Abstract
Objective:
Compared to the general U.S. population, smokers with comorbid psychiatric and/or substance use disorders (SUD) have lower quit rates after evidence-based treatments, and disproportionately high smoking-related deaths. Improved modalities for reducing tobacco-related harm in this subpopulation are needed. Because electronic cigarettes (e-cigarettes) can now deliver physiologically relevant levels of nicotine to consumers, they represent an additional nicotine delivery system that could be used in cessation interventions. While current data suggests that the use of e-cigarettes by smokers promotes a reduction in combustible cigarette use, smoking quit rates through use of e-cigarettes appears to be low. The goal of this study was to examine impact of e-cigarette use on combustible tobacco use, as well as on the readiness to quit smoking and changes in nicotine dependence, in a multi-morbid population.
Methods:
We conducted a 4-week, open label study in 43 military veteran smokers who had no immediate intention to stop smoking and were currently receiving psychiatric services from the Department of Veterans Affairs (VA) healthcare system. Participants were provided with a study e-cigarette they could use ad libitum along with other tobacco products, and were encouraged to attend weekly laboratory visits and a one-month follow-up visit. Main outcome measures were number of cigarettes smoked per day (CPD), the frequency of e-cigarette use, the amount of money spent on combustible cigarettes (U.S. dollars/week), alveolar carbon monoxide (CO) levels, and urine cotinine levels.
Results:
Mean e-cigarette use was 5.7 days per week and only 9% of participants used the e-cigarette for less than 4 days per week. Significant reductions in breath CO (9.3 ppm to 7.3 ppm, p < 0.02) and CPD (from 16.6 to 5.7, p < 0.001) were observed across study weeks, and no serious adverse events were reported. Three participants (10% of completers) reported smoking cessation that was corroborated biochemically. At one-month follow-up, motivation to quit smoking remained significantly higher, and the level of nicotine dependence was significantly lower, than at baseline.
Conclusions:
E-cigarettes are acceptable to smokers with psychiatric comorbidities, as indicated by sustained and frequent e-cigarette use by 90% of participants, and may promote reduction and/or cessation of combustible cigarette use. E-cigarettes appear to be a viable harm reduction modality in smokers with psychiatric comorbidities.
Keywords: Electronic cigarettes, e-cigarettes, cigarettes, smoking, tobacco harm reduction
Introduction
Cigarette smoking is the main cause of preventable death in developed countries (Jamal et al., 2014) and is likely to be a major contributor to the 20 to 25 year reduced life expectancy in smokers with severe comorbid psychiatric disorders (Colton & Manderscheid, 2006). Smokers with psychiatric comorbidities begin smoking at an earlier age, consume more cigarettes, are more dependent on tobacco, and are less likely to quit smoking, than healthy comparators (Hall & Prochaska, 2009; Hays et al., 1999; Jamal et al., 2014; Kelly et al., 2012; Smith, Mazure, & McKee, 2014). Given the especially low success rate of smoking cessation in this comorbid population, novel treatment approaches are needed.
While a variety of cross-sectional (Brown et al.,2014) uncontrolled prospective (Adriaens et al., 2014; Beiner & Hargraves, 2015; James et al., 2016; Nolan et al., 2016; Polosa et al., 2014; Polosa et al., 2011) and preliminary controlled studies (Bullen et al., 2013; Caponnetto et al., 2013; Tseng et al., 2016) suggest that electronic cigarettes (e-cigarettes) may be useful in reducing tobacco-related harm, quantifying the nature and magnitude of any net population health benefits from the use of e-cigarettes by smokers is a complex endeavor (Kalkhoran & Glantz, 2015; Levy et al., 2017; Polosa et al., 2017). However, numerous chemical analytic studies have demonstrated that e-cigarettes contain significantly lower levels of toxic and carcinogenic substances compared to mainstream smoke from combustible cigarettes. In addition, clinical studies have demonstrated a reduction in tobacco-related toxin exposure in both smokers transitioning to e-cigarette use (Goniewicz et al., 2017; McRobbie et al., 2015), and in former smokers with sustained exclusive e-cigarette use (Shahab et al., 2017). Consequently, e-cigarettes represent a potentially less hazardous nicotine delivery system that could be incorporated into harm-reduction approaches for smokers who have failed evidence-based therapies (Benowitz & Fraiman, 2017; Chen, Bullen, & Dirks, 2017; Goniewicz et al., 2014; McAuley et al., 2012).
Among smokers with psychiatric comorbidities, rates of e-cigarette use are high, with rates of dual-use that may be higher than in the general population (Cummins et al., 2014; Hefner et al., 2016; Hefner, Valentine, & Sofuoglu, 2017). This may reflect the more severe nicotine dependence in this subpopulation, socioeconomic factors, or a different set of motivations for using e-cigarettes. For example, while the majority of respondents in cross-sectional studies identify smoking reduction / cessation as a primary reason for using e-cigarettes (Dawkins et al., 2013; Etter & Bullen, 2011; Goniewicz et al., 2017), preliminary cross-sectional data from smokers with psychiatric comorbidities indicate that the ability to use e-cigarettes in non-smoking locations may be most important (Hefner et al., 2016). In addition, although this subpopulation endorses similar beliefs to those of the general population regarding e-cigarette safety and efficacy as a quitting aid, it is unknown whether these beliefs are associated with similar or differential impact of e-cigarette use on smoking cessation.
While there are currently no published randomized-controlled studies of the impact of e-cigarette use on smoking in individuals with psychiatric comorbidities, several small open label studies have been conducted. For example, in methadone-maintained smokers (n = 12) that attempted smoking cessation with concurrent e-cigarette use, e-cigarette adherence rates were high across six weeks, and significant reductions in smoking were observed (Stein et al., 2016). Similarly, smoking reduction was also observed in a 4-week study of smokers with serious mental illness (n = 19) who were provided with e-cigarettes and instruction on how to use them (Pratt et al., 2016), as well as in a one-year prospective study of smokers with chronic schizophrenia (n = 14) who used e-cigarettes without an intention to quit smoking (Caponnetto et al., 2013). However, this latter sample did not include smokers with a history of comorbid substance use disorders (SUD) and the Pratt study did not report on substance use diagnoses. The development of smoking cessation interventions for individuals with comorbid SUD may be particularly significant because compared to those without this comorbidity, smokers with SUD die prematurely (Bandiera et al., 2015; Colton & Manderscheid, 2006). Futhermore, in smokers with remitted SUD, active attempts at smoking cessation can promote sustained abstinence while persistent smoking is correlated with drug relapse (Stuyt, 2014).
The primary purpose of this small, open label, pilot study was to examine the impact of four weeks of ad libitum e-cigarette use on smoking behavior in military veterans with comorbid psychiatric and SUD, who were not currently attempting to quit smoking. In addition, because the validity of self-reported e-cigarette use has not been established, and may be especially problematic in e-cigarette-naïve users, a secondary aim was to assess e-cigarette use frequency in a naturalistic setting by using a commercial e-cigarette that stores use data, thereby maximizing the precision and ecological validity of this measure. Finally, self-report and biochemical measures of smoking-related behavior were collected across four weeks of e-cigarette use, and at one month follow-up, to document changes in combustible tobacco use.
