Abstract
Background
Up to 10% of individuals with breast cancer (BC) belong to families with hereditary syndromes. The aim of this study was to develop an instrument to identify individuals/families at high‐hereditary risk for BC and offer dedicated surveillance programs according to different risks.
Methods
The instrument consisted of a primary questionnaire collecting history of BC and ovarian cancer (OC). This questionnaire was applied to women enrolled in the Emilia‐Romagna Breast Cancer Screening Program. General practitioners (GPs) and specialists could propose the same questionnaire too. Women with a score of ≥ 2, were invited to complete an oncogenetic counseling. According to the Tyrer‐Cuzick evaluation, women considered at high risk were invited to involve the most representative alive individual of the family affected with BC/OC for BRCA1/2 genetic testing.
Results
Since January 2012 and December 2016, 660 040 women were evaluated by the regional screening program, of which 22 289 (3.5%) were invited to the Spoke evaluation, but only 5615 accepted (25.2%). Totally, also considering women sent by GPs and specialists, 11 667 were assessed and 5554 were sent to the Hub evaluation. Finally, 2342 (42.8%) women fulfilled the criteria for genetic testing, and 544 (23.2%) resulted BRCA1/2 mutation carriers.
Conclusions
To our knowledge, this is the first regional population‐based multistep model that is aimed to identify individuals with BRCA1/2 mutations and to offer an intensive surveillance program for hereditary‐high risk women. This tool is feasible and effective, even if more efforts must be performed to increase the acceptance of multiple assessments by the study population.
Keywords: hereditary breast ovarian cancer, population‐based screening, Tyrer‐Cuzick model
This is, to our knowledge, the first multistep approach, including regional breast cancer screening program, general practitioners and specialists, aimed to identify individuals with BRCA1/2 mutations and to offer an intensive surveillance program for hereditary, high and intermediate risk women. Our results show that a regional population‐based model is feasible, even if more efforts have to be yielded to increase the acceptance of subsequent assessments from women at risk.
1. INTRODUCTION
Hereditary breast ovarian cancer (HBOC) can be defined as a genetic disorder in which breast and ovarian malignant tumors seem to cluster within families.1 The main factors that suggest a hereditary cancer predisposition syndrome are young age at cancer diagnosis, multiple tumors, bilateral tumors, presence of rare tumors, several cancer‐affected relatives, autosomal dominant inheritance and, in some cases, ethnicity.2, 3, 4, 5, 6, 7 Families with these characteristics should be referred to specialized hospital/centers that offer cancer risk assessment and genetic counseling8, 9, 10
In Italy, most cancer genetic services are largely distributed in the country but they are not regulated by the national health system rules.11
Therefore, there is limited knowledge regarding the prevalence of HBOC predisposition syndromes in the Italian population, which has already reached more than 60 million people.12 Moreover, considering the high probability of developing cancer in individuals with hereditary cancer predisposition and the fact that presymptomatic identification of at‐risk individuals offers enormous potential for reducing cancer‐related risk,13 a better understanding of the prevalence of hereditary cancer predisposition syndromes in Italy is imperative.
In this context, the goal of this study was to develop a pilot instrument for identifying individuals and families who are at risk for hereditary BC in a regional screening population‐based sample from Emilia‐Romagna, Italy.
2. PATIENTS AND METHODS
2.1. Ethical aspects
Our program was approved and deliberated by the Emilia‐Romagna region under the protocol number RER 220/2011.14 Furthermore, an informed consent form was signed by each woman who participated in the study for the genetic testing analysis and it was approved by the Ethics Committee of each Hub center.
2.2. Population of the study
Since January 2012 until December 2016, the regional program for HBOC started in Emilia‐Romagna. Women attending the Regional Breast Cancer Screening Program (RBCSP), which begins at 45 years with an annual mammogram until 50 years and then continues until 74 years with biyearly mammogram, were interviewed by a questionnaire regarding their personal or family history of BC and OC from radiologist technicians. The interview was repeated every screening round to verify changes in the family history. At January 2019, women living in Emilia‐Romagna region, aged between 45 and 74 years, are 960 792. About 69% of them adhere to the RBCSP that is distributed in 8 different units along the region.15
Women who asked for their risk at the General Practioner (GP) received the same questionnaire too. Finally, also some specialists, the gynecologists, oncologists, and radiologists, could administer the same questionnaire in the case of positive family history.
