Abstract
RNA-protein interactions play a critical role in post-transcriptional gene regulation. Characterizing these interactions in their native context has been challenging, however advances in RNA sequencing and mass spectrometry-based proteomics combined with innovative chemical biological tools have heralded the development of robust strategies for performing biochemistry on a cellular scale. Herein, we review recent advances in the development and application of proteomic and transcriptomic approaches to profile cellular RNA-protein interactions, focusing on sequencing-based strategies and proteomic analysis of RNA-binding proteins (RBPs), as well as approaches to address the role of RNA modifications in protein-RNA binding events.
Introduction
Post-transcriptional gene regulation plays an important role in biological processes. In large part, the underlying molecular mechanisms are mediated by physical interactions between RNA transcripts and a large complement of RNA-binding proteins (RBPs)1 that regulate RNA splicing, stability, nuclear export, and translation, among other properties. Therefore, characterizing the RNA-binding preferences of RBPs and mapping the RBP proteome can reveal fundamental insights into the biological function of RNA-protein binding events. In addition, recent studies have implicated RNA modifications (the “epitranscriptome”)2 as regulators of RNA-protein complexes, adding an additional dimension of complexity to these interaction networks. While early studies of RNA-protein interactions were limited to studies of individual complexes3, technological advances in RNA sequencing and mass spectrometry-based proteomics have enabled transcriptome and proteome wide analysis of these processes. Notably, UV-mediated photocrosslinking of protein-RNA complexes, which can be performed on intact biological samples4,5, has played a major role in the development of approaches to study cellular RBP-RNA interactions in a high-throughput fashion. In addition, chemical biology approaches such as metabolic labeling with artificial nucleotides, bioorthogonal chemistry and protein engineering have been applied to aid in the elucidation of the protein-RNA interactome. In this review, we highlight recent methodological developments in the high-throughput characterization of cellular RNA-protein interactions and discuss future directions for this area of research.
Approaches to footprint RNA-protein binding sites
A key step in understanding the molecular mechanisms underlying the biological function of RBPs is to identify the collection of RNA transcripts with which they physically associate; further, the RNA-binding site or “footprint” of the RBP can provide additional insight into its biological activity. Early efforts towards this end relied upon in vitro selection/SELEX6,7 to characterize the inherent sequence-binding preferences of RBPs using libraries of random-sequence RNA. This strategy efficiently identifies high-affinity binding motifs, which can then be used to mine for related sequences in the transcriptome. In vitro selection is operationally straightforward and results can be analyzed using low-throughput Sanger sequencing; however, these experiments are not always directly relevant to physiological RNA-protein interactions, and therefore, most approaches have focused on the direct characterization of protein-RNA complexes isolated from living systems.
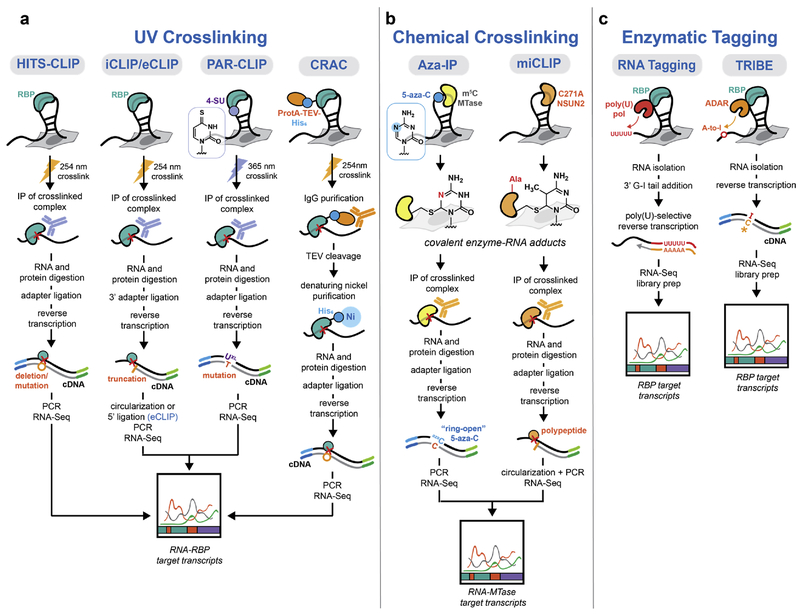
The first widely accepted strategy to characterize RNA-protein interactions en masse was reported by Darnell and co-workers in 20088. Known as HITS-CLIP (also CLIP-seq) (Figure 1a), this approach combined several key methods for the isolation of native RNA-protein complexes4,5,9,10 by crosslinking and immunoprecipitation (CLIP) with high-throughput RNA sequencing (a new technology at that time). Central to the method is UV-induced photocrosslinking, which can be performed on intact cells and crosslinks RNA-protein interactions with much greater efficiency than protein-protein species (thereby favoring crosslinking of RNA with only directly associated RBPs). Once RNA-protein crosslinks have been generated, even low affinity protein-RNA interactions can be isolated under stringent immunoprecipitation conditions, and the associated RNA identified by reverse-transcription and sequencing. RNA is typically subject to partial enzymatic digestion before and after immunoprecipitation in order to map the RBP footprint with greater precision. HITS-CLIP was the forerunner to a number of UV-photocrosslinking based CLIP methods designed to map the transcriptome-wide RNA binding of a single RBP. These include CRAC11, PAR-CLIP12, iCLIP13, and eCLIP14, as well as improved HITS-CLIP methodology15 (Figure 1a). The improvements reported in these modified CLIP methods have generally fallen into the following categories: 1) enhanced photocrosslinking facilitated by metabolic labeling of cellular RNA with 4-thiouridine (4-SU) or 6-thioguanosine (6-SG)12, 2) improved immunoprecipitation and isolation conditions11, and 3) modifications to cDNA library generation and bioinformatic analysis designed to map RBP footprints with single nucleotide resolution13–15 (often relying upon identification of crosslinking-induced mutations, deletions, or truncations). Currently, these improved CLIP strategies represent the state-of-art in mapping RBP footprints transcriptome-wide and have been applied to study numerous RBPs.