Methods
Sixty-three veteran smokers, age 18 years or older were recruited from within the Department of Veterans Affairs (VA) Connecticut Healthcare System by word of mouth. Eligible candidates were without an immediate intention to stop smoking, as assessed by the question ‘Are you currently enrolled in a quit smoking program, or intend to stop smoking in the next 30 days?’ A smoking history of at least five cigarettes per day for the past year was required and active smoking status was confirmed by a semi-quantitative urine cotinine >100 ng/ml (NicAlert). Other exclusionary criteria included current untreated medical or psychiatric and/or SUD as determined by a review of the veteran’s electronic medical record, current use of nicotine replacement or other cessation pharmacotherapies, and use of e-cigarettes or smokeless tobacco products for more than two of the past 30 days. This last criterion was established to avoid excluding veterans reporting recent experimentation or curiosity about e-cigarettes, or occasional use of other nicotine products, while excluding those with more extensive experience administering nicotine from products other than combustible cigarettes, to minimize confounding effects on study outcomes including total nicotine intake and use of tobacco cigarettes. Using these criteria, 50 veterans were eligible for participation. Participants were paid $30 USD for screening, $20 for the adaptation session, $20 for weekly visits and $30 for the follow up visit. At the end of the study, either a study physician or research nurse reviewed available smoking cessation services within the VA. For those participants interested in continued e-cigarette use after study completion, recommendations for reasonably priced, effective e-cigarettes were provided. The study was conducted in accordance with the Declaration of Helskinki and approved by the VA Connecticut Healthcare Human Subjects Subcommittee.
E-cigarette flavor sampling and choice procedure:
In order to maximize ecological validity and e-cigarette acceptability, eligible participants first sampled a menu of six different e-liquids (see flavor details below). Because participants could switch e-liquids at any point during the e-cigarette phase, this sampling also allowed for the detection of acute intolerance to all possible study e-liquid ingredients. Fifty participants sampled the e-liquids and completed the adaptation session (Figure 1).
Figure 1.
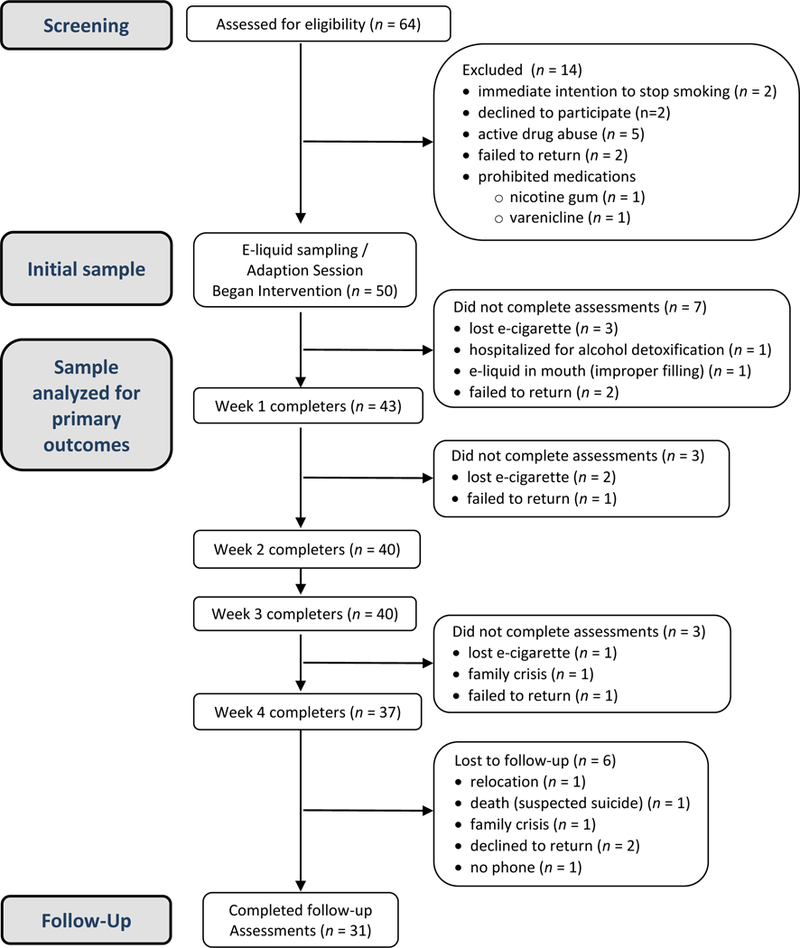
Participant Flow
Adaptation Session:
Participants were taught how to use the study e-cigarette and how to refill the e-liquid tank, and were then given two e-liquid bottles (5 ml each) for the first week. Additional bottles were dispensed as needed for heavier e-cigarette use, or after requests to sample other e-liquids. Participants were informed they could use the study e-cigarette and/or regular tobacco cigarettes, ad libitum, during study participation. The recommended starting power was 10.0 watts (4.2 volts), but it could be easily adjusted to optimize vaping satisfaction.
E-Cigarette:
In order to improve the precision of our use estimate, we selected the eVic Supreme (Joyetech™ USA, Irvine, CA), a commercial, variable power, tank-type device that allows for the downloading of the time, duration and power setting of each puff using a laptop PC and proprietary software (Joytech, MyVapors). The e-cigarette was equipped with a 6.5 ml tank (Delta 23, Joyetech™) and a C3 triple coil atomizer head (Joyetech™) with a total resistance of 1.8 ohms.
E-liquid Ingredients:
E-liquids were prepared by Pace Engineering Concepts (Delafield, WI, USA) in compliance with International Organization for Standardization (ISO) 9001:2008 and Current Good Manufacturing practices (cGMP). All e-liquids were stored at room temperature in sealed amber glass bottles (for flavor sampling), or 5 ml brown plastic, child-resistant, screw top bottles (for weekly e-cigarette use), until dispensing. The base liquid consisted of approximately 50% propylene glycol (PG) United States Pharmacopeia (USP) grade and 50% vegetable glycerin (VG) USP grade (net wt. %). Although the PG:VG ratio is a matter of taste, a 50:50 ratio is widely used in commercial products and provides both the ‘throat hit’, and the visual/sensory experiences of smoking that are critical determinants for e-cigarette acceptability by smokers transitioning to e-cigarette use (Barbeau, Burda, & Siegel, 2013).
For mentholated e-liquids, USP natural menthol flavor at 2.7% (net wt. %) was used to produce an overall sensory impact similar to that expected by menthol-preferring smokers. For tobacco-flavored e-liquids, a ‘burley’ tobacco flavor simulating the robust taste of many popular tobacco cigarettes, and a ‘slim’ tobacco flavor that is sweeter and lighter for smokers who prefer less intense tobacco flavor, were prepared. L-Nicotine USP/EP (European Pharmacopoeia) grade nicotine at concentrations of 12 and 24 mg/ml (net wt. %) were added to each of the three flavors. These nicotine levels are squarely within the range of marketed e-cigarette products (3 mg/ml to 36 mg/ml) and are sufficiently high that plasma nicotine levels similar to that of smoking tobacco cigarettes might be achieved, even by inexperienced e-cigarette users (Lopez et al., 2016; Vansickel, Weaver, & Eissenberg, 2012). While nicotine concentration is not the only determinant of nicotine delivery and subsequent plasma nicotine levels, it is an important factor that can be easily controlled experimentally (Lopez et al., 2016; Ramoa et al., 2016).