2.3. Primary screening questionnaire
The primary questionnaire was based on typical criteria for hereditary breast and ovarian cancer, that is, young age at onset, bilateral breast cancer (BC), association with ovarian cancer (OC) and relationship with other affected patients. These criteria were already considered as pathognomonic of hereditary breast cancer syndrome by Lynch.16 The questionnaire contained a grid that assigned a score from 0 to 2; women who reached the total score of ≥ 2 were invited to ask for the Spoke evaluation. The grid was adopted since 2000, by the Biosciences Laboratory of Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST), in Meldola, identifying 22% of patients with BRCA1/2 mutations among families with characteristics of hereditary breast and ovarian cancer.17 This rate was considered cost‐effective as indicated by the ASCO guidelines.18
In addition, a personal or family history of male BC, of BC and OC in the family or in the same patient, of early onset BC (≤35 years), of bilateral BC at ≤50 years, of not mucinous and not borderline sporadic OC, of two first‐degree relatives affected by BC, of which one arisen at ≤40 years or bilateral, and of triple negative BC (≤ 60 years), represented a direct criterion for Hub evaluation. The majority of the primary questionnaires was administered by the radiology technicians at the single unit of the RBCSP, uploading the grid score whenever the patient entered into the mammography room. All questionnaires were collected on a dedicated software program that calculates the score in real time and collects the results on a specific single database for each patient. Also, GPs had the same grid uploaded on their regional electronic health system and autonomously calculated the score when the patient was visited for the first time. The grid was also held by other specialists such as gynecologists, oncologists, and radiologists, but they rarely offered this evaluation to their patients. No informed consent was signed at this time. No central evaluation was performed to calculate the score obtained by different patients.
The questionnaire is shown in Table 1.
Table 1.
Family risk evaluation
| Age at Onset | BC | OC | ||||
|---|---|---|---|---|---|---|
| <40 y | 40‐49 y Bilateral | 40–49 y Monolateral | 50‐59 y | >60 y | Every | |
| Woman | 2 | 2 | 1 | 1 | 0 | 2 |
| Mother | 2 | 2 | 1 | 1 | 0 | 1 |
| Sister | 2 | 2 | 1 | 1 | 0 | 1 |
| Daughter | 2 | 2 | 1 | 1 | 0 | 1 |
| Paternal Grandmother | 2 | 2 | 1 | 1 | 0 | 1 |
| Paternal Aunt | 2 | 2 | 1 | 1 | 0 | 1 |
| Maternal Grandmother | 1 | 1 | 1 | 0 | 0 | 1 |
| Maternal Aunt | 1 | 1 | 1 | 0 | 0 | 1 |
| Relative with MBC | 2 | 2 | 2 | 2 | 2 | ‐ |
| Cousin (daughter of father's brother) | 1 | 0 | 0 | 0 | 0 | 1 |
| Nephew | 1 | 1 | 1 | 0 | 0 | 1 |
Abbreviations: BC, breast cancer; MBC, male breast cancer; OC, ovarian cancer.
2.4. Spoke model
All healthy women with score ≥ 2 or patients affected with BC at the age ranging between 36 and 40 years were invited to refer to their own Spoke center. In case of women attending the RBCSP eligible for further assessment, they received a letter with the mammography result and the phone number for calling the own Spoke center. In case of eligibility defined by GPs or other specialists, the appropriate Spoke center phone number was directly given to the patient. Totally 13 centers in Emilia‐Romagna were accredited as Spoke centers. At the Spoke Center, either geneticists or oncologists trained in oncogenetic counseling collected information on the cancer family history and drawn the pedigree. In both cases a specific informed consent was obtained aimed to receive family history information. The risk of BC was calculated with the Tyrer‐Cuzick program.19
2.5. Tyrer‐Cuzick risk calculation
The Tyrer‐Cuzick evaluation collects personal information regarding woman's age, menarche's age, height and weight measurements, parity, previous breast lesions, and menopausal status. Further information with regard to family history of BC or OC in the relatives is also collected. The final calculation provides the 10‐year risk and the life‐time risk for BC in individual and general population, respectively.
Women with a life‐time risk of developing BC at most 1 time more than general population were considered at low risk (LR) and were offered RBCSP. Women with a risk ranging between two and three times more than general population, were considered at intermediate profile and followed with an annual mammogram, associated with ultrasound, in the case of dense breasts since 40 until 45 years and then referred to the RBCSP. Women with a risk more than three times (profile 3 or high) were evaluated for referring to the Hub center with the aim to perform gene test analysis.
This model assessment was chosen among different risk calculators, that is, Gail, Claus, Ford, and manual model. It looked to be likely the most sensitive model to select women at high risk, apart from the lack of male breast cancer family history evaluation.20 However, women who were sent to the Hub center were further evaluated for BRCA1/2 genetic testing according to the Modena criteria.21
2.6. Hub model
Four regional Hub centers were accounted in Emilia‐Romagna. The referral to the Hub centers was proposed in the case of profile 3 at the Spoke evaluation or in the case of direct criteria at the primary screening, as previously mentioned.
