RC. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: An unbiased cell-counting study. Synapse. 1999;34(2):95–102. doi: 10.1002/(SICI)1098-2396(199911)34:2<95::AID-SYN2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 73.Kirkpatrick B, Messias NC, Conley RR, Roberts RC. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J Nerv Ment Dis. 2003;191(9):563–7. doi: 10.1097/01.nmd.0000087181.61164.e1. [DOI] [PubMed] [Google Scholar]
- 74.Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull. 2008;34(1):72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised White Matter Tract Integrity in Schizophrenia Inferred from diffusion Tensor imaging. Arch Gen Psychiatry. 1999;56:367–74. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 76.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 77.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zalesky A, Fornito A, Seal ML, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69(1):80–9. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seal ML, Yucel M, Fornito A, et al. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res. 2008;101(1–3):106–10. doi: 10.1016/j.schres.2007.12.489. [DOI] [PubMed] [Google Scholar]
- 80.Petrides M, Pandya DN. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. Association pathways of the prefrontal cortex and functional observations. [Google Scholar]
- 81.Szeszko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33(5):976–84. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 82.Karlsgodt K, van Erp TG, Poldrack R, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63(5):512–8. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 83.Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167(4):451–8. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 84.Luck D, Buchy L, Czechowska Y, et al. Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. J Psychiatr Res. 2011;45(3):369–77. doi: 10.1016/j.jpsychires.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126(Pt 3):610–22. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- 86.Polli FE, Barton JJ, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(Pt 4):971–86. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 87.Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PC. MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- 88.Agartz I, Andersson JLR, Skare S. Abnormal brain white matter in schizophrenia: A diffusion tensor imaging study. Neuroreport: For Rapid Communication of Neuroscience Research. 2001;12(10):2251–4. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- 89.Kalus P, Buri C, Slotboom J, et al. Volumetry and diffusion tensor imaging of hippocampal subregions in schizophrenia. Neuroreport. 2004 Apr 9;15(5):867–71. doi: 10.1097/00001756-200404090-00027. [DOI] [PubMed] [Google Scholar]
- 90.Kubicki M, Westin C, Nestor PG, et al. Cingulate fasiculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003 doi: 10.1016/s0006-3223(03)00419-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang F, Sun Z, Cui L, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161(3):573–5. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- 92.Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scand J Psychol. 2001;42(3):239–50. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- 93.Fujiwara H, Namiki C, Hirao K, et al. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2007;95(1–3):215–22. doi: 10.1016/j.schres.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 94.White T, Kendi AT, Lehericy S, et al. Disruption of hippocampal connectivity in children and adolescents with schizophrenia--a voxel-based diffusion tensor imaging study. Schizophr Res. 2007;90(1–3):302–7. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 95.Schlosser RG, Nenadic I, Wagner G, et al. White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res. 2007;89(1–3):1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 96.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 97.Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6(4):551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 98.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98(20):11650–5. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vidal CN, Rapoport JL, Hayashi KM, et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63(1):25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- 100.Kyriakopoulos M, Perez-Iglesias R, Woolley JB, et al. Effect of age at onset of schizophrenia on white matter abnormalities. Br J Psychiatry. 2009;195(4):346–53. doi: 10.1192/bjp.bp.108.055376. [DOI] [PubMed] [Google Scholar]
- 101.Douaud G, Mackay C, Andersson J, et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132(Pt 9):2437–48. doi: 10.1093/brain/awp126. [DOI] [PubMed] [Google Scholar]
- 102.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–8. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 103.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29(3):148–59. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muetzel RL, Collins PF, Mueller BA, AMS, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39(4):1918–25. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hao Y, Yan Q, Liu H, et al. Schizophrenia patients and their healthy siblings share disruption of white matter integrity in the left prefrontal cortex and the hippocampus but not the anterior cingulate cortex. Schizophr Res. 2009;114(1–3):128–35. doi: 10.1016/j.schres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 106.Munoz Maniega S, Lymer GK, Bastin ME, et al. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophr Res. 2008;106(2–3):132–9. doi: 10.1016/j.schres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 107.DeLisi LE, Szulc KU, Bertisch H, et al. Early detection of schizophrenia by diffusion weighted imaging. Psychiatry Res. 2006;148(1):61–6. doi: 10.1016/j.pscychresns.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karlsgodt K, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66(6):562–9. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoptman MJ, Nierenberg J, Bertisch HC, et al. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res. 2008 Dec;106(2–3):115–24. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 110.Winterer G, Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N. Association of 5′ end neuregulin-1 (NRG1) gene variation with subcortical medial frontal microstructure in humans. Neuroimage. 2008;40(2):712–8. doi: 10.1016/j.neuroimage.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 111.Stefansson H, Sarginson J, Kong A, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72(1):83–7. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McIntosh AM, Moorhead TW, Job D, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Mol Psychiatry. 2008;13(11):1054–9. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- 113.Zuliani R, Moorhead TW, Bastin ME, et al. Genetic variants in the ErbB4 gene are associated with white matter integrity. Psychiatry Res. 2011;191(2):133–7. doi: 10.1016/j.pscychresns.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Voineskos AN, Lerch JP, Felsky D, et al. The ZNF804A gene: characterization of a novel neural risk mechanism for the major psychoses. Neuropsychopharmacology. 2011;36(9):1871–8. doi: 10.1038/npp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67(5):735–48. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bloemen OJ, de Koning MB, Schmitz N, et al. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol Med. 2010;40(8):1297–304. doi: 10.1017/S0033291709991711. [DOI] [PubMed] [Google Scholar]