By providing participants with a choice of flavors and nicotine concentrations, and an e-cigarette with variable power, we intended to maximize the possiblity that a ‘sweet-spot’ would be identified by each participant that would ensure the e-cigarettes were sufficiently appealing, satisfying and consistent with a naturalistic experience (Hajek et al., 2017). The nicotine concentration in all e-liquids were verified by in-house assay.
Quantitative Measures
The main outcomes included the number of cigarettes smoked per day (CPD), the frequency of e-cigarette use (mean days/week), the amount of money spent on combustible cigarettes (U.S. dollars/week), and biochemical measures of alveolar (breath) carbon monoxide (CO) levels (ppm), and of urine cotinine levels (ng/ml).
CO levels were obtained using a factory-calibrated CO monitor (Vitalograph Inc., Lenexa, KS). Quantitative urine cotinine levels were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) employing a deuterated internal standard as reported (Sofuoglu et al., 2012), but adapted to urine. Between day coefficients of variation ranged from 4.5% (at 10 ng/mL) to 2.3% (at 2,000 ng/mL). Urine specimens were stored at −20 degrees Celsius until analysis.
E-cigarette use per day was operationalized as at least five consecutive e-cigarette activations of longer than 2 seconds in duration within a 30 minute window for a given 24 hour period. It is highly unlikely that e-cigarette use data meeting this criterion would occur by inadvertent or accidental e-cigarette activations. Conversely, organized attempts to receive nicotine from the e-cigarette would not likely be represented by short duration (0.5 to 1.0 second) activations randomly scattered throughout an individual use record. Therefore, we believe that this minimal use criterion captures a behavioral record of deliberate intent to self-administer nicotine from the e-cigarette and is consistent with previously published e-cigarette topography studies (Behar, Hua & Talbot, 2014). CPD and money spent on cigarettes were assessed with Time Line Follow Back (TLFB) methods (Miller & Del Boca, 1994). All assessments were completed during weekly visits. Secondary measures included the Fagerstrom Test of Nicotine Dependence (FTND) (Heatherton et al.,1991) and the Contemplation Ladder, a validated measure of the readiness to consider smoking cessation (Biener & Abrams, 1991).
Qualitative Measures
Participants completed an e-cigarette Questionnaire at baseline (Pre) and follow-up (Post). The questionnaire items were developed by the research team and colleagues and/or adapted from published surveys (Camenga et al., 2015; Hefner et al., 2016; Kong et al., 2015). They assessed changes in perceptions about e-cigarettes (e.g., harmfulness, benefits, cost), motivations to use (or not use) them, and the reasons for e-cigarette or combustible cigarette preferences. At follow-up, the Post-E-cigarette Questionnaire also assessed whether participants had purchased, or planned to purchase an e-cigarette.
Data Analysis
All quantitative outcome measures were assessed for normality and analyzed using models for repeated measures (linear mixed effects models for all variables except days of e-cigarette use which was analyzed using Generalized Estimating Equations (GEE) with binomial distribution and logit link). Transformations were applied to variables with positive skewness before fitting mixed models. In all models there was a fixed effect of time. The best fitting variance-covariance structure was selected based on Akaike’s Information criterion. Post-hoc pairwise comparisons among time points were performed to explain significant effects in the models. Least square means and 95% confidence intervals by time point are also presented.
Results
Baseline demographics are presented in Table 1. Menthol cigarettes were preferred by 86% of participants who were 93% male, 67% African-American, with a mean (SD) age of 56.9 (8.0) and a smoking intensity of 16.6 (9.4) cigarettes/day. The baseline FTND score was 4.9 (2.1) and the exhaled CO level was 9.3 (7.1). Participants had multiple medical, psychiatric and substance use disorders.
Table 1.
Baseline Measures
| Baseline Demographics (N = 43) | N (%) |
|---|---|
| Sex | |
| Male | 40 (93%) |
| Female | 3 (7%) |
| Race | |
| African American | 29 (67%) |
| Caucasian | 12 (28%) |
| Pacific Islander | 1 (2%) |
| American Indian | 1 (2%) |
| Menthol-preferring smokers | 37 (86%) |
| Major Psychiatric Disorders | |
| Posttraumatic Stress Disorder | 18 (42%) |
| Depression | 15 (35%) |
| Anxiety | 7 (16%) |
| Schizoaffective | 4 (8%) |
| Bipolar | 2 (5%) |
| Attention Deficit Hyperactivity Disorder | 2 (5%) |
| Schizophrenia | 1 (2%) |
| Substance Use Disorders | |
| Alcohol | 22 (51%) |
| Cocaine | 19 (45%) |
| Opiate | 16 (37%) |
| Opiate Agonist Therapy | 14 (33%) |
| Cannabis | 7 (16%) |
| Sedative | 1 (2%) |
| Medical Diagnoses | |
| Hypertension | 23 (53%) |
| Chronic neck / back pain | 18 (42%) |
| Hepatitis C | 18 (42%) |
| Chronic obstructive pulmonary disease / emphysema | 13 (30%) |
| Diabetes | 11 (26%) |
| Peripheral neuropathy | 11 (26%) |
| Obstructive sleep apnea | 10 (23%) |
| Gastro-esophageal reflux | 9 (21%) |
| Cardiovascular disease | 4 (14%) |
| Human immunodeficiency virus | 4 (9%) |
| * Standard Deviation | Mean (SD*) |
| Age, years | 56.9 +/− 8.0 |
| Baseline Biochemical and Self-Reported Smoking Indices | |
| Expired carbon monoxide (CO), ppm | 9.3 (7.1) |
| Urine cotinine, ng/ml | 1161 (975.2) |
| Cigarettes smoked / day | 16.6 (9.4) |
| Longest estimated abstinence (months) | 13.9 (21.7) |
| Number of self-reported quit attempts | 15.7 (27.4) |
| Baseline Fagerstrom Test for Nicotine Dependence (FTND) score | 4.9 (2.1) |
Note. CO = Carbon monoxide; FTND = Fagerstrom Test for Nicotine Dependence; SD = Standard deviation.
Mean e-cigarette use frequency during each week of the study period was 5.7 (1.6) days per week. The cumulative percentage of e-cigarette use per day by participants across the 4 study weeks was 9% (less than four days), 26% (4–5 days), and 65% (6–7 days). There were no significant differences in mean daily e-cigarette use per week over the 4-week study period [χ2 = 5.45, p = 0.14)].
CO levels decreased over the 4-week study period [main effect of time: F(5,183) = 2.64, p = 0.02)], with a significant decrease from baseline to week’s 1–4 (p < 0.02) (Figure 2). The difference between CO levels at baseline and follow-up assessment was not statistically significant (p = 0.14). For cotinine levels, no significant changes were observed at any time point (Figure 3) [main effect of time: F(5,33.4) = 1.78, p = 0.14)].