At the Hub evaluation, a family history of:
Early Onset BC
BC and OC in the same patient or in other members
Male BC
Triple Negative BC at ≤60 years
OC not mucinous not borderline
Two or more BC in the family with a first‐degree relationship each other and young age at onset (≤40 years) or bilateral BC
was considered eligible for genetic testing and the most indicative affected patient in the family was invited to undergo pretest oncogenetic sessions, aimed to perform BRCA1/2 analysis. In a period of 3‐4 weeks, the BRCA1/2 results were released along with a posttest genetic session. When a positive test was found, relatives of the index case were invited to undergo specific mutation analysis. All positive women already affected or not, were offered a screening protocol with six‐monthly breast ultrasound since 18 to 69 years, annual breast MRI since 25 until 74 years, annual mammogram since 35 to 69 years and biennial in the 70‐74 years range. In the case of bilateral mastectomy, only breast ultrasound every six months was proposed since the residual cancer risk remains equal to 4%‐5% and no guidelines are provided for breast MRI screening utility in this group of women. Breast ultrasound seemed to have a good ratio between cost and effectiveness rather than a solely clinical examination in order to recognize little foci of cancer upon the protheses. For OC screening, a six‐monthly transvaginal ultrasound plus Ca.125 marker dosage was proposed, when patients refused to consider the prophylactic oophorectomy or when they were too young to offer. The six‐monthly transvaginal ultrasound plus Ca.125 marker dosage was offered on the basis of data obtained by a phase II study in which OC screening performed more frequently than annually with prompt surgical intervention seemed to offer a better chance of early‐stage detection in high‐risk women.22 In the case of previous OC, only Ca.125 marker dosage every six months was offered.14
However, when criteria or individual disposable for gene testing lacked, a dedicated screening protocol was offered to women with different risk profiles; in the case of high risk (profile 3) it starts at 25 years of age with six‐monthly ultrasound until 49, annual mammogram since 35 to 69 years and then biennial until 74 years. For LR and intermediate risk (IR), the screening protocol was described above.14 The surveillance protocol is detailed in Table 2. The process mapping of patients through Spoke and Hub screening procedure is shown in Figure 1.
Table 2.
Surveillance program for different risk profiles
| Risk profile | Start | US | MX | MRI |
|---|---|---|---|---|
| Profile 1 | 45 y | If suspected mammogram image |
45‐50 y Aa 51‐74 y Bb (Population Screening) |
|
| Profile 2 |
25 y (if relative with EOBCc) 36 y |
>41 y if high breast density or suspected mammogram image |
40‐50 y Aa 51‐74 y Bb (Population Screening) |
According to EUSOMA guidelines |
| Profile 3 (without detected mutations) | 25 y | 25‐60 y Sd |
35‐69 y Aa 70‐74 y Bb |
According to EUSOMA guidelines |
| Profile 3 (with detected mutations)e | From the mutation detection | From the mutation detection‐69 y Sd |
35‐69 y Aa 70‐74 y Bb |
>25 y A |
Annual
Biennial
Early Onset Breast Cancer
Semestral
Ca.125 and Transvaginal Ultrasound every 6‐months was also added
Figure 1.
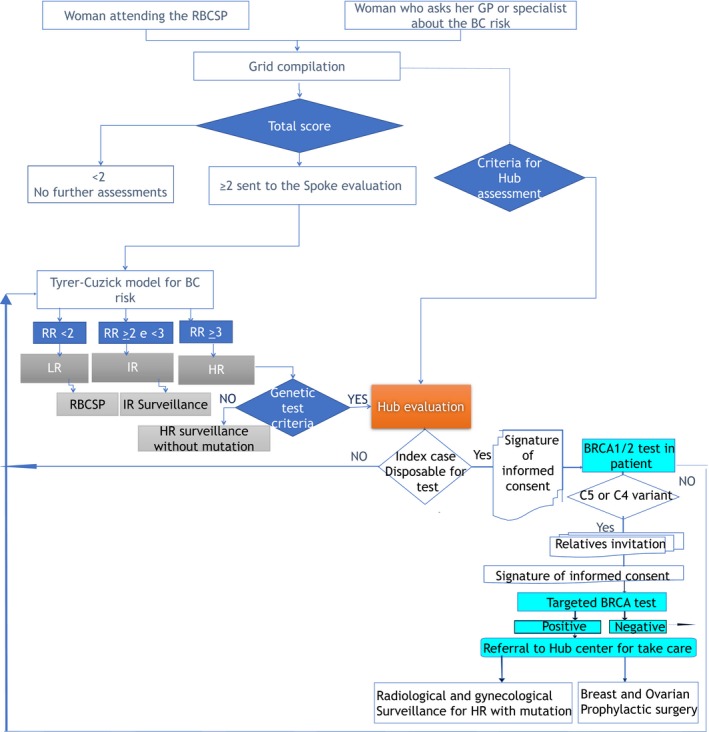
The flowchart of patients through the Spoke and Hub screening procedure. BC, breast cancer; GP, general practitioner; HR, high risk; IR, intermediate risk; LR, low risk; RBCSP, Regional Breast Cancer Screening Program
2.7. Genetic testing
Genetic testing for identifying BRCA1 and BRCA2 mutations included the Next Generation Sequencing (NGS) on the entire codifying sequence and the Multiplex Ligation Polymerase Analysis (MLPA) for rearrangements of BRCA1/2 gene. The variants were divided, according to the ENIGMA classification, in five classes: C5 and C4 were considered as positive results and carriers were followed as previous reported. In the case of C3, C2, and C1 results, the screening was based on the risk profile defined by the Tyrer‐Cuzick risk model. For the C3 classes, a customized pipeline for variant calling was questioned every month in order to find an eventual reclassification.