- 117.Jacobson S, Kelleher I, Harley M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11–13 year old schoolchildren. Neuroimage. 2010;49(2):1875–85. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 118.Witthaus H, Brune M, Kaufmann C, et al. White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophr Res. 2008;102(1–3):141–9. doi: 10.1016/j.schres.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 119.Fusar-Poli P, Crossley N, Woolley J, Carletti F, Perez-Iglesias R, Broome M, et al. White matter alterations related to P300 abnormalities in individuals at high risk for psychosis: an MRI-EEG study. J Psychiatry Neurosci. 2011;36(4):239–48. doi: 10.1503/jpn.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ziermans TB, Schothorst PF, Schnack HG, et al. Progressive Structural Brain Changes During Development of Psychosis. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fusar-Poli P, Crossley N, Woolley J, et al. White matter alterations related to P300 abnormalities in individuals at high risk for psychosis: an MRI-EEG study. J Psychiatry Neurosci. 2011 Jul;36(4):239–48. doi: 10.1503/jpn.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCarley RW, Shenton ME, O’Donnell BF, et al. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993 Mar;50(3):190–7. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- 123.McCarley RW, Salisbury DF, Hirayasu Y, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002 Apr;59(4):321–31. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- 124.Frommann I, Brinkmeyer J, Ruhrmann S, Hack E, et al. Auditory P300 in individuals clinically at risk for psychosis. Int J Psychophysiol. 2008;70(3):192–205. doi: 10.1016/j.ijpsycho.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 125.Peters BD, de Haan L, Dekker N, et al. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58(1):19–28. doi: 10.1159/000154476. [DOI] [PubMed] [Google Scholar]
- 126.Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J Psychiatr Res. 2010;44(15):993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 127.Ward RE, Huang W, Curran OE, Priestley JV, Michael-Titus AT. Docosahexaenoic acid prevents white matter damage after spinal cord injury. J Neurotrauma. 2010;27(10):1769–80. doi: 10.1089/neu.2010.1348. [DOI] [PubMed] [Google Scholar]
- 128.King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26(17):4672–80. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg. 2003;38(3):564–75. doi: 10.1016/s0741-5214(03)00473-7. [DOI] [PubMed] [Google Scholar]
- 130.Dyall SC, Michael-Titus AT. Neurological benefits of omega-3 fatty acids. Neuromolecular Med. 2008;10(4):219–35. doi: 10.1007/s12017-008-8036-z. [DOI] [PubMed] [Google Scholar]
- 131.Peet M, Stokes C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. 2005;65(8):1051–9. doi: 10.2165/00003495-200565080-00002. [DOI] [PubMed] [Google Scholar]
- 132.Peet M. Eicosapentaenoic acid in the treatment of schizophrenia and depression: rationale and preliminary double-blind clinical trial results. Prostaglandins Leukot Essent Fatty Acids. 2003;69(6):477–85. doi: 10.1016/j.plefa.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 133.Sethom MM, Fares S, Bouaziz N, et al. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2010;83(3):131–6. doi: 10.1016/j.plefa.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 134.Fenton WS, Hibbeln J, Knable M. Essential fatty acids, lipid membrane abnormalities, and the diagnosis and treatment of schizophrenia. Biol Psychiatry. 2000;47(1):8–21. doi: 10.1016/s0006-3223(99)00092-x. [DOI] [PubMed] [Google Scholar]
- 135.Fusar-Poli P, Berger G. Eicosapentaneioc acid interventions in schizophrenia: meta analysis of randomized placebo controlled studies. J Clin Psychiatry. doi: 10.1097/JCP.0b013e318248b7bb. in press. [DOI] [PubMed] [Google Scholar]
- 136.Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–54. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 137.Fusar-Poli P, Crossley N, Woolley J, et al. Gray matter alterations related to P300 abnormalities in subjects at high risk for psychosis: longitudinal MRI-EEG study. Neuroimage. 2011;55(1):320–8. doi: 10.1016/j.neuroimage.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 138.Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis--a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34(8):1207–22. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 139.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31(4):465–84. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 140.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–8. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia--a systematic review and meta-analysis. Biol Psychiatry. 2011 Mar 1;69(5):495–503. doi: 10.1016/j.biopsych.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 142.Berger GE, Wood SJ, Wellard RM, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study Neuropsychopharmacology. 2008;33(10):2467–73. doi: 10.1038/sj.npp.1301628. [DOI] [PubMed] [Google Scholar]
- 143.Sinha RA, Khare P, Rai A, et al. Anti-apoptotic role of omega-3-fatty acids in developing brain: perinatal hypothyroid rat cerebellum as apoptotic model. Int J Dev Neurosci. 2009;27(4):377–83. doi: 10.1016/j.ijdevneu.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 144.Berger GE, Wood SJ, Pantelis C, Velakoulis D, Wellard RM, McGorry PD. Implications of lipid biology for the pathogenesis of schizophrenia. Aust N Z J Psychiatry. 2002;36(3):355–66. doi: 10.1046/j.1440-1614.2001.01021.x. [DOI] [PubMed] [Google Scholar]
- 145.Stone JM, Day F, Tsagaraki H, et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66(6):533–9. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 146.Fusar-Poli P, Stone JM, Broome MR, et al. Thalamic Glutamate Levels as a Predictor of Cortical Response During Executive Functioning in Subjects at High Risk for Psychosis. Arch Gen Psychiatry. 2011 May 2; doi: 10.1001/archgenpsychiatry.2011.46. [DOI] [PubMed] [Google Scholar]
- 147.Stone JM, Howes OD, Egerton A, et al. Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biol Psychiatry. 2010;68(7):599–602. doi: 10.1016/j.biopsych.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 148.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292(5518):923–6. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 149.Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55(2):145–52. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 150.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1966. pp. 3–70. [Google Scholar]