Figure 2.
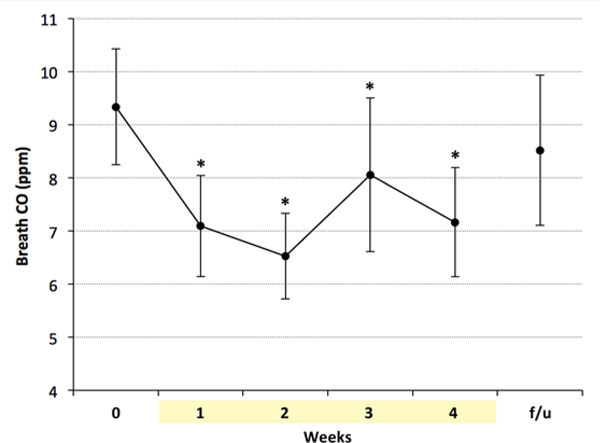
Breath CO levels were significantly lower than baseline during 4 weeks of e-cigarette use. * indicates p < 0.02 vs. baseline. CO = Carbon monoxide; f/u = follow-up.
Figure 3.
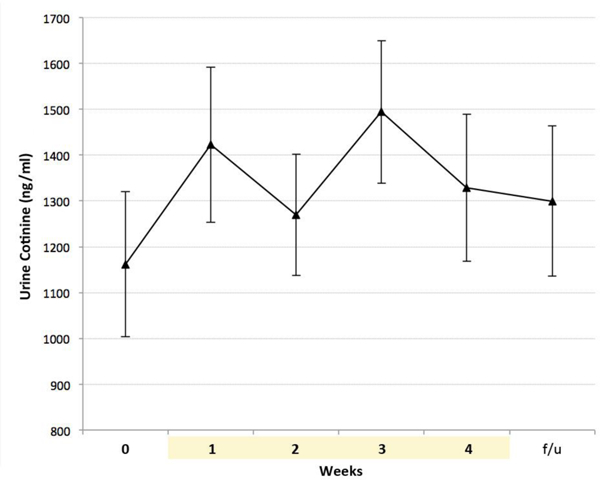
All values for urine cotinine levels were non-significantly higher as compared to baseline levels. f/u = follow-up
There was a significant reduction in CPD over time (Figure 4) [main effect of time: F(5,36.4) = 19.26, p < 0.0001, with a significant decrease from baseline to week’s 1–4, and at follow-up (p < .0001). CPD at follow-up were significantly lower than at baseline, but significantly higher than at weeks 2–4 (p < .01).
Figure 4.
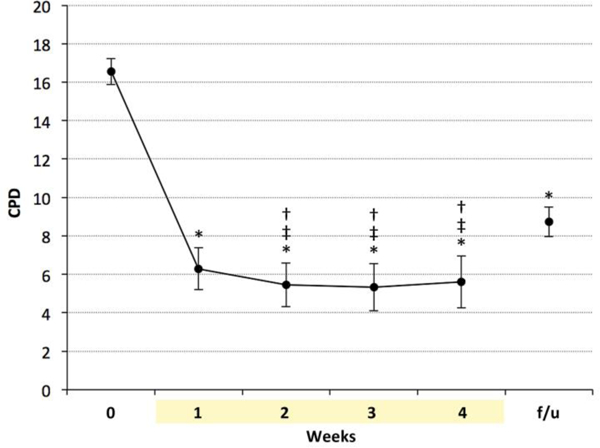
Self-reported CPD during e-cigarette use were significantly lower than baseline, and remained significantly lower at one-month follow-up. * indicates p < 0.0001 vs. baseline, ‡ indicates p < 0.02 baseline, † indicates p < 0.01 vs. follow-up. CPD = cigarettes per day; f/u = follow-up.
Participants reported spending less money on cigarettes over time (Figure 5) [main effect of time (F(5,35) = 10.16, p < 0.0001), with a significant decrease from baseline to week’s 1–4 and follow-up (p<.0001). Money spent at follow-up was significantly lower than at baseline, but significantly higher than at weeks 3 and 4 (p < .01).
Figure 5.
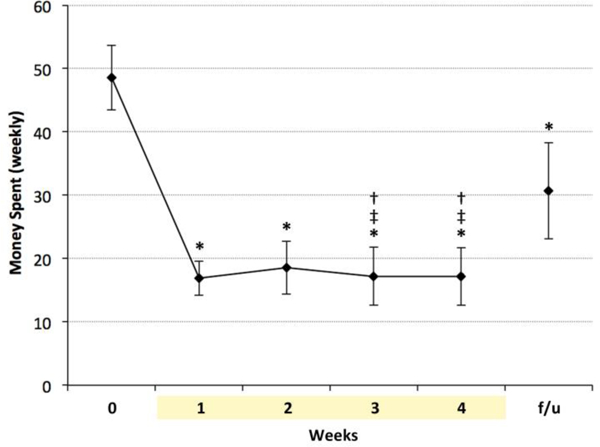
Money spent per week on cigarettes during e-cigarette use was significantly lower than at baseline, and remained significantly lower at one-month follow-up. * indicates p < 0.0001 vs. baseline, ‡ indicates p < 0.05 vs week 1, † indicates p < 0.005 vs. follow-up. f/u = follow-up
There was a statistically significant effect of time (F(5,38.8) = 4.43, p = 0.003) on motivation to quit smoking (Figure 6), with a significant increase in Contemplation Ladder scores from baseline and week one to week’s 2–4 (p < .05). Motivation to quit remained higher at follow-up as compared to baseline (p = 0.01). In addition, there was a significant decrease in FTND from baseline to follow-up (F(1,31.2) = 10.4, p = 0.003) (Figure 7).
Figure 6.
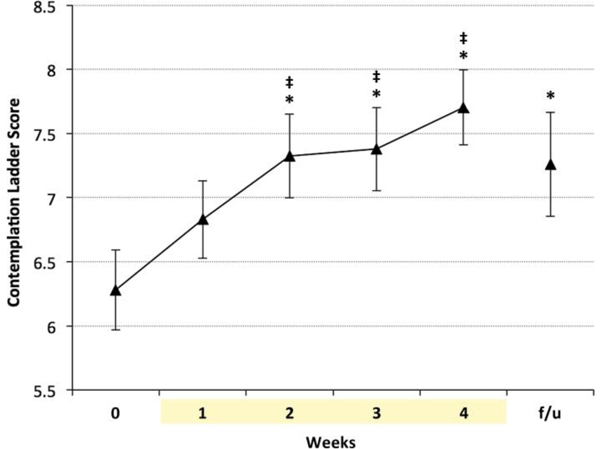
Readiness to quit smoking was significantly higher at weeks 2, 3 and 4 and remained higher at one- month follow-up. * indicates p < 0.0001 vs. baseline, ‡ indicates p < 0.05 vs. week 1. f/u = follow-up.
Figure 7.