3. RESULTS
3.1. Primary screening questionnaire
From January 2012 to December 2016, among 660 040 women participating in the RBCSP, 659 747 women answered the questionnaire, whereas 293 (0.04%) refused to compile the same. Totally, 22 289 women (3.5%) received the letter and were invited to refer to the own Spoke center. By dividing all women in quintiles for age from 45 to 74 years, 8518 of those invited to call on the Spoke evaluation ranged between 45 and 49 years (38.2%). Detailed information on classes of age, number, and percentage of patients referred to the Spoke center by RBCSP can be found in Table 3.
Table 3.
Women refer to Spoke evaluation by RBCSP according to the age
| Total No | Age | % |
|---|---|---|
| 8518 | 45‐49 | 38.2 |
| 3836 | 50‐54 | 17.2 |
| 2856 | 55‐59 | 12.8 |
| 2511 | 60‐64 | 11.3 |
| 2354 | 65‐69 | 10.6 |
| 2214 | 70‐74 | 9.9 |
| 22 289 | All | 100 |
3.2. Spoke evaluation
Among all, 22 289 women were referred to the Spoke by the RBCSP, only 5615 (25.2%) phoned for having a Spoke evaluation. All requests were held in about 2 weeks. In addition, 2258 (19.4%) women were referred by GPs and 3794 (32.5%) by specialists to the Spoke centers, with the remaining women (48.1%) coming from RBCSP. Women referred by GPs were, in about one‐quarter of cases, younger than 35 years of age (560/2258, 24.8%), whereas specialists identified more patients in the age 40‐44 years (887/3794, 23.4%). Totally, 11 667 women arrived at the Spoke centers. Among those, 330 refused to complete the assessment, and the remaining 11 337 women were evaluated. Totally, 4627 women evaluated were considered eligible for the Hub referral (40.8%), whereas 6710 (59.2%) had an IR or LR profile. Data on source, age and risk profile are reported in Table 4.
Table 4.
Characteristics of women arrived at the Spoke centers
| Age | GPs | Specialists | RBCSP | Total | % | Refuse | LR | IR | HR |
|---|---|---|---|---|---|---|---|---|---|
| <35 | 560 | 840 | 0 | 1400 | 12 | 32 | 237 | 424 | 707 |
| 35‐39 | 403 | 722 | 0 | 1125 | 9.6 | 26 | 191 | 365 | 543 |
| 40‐44 | 509 | 887 | 49 | 1445 | 12.4 | 35 | 309 | 453 | 648 |
| 45‐49 | 246 | 432 | 1832 | 2510 | 21.5 | 96 | 835 | 663 | 916 |
| 50‐54 | 214 | 369 | 1153 | 1736 | 14.9 | 48 | 612 | 423 | 653 |
| 55‐59 | 140 | 234 | 861 | 1235 | 10.6 | 28 | 458 | 260 | 489 |
| 60‐64 | 100 | 146 | 743 | 989 | 8.5 | 26 | 409 | 234 | 320 |
| 65‐69 | 65 | 102 | 599 | 766 | 6.6 | 30 | 339 | 180 | 217 |
| 70‐74 | 21 | 62 | 378 | 461 | 4 | 9 | 210 | 108 | 134 |
| Total | 2258 | 3794 | 5615 | 11 667 | 100 | 330 | 3600 | 3110 | 4627 |
Abbreviations: HR, High Risk; IR, Intermediate Risk; LR, Low Risk.
Among all 11 667 women who made an appointment at the Spoke center, 1400 (12%) were very young (<35 years), 2570 (22%) ranged between 35 and 44 years of age, whereas 7697 (66%) were aged from 45 to 74 years. Out of 3970 women aged less than 45 years, 2449 (61.7%) were sent at the Spoke Center by specialists, 1472 by GPs (37.1%) and 49 by RBCSP (1.2%).
3.3. Hub evaluation
Out of 4627 women assessed as HR (profile 3), only 2815 (60.8%) accepted the Hub evaluation. The most proportion of women eligible for the Hub evaluation was identified by GPs (1205/2241, 53.8%), followed by specialists (1768/3684, 48.0%) and finally by RBCSP (1654/5412, 30.6%) as shown in Table 5.
Table 5.
Characteristics of women assessed at the Hub centers
| <35 | Hub | % | 35‐44 | Hub | % | 45‐74 | Hub | % | Total | Hub referral | % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPs | 556 | 289 | 52.0 | 909 | 473 | 52.0 | 776 | 443 | 57.1 | 2241 | 1205 | 53.8 |
| Specialists | 812 | 418 | 51.5 | 1552 | 699 | 45.0 | 1320 | 651 | 49.3 | 3684 | 1768 | 48.0 |
| RBCSP | 0 | 0 | 0 | 48 | 19 | 39.6 | 5364 | 1635 | 30.5 | 5412 | 1654 | 30.6 |
| Total | 1368 | 717 | 52.4 | 2509 | 1191 | 47.5 | 7460 | 2729 | 36.6 | 11 337 | 4627 | 40.8 |
Additionally, following the seven criteria considered as enough for to the Hub referral at the primary questionnaire, 2739 women were sent directly, without the Spoke assessment. Totally, 5554 women received a Hub evaluation.