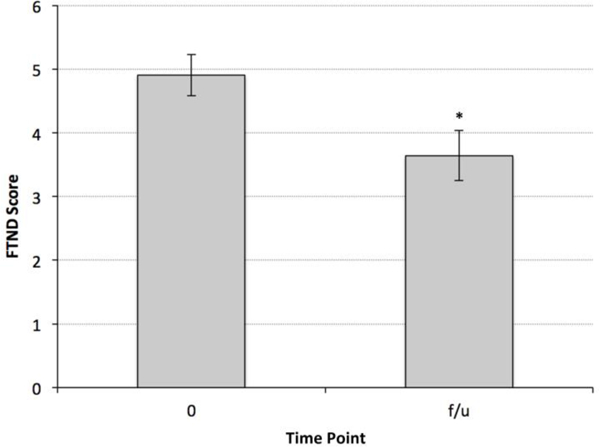
The level of nicotine dependence at one-month follow-up, as assessed by the total FTND score, was significantly lower than baseline. * indicates p < 0.003 vs. baseline. FTND = Fagerstrom Test for Nicotine Dependence; f/u = follow-up.
Survey Results:
Pre- and Post-E-cigarette Questionnaire results are presented in Table 2 and selected findings are highlighted below. The data set includes only those subjects that completed both the e-cigarette phase and one-month follow-up (n = 30). At the end of the study, over two-thirds of participants purchased, or were planning to purchase their own e-cigarette (38% already purchased one), and 43% responded that they now preferred e-cigarette use to smoking (increase from 17% at baseline), while only 7% had no preference (down from 47% at baseline). In contrast, a comparable proportion at baseline (27%) and follow-up (30%) reported a preference for combustible tobacco.
Table 2.
Qualitative Measures
| Pre / Post E-cigarette (EC) questionnaire | N (N = 30) |
Percent of starters |
N (N=30) |
Percent of completers |
|---|---|---|---|---|
| Do you think e-cigarettes are harmful? | ||||
| No | 15 | 50.0 | 19 | 63.3 |
| Yes | 4 | 13.3 | 3 | 10.0 |
| No Opinion | 11 | 30.0 | 3 | 10.0 |
| Do you think e-cigarettes should be regulated? | ||||
| No | 9 | 30.0 | 7 | 23.3 |
| Yes | 10 | 33.3 | 14 | 46.7 |
| No Opinion | 11 | 36.7 | 4 | 13.3 |
| Do you prefer one over the other? | ||||
| E-cigarettes | 5 | 16.7 | 13 | 43.3 |
| Combustible Cigarettes | 8 | 26.7 | 9 | 30.0 |
| No preference | 14 | 46.7 | 2 | 6.7 |
| It depends on the situation | 2 | 6.7 | 1 | 3.3 |
| Why do you prefer one over the other? (select all that apply) | ||||
| Strength | 5 | 16.7 | 5 | 16.7 |
| Smoothness | 6 | 20.0 | 9 | 30.0 |
| Taste | 9 | 30.0 | 6 | 20.0 |
| Social acceptability | 4 | 13.3 | 0 | 0.0 |
| Cost | 9 | 30.0 | 8 | 26.7 |
| Other | 0 | 0.0 | 5 | 16.7 |
| Have you used EC to reduce smokeless tobacco use? (yes) | 10 | 33.3 | 13 | 43.3 |
| Do you believe EC are as addictive as tobacco? (yes) | 7 | 23.3 | 14 | 46.7 |
| Reasons why you use, or may use, an e-cigarette (select all that apply): | ||||
| Save Money | 17 | 56.7 | 21 | 70.0 |
| To use in places where I can’t smoke | 14 | 46.7 | 15 | 50.0 |
| E-cigarettes are better for my health | 13 | 43.3 | 15 | 50.0 |
| E-cigarette vapor is less harmful to others | 10 | 33.3 | 17 | 56.7 |
| My family/friends prefer my use of e-cigarette | 7 | 23.3 | 9 | 30.0 |
| E-cigarettes taste better / enjoy a variety of flavors | 6 | 20.0 | 10 | 33.3 |
| E-cigarettes set a better example for others | 4 | 13.3 | 5 | 16.7 |
| Less judgment by others | 6 | 20.0 | 4 | 13.3 |
| E-cigarettes reduce the amount that I smoke | 13 | 43.3 | 18 | 60.0 |
| E-cigarettes allowed me to completely quit smoking | 5 | 16.7 | 5 | 16.7 |
| E-cigarettes reduce the amount I use other tobacco products | 9 | 30.6 | 12 | 40.0 |
| How important is the availability of flavors in using e-cigarettes? | ||||
| Not important | 10 | 33.3 | 6 | 20.0 |
| Somewhat important | 7 | 23.3 | 6 | 20.0 |
| Very important | 9 | 30.0 | 13 | 43.3 |
Note. EC = E-cigarette
In addition, 10% of the participants that completed all 4 weeks and all follow-up visit ratings (3/30) reported they had quit smoking. All three participants reported stopping during the first week of the study, and this self-report was corroborated by the progressive decrease in mean breath CO levels from a baseline of 9.3 (1.5) to 1.8 (2.1) over the 4 weeks of reported exclusive e-cigarette use. Two of these participants reported continued smoking cessation at follow-up, and sustained cessation was supported by measuring expired CO levels of 1 ppm.
Motivations for using e-cigarettes were largely consistent across the study period, although at follow-up, a larger proportion indicated that e-cigarettes are less harmful to others than smoking (57% vs. 33% at baseline), reduce one’s smoking (60% vs. 43%), save money (70% vs. 57%), taste better than cigarettes (33% vs 20%), incur less judgment from others (20% vs. 13%), and reduce other tobacco use (40% vs. 31%). Approximately one-half of participants indicated at both baseline and follow-up, that use of e-cigarettes where one cannot smoke is a reason for e-cigarette use.
Nearly half (47%) of participants at follow-up felt e-cigarettes should be regulated like tobacco (increase from 33% at baseline), although 63% of participants indicated believing e-cigarettes are not harmful (increase from 50% at baseline).
At baseline, 33% of participants indicated flavor variety was ‘not important’ to their e-cigarette use and 30% indicated flavors were ‘very important’. At follow-up, the proportion indicating that e-cigarette flavor was ‘very important’ increased to 43%, and the belief that e-cigarettes were as addictive as tobacco products increased from 23% to 47%.
Discussion
In this small, unblinded, uncontrolled longitudinal study, e-cigarette use was acceptable to smokers with psychiatric comorbidities who continued to use combustible cigarettes (i.e. dual-use). The mean frequency of e-cigarette use was 5.7 days per week with over 90% of the participants using the e-cigarette at least four days per week. No serious adverse events were reported, however we could not ascertain whether adverse events were a factor in the attrition of four participants who did not return to our research clinic. E-cigarette use was associated with significant decreases in combustible cigarette use, decreases in breath CO levels, and an increase in motivation to stop smoking. Moreover, for 10% of completers, e-cigarette use appeared to have facilitated cessation, despite the lack of an immediate intention to stop smoking at study entry. Collectively, these findings are consistent with prior studies demonstrating that e-cigarette use by smokers can assist with an overall reduction in combustible cigarette use, and may promote cessation in a minority of adult smokers. However, our findings are particularly promising because our convenience sample of smokers with psychiatric and medical comorbidities represents a subpopulation that would typically be excluded from clinical trials examining novel pharmacological interventions for smoking cessation, and for whom existing evidence-based treatments are especially ineffective.
Although a full understanding of the long-term consequences of either exclusive use of e-cigarettes, or of dual-use, will require prospective studies, our findings demonstrate the acceptability and short-term tolerability of dual-use within a multi-morbid population. Additional data will be required to understand the factors driving different patterns of e-cigarette use once it has been initiated, and to identify the effects on individual and population health from variations in the amount of tobacco product exposure along the continuum of e-cigarette / combustible cigarette use. Interestingly, in the present study, over 43% of participants preferred e-cigarette to smoking at follow-up, yet only 10% stopped smoking suggesting that initiation of e-cigarette use alone is insufficient to produce cessation in the large majority of smokers. However, the use of e-cigarette by smokers may increase the probability of quitting at a later date. For example, smokers who also use e-cigarette daily are more interested in quitting smoking, or trying to reduce their smoking, than those who do not use e-cigarettes, and survey respondents frequently report the use of e-cigarettes for quitting or reducing cigarette smoking (Dawkins et al., 2013). Our finding of a significantly increased motivation to quit smoking that was sustained at one-month follow-up, suggests the possibility that for some smokers, dual-use represents an interim state of harm reduction which may lead to cessation (Rutten et al., 2015).
Because e-cigarettes closely mimic the form and function of combustible cigarettes, and allow for the preservation of valued ritualistic and social behavior associated with nicotine self-administration from smoking, the utility of e-cigarettes as a form of nicotine replacement is theoretically superior to other forms of nicotine replacement therapies (NRT) (Barbeau et al., 2013; Caponnetto et al., 2017; Franck et al., 2016; Polosa et al., 2013). In addition, because e-cigarettes can produce plasma nicotine levels comparable to those of smoked tobacco products, they may be superior to NRT that have a slower rate of nicotine delivery and/or yield lower levels of plasma nicotine (Aubin, Luquiens, & Berlin, 2014). Although plasma nicotine levels were not measured in this study, urine cotinine levels increased non-significantly during e-cigarette use (all weekly mean values were higher than baseline). This stability of cotinine levels may represent nicotine self-titration by participants who used the e-cigarette to deliver behaviorally-relevant levels of nicotine to offset the reduction in nicotine intake that resulted from a decrease in combustible cigarette use. While nicotine, itself, has potential adverse health effects (Benowitz & Fraiman, 2017), given the well-established and serious negative health consequences from combustible use, net public health benefits may accrue from e-cigarette use despite the absence of any overall change in exposure to nicotine within this otherwise treatment-refractory subpopulation of smokers.
This study has several significant strengths. First, self-reported reduction in smoking was verified biochemically, and quantification of e-cigarette use did not rely upon a measure with unknown validity. Second, the measurement of cotinine levels provide additional insight into the role e-cigarettes may play in harm reduction approaches by demonstrating stable overall nicotine intake despite reduced combustible cigarette use, a pharmacological profile consistent with a basic tenet of tobacco-harm reduction that seeks to reduce smoking-related morbidity and mortality while accepting the likelihood of continued exposure to nicotine. Finally, our observations have improved ecological validity over prior studies that used a single e-cigarette condition because participants were able to select e-liquids from a ‘menu’ of options, as they might at a vape retailer. This may have contributed to the wide appeal of e-cigarettes among study completers, many of whom ended up preferring e-cigarettes to combustible tobacco, and either purchased, or planned to purchase, their own e-cigarette. Indeed, flavor variety was as a more important aspect of participant’s e-cigarette experience at follow-up. However, until studies are performed that utilize a randomly assigned comparator, the magnitude of any effects e-liquid choice, and the ability to adjust the performance of the e-cigarette, may have on subsequent smoking behavior remains unknown.
A primary limiting factor of this study is the absence of a control group that would exclude the possibility that factors unrelated to e-cigarette use were responsible for the observed changes. However, this limitation is partially mitigated by our finding of stable cotinine levels, despite significantly decreased combustible use, suggesting that changes in smoking behavior were partly due to the e-cigarette effectively delivering nicotine. This assertion is supported by the 100% increase from baseline in the number of participants that believed e-cigarettes were as addictive as tobacco products. Finally, we used relatively high nicotine concentrations and an e-cigarette with improved performance capabilities to increase the probability that participants maintained, or achieved, physiologically relevant plasma levels of nicotine from e-cigarette use (Hajek et al., 2017). However, we cannot exclude the possibility that e-cigarette use was also motivated by economic factors due to the availability of a free source of nicotine. This possibility is consistent with the finding that both self-reported CPD and CO levels increased at follow-up, and this may be secondary to increased combustible cigarette use after an economic incentive for e-cigarette use had been removed. Future studies need to address this important confound using designs that minimize the effect of economic variables.
An additional study limitation is the disparity in race (67% African American), gender (93% male) and menthol preference (86% menthol smokers) within our study population. Although these demographic variables are representative of the veteran population, they limit the generalizability of our findings to non-African American, female, and non-menthol smokers. In addition, the interpretation of the changes in CPD and cotinine levels should take into account the potential impact of menthol, race and gender on the amount of combustible cigarette use, CO levels and nicotine clearance (Ross et al, 2016; Shiffman et al., 2014; Watson et al, 2017). Finally, baseline expired CO levels were lower than might be expected based upon self-reported CPD. This may be due to exaggerated reports of cigarette use to assure study entry and/or continued participation, or to constraints on smoking behavior while on the VA hospital premises where no-smoking zones are strictly enforced, therefore contributing to the clearance of CO as participants engaged VA services prior to their weekly study visits. In addition, one-third of our study population frequently presented early in the morning due to restricted methadone clinic hours which may have biased our measurements toward time points when CO levels were at a nadir due to overnight abstinence. Because we did not assess the interval between last cigarette use and expired CO measurements, expired CO levels in this study cannot be considered a very exact quantitative measure of total cigarette consumption.
In summary, this study extends previous findings of reduced combustible cigarette use in smokers who use e-cigarettes, to a subpopulation of smokers with psychiatric comorbidities and no immediate intention to stop smoking. Additional well-controlled, prospective studies of e-cigarettes as a tobacco harm reduction modality in heavy smokers with psychiatric comorbidities are needed to further evaluate the risk-reduction profile of e-cigarette use within this notably difficult to treat population.
Acknowledgments:
We thank Stacy Minnix, Michael J. Stevens, Lance Barnes, Christopher Cryan, Jeremy Merrel, and Ellen Mitchell for their important contributions to this study, including recruitment, administrative support, clinical evaluation of participants and data collection/management. In addition, we thank Tony Pace, PhD, for his contribution of e-liquid manufacturing expertise during the design phase of this study.
Funding: This research was supported by the New England Mental Illness Research, Education and Clinical Center (MIRECC) and the U.S. Department of Veterans Affairs. Statistical analyses, biochemical assays and analyses of e-cigarette solutions were supported by the Administrative and Laboratory cores of P50DA036151 (Yale TCORS) from the National Institutes of Health and the U.S. Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of the U.S. Food and Drug Administration.