In 2342 cases (42.2%), a genetic test was performed, whereas 3212 (57.8%) women did not meet criteria for BRCA1/2 analysis. Five hundred and forty‐four women (23.2%), resulted BRCA1/2 mutation carriers. As expected, among BRCA1/2 mutated women, the highest rate of mutations was found in very young women, aged less than 30 years, (35%); the positive rate decreased until 18% in women ranging between 55 and 59 years. Women without mutation ascertained were classified as having profiles 1, 2, or 3 according to the previous Tyrer‐Cuzick risk calculation. Table 6 reports data on women who underwent a genetic test. The number of patients along the process mapping is depicted in Figure 2.
Table 6.
Characteristics of women underwent BRCA1/2 gene analysis
| Age | N° LR | % LR | N° IR | % IR | N° HR | % HR | N° BRCA1/2 | % BRCA1/2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| <25 | 44 | 40 | 23 | 21 | 8 | 7 | 36 | 32 | 111 |
| 25‐29 | 23 | 23 | 19 | 19 | 24 | 24 | 35 | 35 | 101 |
| 30‐34 | 44 | 25 | 24 | 14 | 58 | 33 | 49 | 28 | 175 |
| 35‐39 | 68 | 24 | 38 | 13 | 115 | 41 | 62 | 22 | 283 |
| 40‐44 | 64 | 21 | 44 | 15 | 115 | 38 | 80 | 26 | 303 |
| 45‐49 | 83 | 26 | 45 | 14 | 123 | 39 | 66 | 21 | 317 |
| 50‐54 | 74 | 26 | 24 | 8 | 132 | 46 | 58 | 20 | 288 |
| 55‐59 | 57 | 26 | 15 | 7 | 107 | 49 | 38 | 18 | 217 |
| 60‐64 | 41 | 22 | 14 | 8 | 92 | 50 | 37 | 20 | 184 |
| 65‐69 | 40 | 25 | 3 | 2 | 82 | 51 | 35 | 22 | 160 |
| 70‐74 | 51 | 25 | 3 | 1 | 101 | 50 | 48 | 24 | 203 |
| Total | 589 | 25.1 | 252 | 10.8 | 957 | 40.9 | 544 | 23.2 | 2342 |
Figure 2.
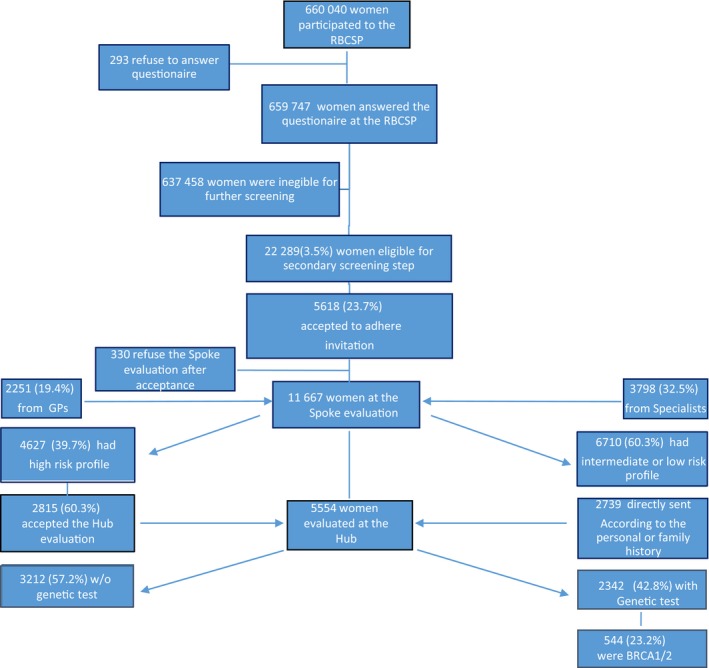
Number of patients evaluated along the process mapping
4. DISCUSSION
Our model is an effective tool to identify individuals/families at risk for BC, in a population‐based sample. In fact, nearly all women attending the RBCSP, with the exception of 293 cases (0.04%), compiled the primary questionnaire, also taking into account the change of family history along time. This multistep tool, proposed by a healthcare staff, provides more awareness about hereditary BC than a recently developed nurse navigator approach, by which a low participation of screening patients requesting HBOC education and evaluation occurred (9%).23
Hoskins et al,24 previously validated a tool for each case of BC or OC arbitrarily selected, weighting more points for patients with features associated with a higher probability that a BRCA mutation is present: early age of BC diagnosis, OC diagnosis, male BC in the family.25, 26, 27, 28, 29 The authors analyzed a total of 3906 women without a personal history of BC presenting for a screening mammogram at a community hospital, identifying 86 (2.2%) women with a family history indicative of a high probability (>10%) that a BRCA mutation was present. The percentage of at‐risk families was superimposable to our selection model in which 22 289 (3.5%) women were eligible for a secondary screening step.
An added value of this multistep process has been provided by GPs and specialists involvement. A recent systematic review on 40 studies published between 1996 and 2017, has evaluated the effects on patients of genetic cancer risk assessment in general practice.30 A variety of testing and screening tools were available for genetic cancer risk assessment in general practice, principally for breast‐ovarian, but GPs often reported low knowledge about hereditary cancers even if, time along, they were increasingly interested. In our experience too, the lowest percentage of women sent to the Spoke evaluation, came from GPs (19.4%) with respect to specialists (32.5%) and RBCSP (48.1%). However, GPs and specialists were needed to identify young high‐risk individuals, since the RBCSP begins at 45 years. In fact, 24.8% of women referred to the Spoke centers by GPs were younger than 35 years, such as women sent by specialists were mostly inclusive between 40 and 44 years (23.4%). As already seen in a recent paper that investigated the young women's perceptions regarding communication with healthcare providers about BC risk, people aged 18‐29 years asked more than doctors about their risk, whereas women in the age group between 30 and 44 years were likely queried by GPs about their family history.31 Totally, only 34% of all women evaluated at the Spoke centers aged less than 45 years and this could represent a critical issue, in order to offer a very preventive strategy in young individuals at hereditary‐HR for BC.
Another weakness was shown by the low rate of adhesion to the Spoke evaluation among women eligible by the RBCSP (25.2%). The low rate of adherence to the second screening step could depend by the fact that the invitation was provided by the same letter in which the mammogram result was delivered. Probably by this way, women focused their attention on negative mammogram results rather than on the deepening of their BC risk. An attempt to recover women eligible for the second screening step has been performed at the subsequent round, where the grid was repeated with a little increased rate of acceptance (0.3%). In a Brazilian study performed on 20 000 women attending the mammogram screening program, a questionnaire regarding BC family history was proposed and 3121 (15.6%) were invited to the second phase of the study by a direct approach or by letter or by phone. The first two modalities of invitation provided a very low rate of adhesion (11.4% and 16%, respectively), lower than our rate of acceptance. The highest rate of adherence was reached by the phone call (72.6%), demonstrating the validity for this approach for identifying families at risk for BC.32 However, the telephone approach needs dedicated personnel, and expensive costs for a large regional screening program. Also, considering the recall carried out at the subsequent round, we concluded that women who did not attend the Spoke assessment did not want to know their BC risk, with respect to the nondirectiveness defined as procedures that promote and enhance the autonomy and self‐control of patients.33
Nevertheless, among 11 337 women who received the Spoke evaluation, about 40% were HR, whereas 60% were considered as IR or LR. All LR (3600, 31.8%) and young IR women (aged 45 years or more, that is, 1868 equal to 16.6%), did not receive any personal prevention screening program but were invited to attend the RBCSP starting at 45 years. Only IR women aged less than 45 years (1294, 11.4%) and all HR individuals (4627, 40.8%) were offered a dedicated screening program for no hereditary risk of BC. Totally, the regional Spoke assessment identified about half of the women (5921, 52.2%) who needed an intensive screening program. A recent analysis performed on 2177 women at HR and IR followed at the Modena spoke center showed a BC detection rate of 8.5 and 16.1 × 1000 persons‐years, respectively, which clearly increases in comparison with the BC detection rate provided by the RBCSP.34 This data confirms the usefulness of an intensive screening program for at‐risk women and justifies a massive effort to identify the various parameters.
Once again, only 60% of women sent to the Hub centers accepted to be evaluated, but the rate of adherence was increased with respect to the previous step, underlying more interest toward the knowledge of hereditary conditions. Of notice, 49% of women evaluated at the Hub centers skipped the Spoke assessment according to specific criteria at the primary questionnaire. Among 5554 women evaluated at the Hub centers, 42.8% were eligible for gene testing and 23.2% resulted positive. This multistep approach provides a long patient journey, deriving a very high benefit in terms of BRCA mutation carrier identification. In fact, the step‐by‐step process is able to select a shrinking number of women to be investigated by gene test analysis: this approach looks to be really cost‐effective, although a recent study comparing BRCA1, BRCA2, PALB2, RAD51C, RAD51D, and BRIP1 analysis performed in selected and unselected women, seems to prevent more BC and OC in the general population, without previous selection.35 However, our mutation rate is in line with other series based on different eligibility criteria for HBOC, as referred in a recent review where the BRCA1/2 mutation rate in early onset BC, triple negative BC, bilateral BC, and family history of BC is about 30%.36 It also means that direct criteria for the Hub evaluation represent a significant way to collect women who are potentially at risk for HBOC syndrome, avoiding a multistep process and saving time and important costs.