Footnotes
Disclosures: Ralitza Gueorguivea, PhD, discloses consulting fees for Palo Alto Health Sciences and Mathematica Policy Research and a provisional patent submission by Yale University: Chekroud, AM, Gueorguieva, R & Krystal, KH. “Treatment Selection for Major Depressive Disorder” (filing date 3rd June 2016, USPTO docket number Y0087.70116US00). The authors report no other financial relationships with commercial interests.
References:
- Adriaens K, Van Gucht D, Declerck P, & Baeyens F (2014). Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. International Journal of Environmental Research and Public Health, 11(11), 11220–11248. doi: 10.3390/ijerph111111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin HJ, Luquiens A, & Berlin I (2014). Pharmacotherapy for smoking cessation: Pharmacological principles and clinical practice. British Journal of Clinical Pharmacology, 77(2), 324–336. doi: 10.1111/bcp.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera FC, Anteneh B, Le T, Delucchi K, & Guydish J (2015). Tobacco-related mortality among persons with mental health and substance abuse problems. PLoS One, 10(3), 1–14. doi: 10.1371/journal.pone.1210581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau AM, Burda J, & Siegel M (2013). Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: A qualitative approach. Addiction Science & Clinical Practice, 8(5),1–7. doi: 10.1186/1940-0640-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar RZ, Hua M, & Talbot P (2014). Puffing topography and nicotine intake of electronic cigarette users. PLoS One, 10(2), e0117222. doi: 10.1371/journal.pone.117222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, & Fraiman JB (2017). Cardiovascular effects of electronic cigarettes. Nature Reviews Cardiology, 14(8), 447–456. doi: 10.1038/nrcardio.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, & Abrams DB (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10(5), 360–365. [DOI] [PubMed] [Google Scholar]
- Biener L, & Hargraves JL (2015). A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: Association with smoking cessation and motivation to quit. Nicotine & Tobacco Research, 17(2), 127–133. doi: 10.1093/ntr/ntu200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Beard E, Kotz D, Michie S, & West R (2014). Real-world effectiveness of e-cigarettes when used to aid smoking cessation: A cross-sectional population study. Addiction, 109(9), 1531–1540. doi: 10.1111/add.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, & Walker N (2013). Electronic cigarettes for smoking cessation: A randomised controlled trial. Lancet, 382(9905), 1629–1637. doi: 10.1016/s0140-6736(13)61842-5 [DOI] [PubMed] [Google Scholar]
- Bullen C,McRobbie H, Thornley S,Glover M, Lin R, & Laugesen M (2010). Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomised cross-over trial. Tobacco Control, 19(2), 98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- Camenga DR, Cavallo DA, Kong G, Morean ME, Connell CM, Simon P, . . . Krishnan-Sarin S. (2015). Adolescents’ and young adults’ perceptions of electronic cigarettes for smoking cessation: A focus group study. Nicotine & Tobacco Research, 17(10), 1235–1241. doi: 10.1093/ntr/ntv020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R (2013). Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: A prospective 12-month pilot study. International Journal of Environmental Research and Public Health, 10(2), 446–461. doi: 10.3390/ijerph10020446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, & Polosa R (2013). EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: A prospective 12-month randomized control design study. PLoS One, 8(6), e66317. doi: 10.1371/journal.pone.0066317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Maglia M, Cannella MC, Inguscio L, Buonocore M, Scoglio C, . . . Vinci V. (2017). Impact of different e-cigarette generation and models on cognitive performances, craving and gesture: A randomized cross-over trial (CogEcig). Frontiers in Psychology, 8, 127. doi: 10.3389/fpsyg.2017.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Bullen C, & Dirks K (2017). A comparative health risk assessment of electronic cigarettes and conventional cigarettes. International Journal of Environmental Research and Public Health, 14(4), e382. doi: 10.3390/ijerph14040382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CW, & Manderscheid RW (2006). Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Preventing Chronic Disease, 3(2), A42. [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3(1), 7–16. doi: 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- Cummins SE, Zhu SH, Tedeschi GJ, Gamst AC, & Myers MG (2014). Use of e-cigarettes by individuals with mental health conditions. Tobacco Control, 23(Suppl 3), iii48–53. doi: 10.1136/tobaccocontrol-2013-051511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Roberts A, & Soar K (2013). ‘Vaping’ profiles and preferences: An online survey of electronic cigarette users. Addiction, 108(6), 1115–1125. doi: 10.1111/add.12150 [DOI] [PubMed] [Google Scholar]
- Eissenberg T (2010). Electronic nicotine delivery devices: Ineffective nicotine delivery and craving suppression after acute administration. Tobacco Control, 19(1), 87–88. doi: 10.1136/tc.2009.033498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, & Bullen C (2011). Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction, 106(11), 2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x [DOI] [PubMed] [Google Scholar]
- Franck C, Filion KB, Kimmelman J, Grad R, & Eisenberg MJ (2016). Ethical considerations of e-cigarette use for tobacco harm reduction. Respiratory Research, 17(1), 53. doi: 10.1186/s12931-016-0370-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, . . . Benowitz N (2014). Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control, 23(2), 133–139. doi: 10.1136/tobaccocontrol-2012-050859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Lingas EO, & Hajek P (2013). Patterns of electronic cigarette use and user beliefs about their safety and benefits: An internet survey. Drug and Alcohol Review, 32(2), 133–140. doi: 10.1111/j.1465-3362.2012.00512.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Przulj D, Phillips A, Anderson R, & McRobbie H (2017). Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology, 234(5), 773–779. doi: 10.1007/s00213-016-4512-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, & Prochaska JJ (2009). Treatment of smokers with co-occurring disorders: Emphasis on integration in mental health and addiction treatment settings. Annual Review of Clinical Psychology, 5, 409–431. doi: 10.1146/annurev.clinpsy.032408.153614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, & Hajek P (2016). Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews, 9, 1–93. CD010216. doi: 10.1002/14651858.CD010216.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JT, Schroeder DR, Offord KP, Croghan IT, Patten CA, Hurt RD, . . . Fiore MC. (1999). Response to nicotine dependence treatment in smokers with current and past alcohol problems. Annals of Behavioral Medicine, 21(3), 244–250. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hefner K, Rosenheck R, Merrel J, Coffman M, Valentine G, & Sofuoglu M (2016). E-cigarette use in veterans seeking mental health and/or substance use services. Journal of Dual Diagnosis, 12(2), 109–117. doi: 10.1080/15504263.2016.1172895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Valentine G, & Sofuoglu M (2017). Electronic cigarettes and mental illness: Reviewing the evidence for help and harm among those with psychiatric and substance use disorders. American Journal on Addictions, 26(4), 306–315. doi: 10.1111/ajad.12504 [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43(3), 289–294. [DOI] [PubMed] [Google Scholar]
- Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, & Neff L (2014). Current cigarette smoking among adults--United States, 2005–2013. Morbidity and Mortality Weekly Report, 63(47), 1108–1112. [PMC free article] [PubMed] [Google Scholar]
- James SA, Meier EM, Wagener TL, Smith KM, Neas BR, & Beebe LA (2016). E-cigarettes for immediate smoking substitution in women diagnosed with cervical dysplasia and associated disorders. International Journal of Environmental Research and Public Health, 13(3), 288. doi: 10.3390/ijerph.13030288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoran S, & Glantz SA (2015). Modeling the health effects of expanding e-cigarette sales in the United States and United Kingdom: A Monte Carlo analysis. JAMA Internal Medicine, 175(10), 1671–1680. doi: 10.1001/jamainternmed.2015.4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PJ, Baker AL, Deane FP, Kay-Lambkin FJ, Bonevski B, & Tregarthen J (2012). Prevalence of smoking and other health risk factors in people attending residential substance abuse treatment. Drug and Alcohol Review, 31(5), 638–644. doi: 10.1111/j.1465-3362.2012.00465.x [DOI] [PubMed] [Google Scholar]
- Kong G, Morean ME, Cavallo DA, Camenga DR, & Krishnan-Sarin S (2015). Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine & Tobacco Research, 17(7), 847–854. doi: 10.1093/ntr/ntu257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Borland R, Fong GT, Villanti AC, Niaura R, Meza R, . . . Abrams DB. (2017). Developing consistent and transparent models of e-cigarette use: Reply to Glantz and Soneji et al. Nicotine & Tobacco Research, 19(2), 268–270. doi: 10.1093/ntr/ntw236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JC, Bullen C, Newcombe R, Walker N, & Walton D (2013). The use and acceptability of electronic cigarettes among New Zealand smokers. The New Zealand Medical Journal, 126(1375), 48–57. [PubMed] [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramoa CP, Karaoghlanian NV, Lipato T, . . . Eissenberg T. (2016). Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: A preliminary report. Nicotine & Tobacco Research, 18(5), 720–723. doi: 10.1093/ntr/ntv182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley TR, Hopke P, Zhao J, & Babaian S (2012). Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhalation Toxicology, 24(12), 850–857. doi: 10.3109/08958378.2012.724728 [DOI] [PubMed] [Google Scholar]
- Miller WR, & Del Boca FK (1994). Measurement of drinking behavior using the Form 90 family of instruments. Journal of Studies on Alcohol Supplement, (s12), 112–118. [DOI] [PubMed] [Google Scholar]
- Nolan M, Leischow S, Croghan I, Kadimpati S, Hanson A, Schroeder D, & Warner DO (2016). Feasibility of electronic nicotine delivery systems in surgical patients. Nicotine & Tobacco Research, 18(8), 1757–1762. doi: 10.1093/ntr/ntw003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Maglia M, Morjaria JB, & Russo C (2014). Success rates with nicotine personal vaporizers: A prospective 6-month pilot study of smokers not intending to quit. Biomed Central Public Health, 14, 1–9. doi: 10.1186/1471-2458-14-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, & Russo C (2011). Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: A prospective 6-month pilot study. Biomed Central Public Health, 11, 1–12. doi: 10.1186/1471-2458-11-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Niaura R, & Abrams D (2017). Analysis of e-cigarette use in the 2014 Eurobarometer survey: Calling out deficiencies in epidemiology methods. Internal and Emergency Medicine, 12(6),733–35. doi: 10.1007/s11739-017-1667-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Rodu B, Caponnetto P, Maglia M, & Raciti C (2013). A fresh look at tobacco harm reduction: The case for the electronic cigarette. Harm Reduction Journal, 10, 1–11. doi: 10.1186/1477-7517-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt SI, Sargent J, Daniels L, Santos MM, & Brunette M (2016). Appeal of electronic cigarettes in smokers with serious mental illness. Addictive Behaviors, 59, 30–34. doi: 10.1186/1477-7517-10-19 [DOI] [PubMed] [Google Scholar]
- Ramoa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, . . . Eissenberg T. (2016). Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: A preliminary report. Tobacco Control, 25(e1), e6–9. doi: 10.1136/tobaccocontrol-2015-052447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KC, Gubner NR, Tyndale RF, Hawk LW Jr., Lerman C, George TP, . . . Benowitz NL. (2016). Racial differences in the relationship between rate of nicotine metabolism and nicotine intake from cigarette smoking. Pharmacology Biochemistry & Behavior, 148, 1–7. doi: 10.1016/j.pbb.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten LJ, Blake KD, Agunwamba AA, Grana RA, Wilson PM, Ebbert JO, . . . Leischow SJ. (2015). Use of e-cigarettes among current smokers: Associations among reasons for use, quit intentions, and current tobacco use. Nicotine & Tobacco Research, 17(10), 1228–1234. doi: 10.1093/ntr/ntv003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, . . . West R. (2017). Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: A cross-sectional study. Annals of Internal Medicine, 166(6), 390–400. doi: 10.7326/m16-1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, & Benowitz NL (2014). A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiology Biomarkers & Prevention, 23(7), 1264–1272. doi: 10.1158/1055-9965.epi-13-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonavicius E, McNeill A, Arnott D, & Brose LS (2017). What factors are associated with current smokers using or stopping e-cigarette use? Drug and Alcohol Dependence, 173, 139–143. doi: 10.1016/j.drugalcdep.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Mazure CM, & McKee SA (2014). Smoking and mental illness in the U.S. population. Tobacco Control, 23(e2), e147–153. doi: 10.1136/tobaccocontrol-2013-051466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Nadim H, & Jatlow P (2012). Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology, 37(6), 1509–1516. doi: 10.1038/npp.2011.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Caviness C, Grimone K, Audet D, Anderson BJ, & Bailey GL (2016). An open trial of electronic cigarettes for smoking cessation among methadone-maintained smokers. Nicotine & Tobacco Research, 18(5), 1157–1162. doi: 10.1093/ntr/ntv267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuyt EB (2014). Enforced abstinence from tobacco during inpatient dual-diagnosis treatment improves substance abuse treatment outcomes in smokers. American Journal of Addiction, 24(3), 252–257. doi: 10.1111/j.1521-0391.2014.12179.x [DOI] [PubMed] [Google Scholar]
- Tiffany ST, & Drobes DJ (1991). The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction, 86(11), 1467–1476. [DOI] [PubMed] [Google Scholar]
- Tseng TY, Ostroff JS, Campo A, Gerard M, Kichner T, Rotrosen J, & Shelley D (2016). A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine & Tobacco Research, 18(10), 1937–1943. doi: 10.1093/ntr/ntw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, & Eissenberg TE (2010). A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology Biomarkers & Prevention, 19(8), 1945–1953. doi: 10.1158/1055-9965.epi-10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Weaver MF, & Eissenberg T (2012). Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction, 107(8), 1493–1500. doi: 10.1111/j.1360-0443.2012.03791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CV, Richter P, de Castro BR, Sosnoff C, Potts J, & Clark P (2017). Smoking behavior and exposure:Results of a menthol cigarette cross-over ztudy. American Journal of Health Behavior, 41(3), 309–19. doi: 10.5993/AJHB.41.3.10 [DOI] [PMC free article] [PubMed] [Google Scholar]