CONFLICT OF INTEREST
The authors declare that they have no financial disclosure and conflict of interests.
AUTHOR'S CONTRIBUTION
Laura Cortesi: conceptualization, data curation, formal analysis, writing—original draft, and writing—review and editing. Bruna Baldassarri: conceptualization, funding acquisition, project administration, resources. Stefano Ferretti: funding acquisition, project administration, resources, supervision and writing—review and editing. Elisabetta Razzaboni: conceptualization, data curation, writing—review and editing. Mariangela Bella: data curation, writing—review and editing. Lauro Bucchi: data curation, formal analysis, methodology, and writing—review and editing. Debora Canuti: data curation, formal analysis, and writing—review and editing. Pierandrea De Iaco: conceptualization, data curation, supervision, and writing—review and editing. Giorgio De Santis: investigation, supervision writing—review and editing. Fabio Falcini: conceptualization, data curation, investigation, and writing—review and editing. Vania Galli: data curation, methodology, and writing—review and editing. Lea Godino: data curation, formal analysis, and writing—review and editing. Maurizio Leoni: conceptualization, data curation, formal analysis, supervision, validation, and writing—review and editing. Anna Myriam Perrone: investigation, and writing—review and editing. Marco Pignatti: investigation, and writing—review and editing. Gianni Saguatti: conceptualization, data curation, formal analysis, supervision, and writing—review and editing. Donatella Santini: investigation, and writing—review and editing. Priscilla Sassoli dè Bianchi: data curation, formal analysis, methodology, project administration, software, supervision, validation, and writing—review and editing. Federica Sebastiani: data curation, investigation, and writing—review and editing. Mario Taffurelli: investigation, methodology, and writing—review and editing. Giovanni Tazzioli: investigation, and writing—review and editing. Daniela Turchetti: conceptualization, data curation, formal analysis, investigation, methodology, and writing—review and editing. Claudio Zamagni: investigation, and writing—review and editing. Carlo Naldoni: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, writing—original draft, and writing—review and editing.
ACKNOWLEDGMENTS
We thank and express our gratitude to Tamara Sassi for her editorial assistance.
Cortesi L, Baldassarri B, Ferretti S, et al. A regional population‐based hereditary breast cancer screening tool in Italy: First 5‐year results. Cancer Med. 2020;9:2579–2589. 10.1002/cam4.2824
Funding information
This study was supported by funds from the Department of Health and Welfare of Emilia‐Romagna Region, in Italy.
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Samadder NJ, Giridhar KV, Baffy N, Riegert‐Johnson D, Couch FJ. Hereditary cancer syndromes‐a primer on diagnosis and management: part 1: breast‐ovarian cancer syndromes. Mayo Clin Proc. 2019;94(6):1084‐1098. [DOI] [PubMed] [Google Scholar]
- 2. Gómez‐Flores‐Ramos L, Álvarez‐Gómez RM, Villarreal‐Garza C, Wegman‐Ostrosky T, Mohar A. Breast cancer genetics in young women: What do we know? Mutat Res. 2017;774:33‐45. [DOI] [PubMed] [Google Scholar]
- 3. Cybulski C, Nazarali S, Narod SA. Multiple primary cancers as a guide to heritability. Int J Cancer. 2014;135(8):1756‐1763. [DOI] [PubMed] [Google Scholar]
- 4. Heisey R, Carroll JC. Identification and management of women with a family history of breast cancer: practical guide for clinicians. Can Fam Physician. 2016;62(10):799‐803. [PMC free article] [PubMed] [Google Scholar]
- 5. Eng C. Mendelian genetics of rare–and not so rare–cancers. Ann N Y Acad Sci. 2010;1214:70‐82. [DOI] [PubMed] [Google Scholar]
- 6. Frank TS, Hereditary cancer syndromes. Arch Pathol Lab Med. 2001;125(1):85‐90. [DOI] [PubMed] [Google Scholar]
- 7. Cox DM, Nelson KL, Clytone M, Collins DL. Hereditary cancer screening: case reports and review of literature on ten Ashkenazi Jewish founder mutations. Mol Genet Genomic Med. 2018;6(6):1236‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hampel H, Bennett RL, Buchanan A, Pearlman R, Wiesner GL. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17(1):70‐87. [DOI] [PubMed] [Google Scholar]
- 9. Hilgart JS, Coles B, Iredale R. Cancer genetic risk assessment for individuals at risk of familial breast cancer. Cochrane Database Syst Rev. 2012;(2):CD003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kyne G, Maxwell S, Brameld K, Harrison K, Goldblatt J, O'Leary P. Compliance with professional guidelines with reference to familial cancer services. Aust N Z J Public Health. 2011;35(3):226‐230. [DOI] [PubMed] [Google Scholar]
- 11. D’Andrea E, Marzuillo C, De Vito C, et al. Which BRCA genetic testing programs are ready for implementation in health care? A systematic review of economic evaluations. Genet Med. 2016;18(12):1171‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annuario Statistico Italiano. 2019. ISBN 978-88-458-2003-8. http://www.istat.it/it/archivio
- 13. Jatoi I. Risk‐reducing options for women with a hereditary breast cancer predisposition. Eur J Breast Health. 2018;14(4):189‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferretti S, Naldoni C, Baldessari B, et al. Protocollo assistenziale nelle donne a rischio ereditario di tumore della mammella e/o ovaio. Regione Emilia‐Romagna II edizione Anno. 2016;91:1‐56. [Google Scholar]
- 15. I programmi di screening oncologici della Regione Emilia-Romagna N° 99 - 2018 ISSN 2464 - 9252. http://salute.regione.emilia-romagna.it/documentazione/rapporti/contributi/
- 16. Lynch HT, Watson P, Conway TA, Lynch JF. Clinical/genetic features in hereditary breast cancer. Breast Cancer Res Treat. 1990;15(2):63‐71. [DOI] [PubMed] [Google Scholar]
- 17. Seymour IJ, Casadei S, Zampiga V, et al. Results of a population‐based screening for hereditary breast cancer in a region of North‐Central Italy: contribution of BRCA1/2 germ‐line mutations. Breast Cancer Res Treat. 2008;112(2):343‐349. [DOI] [PubMed] [Google Scholar]
- 18. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K. American Society of Clinical Oncology Policy Statement Update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893‐901. [DOI] [PubMed] [Google Scholar]
- 19. Tyrer J, Duffy SW, Cuzick J, et al. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111‐1130. [DOI] [PubMed] [Google Scholar]
- 20. Amir E, Evans DG, Shenton A, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003;40(11):807‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cortesi L, Turchetti D, Marchi I, et al. Breast cancer screening in women at increased risk according to different family histories: an update of the Modena Study Group experience. BMC Cancer. 2006;17(6):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenthal AN, Fraser L, Manchanda R, et al. Results of annual screening in phase I of the United Kingdom familial ovarian cancer screening study highlight the need for strict adherence to screening schedule. J Clin Oncol. 2013;31:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Appel SJ, Cleiment RJ. Identifying women at risk for hereditary breast and ovarian cancer syndrome utilizing breast care nurse navigation at mammography and imaging centers. J Natl Black Nurses Assoc. 2015;26(2):17‐26. [PubMed] [Google Scholar]
- 24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population‐based screening. Cancer. 2006;107(8):1769‐1776. [DOI] [PubMed] [Google Scholar]
- 25. Turchetti D, Cortesi L, Federico M, et al. BRCA1 mutations and clinicopathological features in a sample of Italian women with early‐onset breast cancer. Eur J Cancer. 2000;36(16):2083‐2089. [DOI] [PubMed] [Google Scholar]
- 26. Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20(6):1480‐1490. [DOI] [PubMed] [Google Scholar]
- 27. The Cancer Genome Atlas Research Network . Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedman LS, Gayther SA, Kurosaki T, et al. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997;60(2):313‐319. [PMC free article] [PubMed] [Google Scholar]
- 29. Ottini L, Silvestri V, Rizzolo P, et al. Clinical and pathologic characteristics of BRCA‐positive and BRCA‐negative male breast cancer patients: results from a collaborative multicenter study in Italy. Breast Cancer Res Treat. 2012;134(1):411‐418. [DOI] [PubMed] [Google Scholar]
- 30. Laforest F, Kirkegaard P, Mann B, Edwards A. Genetic cancer risk assessment in general practice: systematic review of tools available, clinician attitudes, and patient outcomes. Br J Gen Pract. 2019;69(679):e97‐e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lunsford NB, Sapsis KF, Smither B, Reynolds J, Wilburn B, Fairley T. Young women's perceptions regarding communication with healthcare providers about breast cancer, risk, and prevention. J Womens Health (Larchmt). 2018;27(2):162‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campacci N, de Lima JO, Carvalho AL, et al. Identification of hereditary cancer in the general population: development and validation of a screening questionnaire for obtaining the family history of cancer. Cancer Med. 2017;6(12):3014‐3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kessler S. Psychological aspects of genetic counseling. XI. Nondirectiveness revisited. Am J Med Genet. 1997;72(2):164‐171. [DOI] [PubMed] [Google Scholar]
- 34. Cortesi L, Canossi B, Battista R, et al. Breast ultrasonography (BU) in the screening protocol for women at hereditary‐familial risk of breast cancer: has the time come to rethink the role of BU according to different risk categories? Int J Cancer. 2019;144(5):1001‐1009. [DOI] [PubMed] [Google Scholar]
- 35. Manchanda R, Patel S, Gordeev VS, et al. Cost‐effectiveness of population‐based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in unselected general population women. J Natl Cancer Inst. 2018;110(7):714‐725. [DOI] [PubMed] [Google Scholar]
- 36. Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, Aziz H. The role of genetic testing in patients with breast cancer: a review. JAMA Surg. 2017;152(6):589‐594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