Figure 1.
Methods to identify RNA-protein binding sites. (a) Comparison of UV crosslinking-based CLIP methods. RBP footprints are identified by UV crosslinking, immunoprecipitation, and sequencing of covalently linked RNA. Variations on the prototypical HITS-CLIP protocol have been developed including modified photocrosslinking (PAR-CLIP), cDNA library generation (iCLIP/eCLIP), and immunoprecipitation (CRAC). (b) Chemical crosslinking approaches take advantage of the catalytic mechanism of RNA m5C methyltransferases, which involves formation of a covalent adduct with RNA substrates. In the Aza-IP strategy, metabolic incorporation of 5-azacytidine generates stalled covalent adducts at sites of modification. In miCLIP, a mutant RNA m5C methyltransferase lacking the ability to release itself from RNA substrates generates covalently bound complexes. (c) Non-crosslinking methods to map RBP substrates. In RNA Tagging, 3’ poly(U) tails are deposited on RBP-associated RNA by a poly(U) polymerase, PUP-2, fused to the RBP of interest. Following RNA isolation, target transcripts are enriched by reverse transcription with a primer specific for uridylated RNAs. TRIBE utilizes an RBP-ADAR construct to label associated RNA transcripts through adenosine to inosine (A:I) editing, which can then identified by RNA sequencing analysis (I pairs to predominantly to C).
While UV crosslinking is a general strategy that can be applied to almost any RBP, the unique catalytic mechanism of RNA 5-methylcytidine (m5C) methyltransferases16 enables their crosslinking to substrate RNA through chemical means. Two crosslinking-based approaches have been reported for mapping m5C methyltransferase substrates – Aza-IP17 and miCLIP18 (Figure 1b). In the Aza-IP strategy, RNA-protein crosslinking is mediated by 5-azacytidine, which can be incorporated transcriptome-wide through metabolic labeling; for the miCLIP approach, a mutant methyltransferase is utilized which remains bound to its substrate cytidine residue. After chemical crosslinking, immunoprecipitation and RNA sequencing analysis is performed using strategies similar to those employed in UV-based CLIP workflows. While UV CLIP approaches can also be applied to m5C methyltransferases, mechanism-based crosslinking reports on RNA transcripts that are subject to enzymatic methylation, as opposed to simple binding.
Non-crosslinking strategies have also seen application in RBP substrate mapping. Rosbash and co-workers have developed the TRIBE approach19 (Figure 1c, right), based on the ability of adenosine deaminase enzymes (ADARs) to catalyze adenosine to inosine (A:I) editing when brought into close proximity to an RNA transcript (such as through fusion to the RBP of interest). In this strategy, no purification of RNA-protein species is required since inosine is predominantly converted to C during reverse transcription and its presence can be identified directly by sequencing and bioinformatic analysis. An enhanced version of TRIBE (named HyperTRIBE20), which uses a more active ADAR mutant has been recently described. An alternative non-crosslinking based strategy was developed by Wickens and co-workers21. Their approach, named “RNA tagging”, relies upon the fusion of C. elegans poly(U)polymerase PUP-2 to the RBP of interest resulting in the deposition of polyU tails on RBP-associated RNAs (Figure 1c, left). PolyU-tagged RNAs can then be selectively enriched during cDNA library generation. The major advantage of both the TRIBE and RNA tagging approach is that these strategies do not require isolation of RNA-protein complexes. However, they both involve fusing an enzyme to the RBP of interest, which may affect RNA-binding, and are unlikely to provide binding data at nucleotide resolution.
Approaches to profile the RNA-binding proteome
CLIP-based sequencing approaches provide information on the binding behavior of a single RBP, but fail to report on the RNA-binding proteome. To discover new RBPs and characterize their behavior on a proteome-wide level, it is necessary to apply proteomics for RBP analysis. This is generally more challenging than RNA sequencing-based analysis due to the lower throughput and lack of amplification, but in the last decade multiple strategies for proteomic characterization of the cellular RBP complement have emerged. These approaches have built upon advances in RNA-protein crosslinking initially developed for CLIP experiments and are described below.
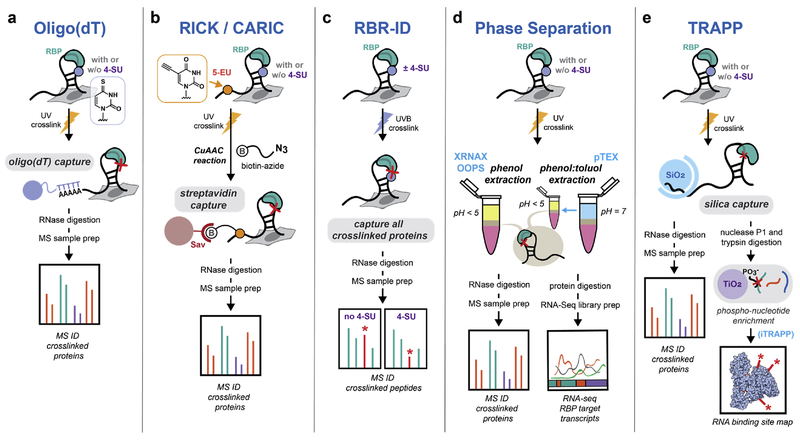
The first strategy to isolate RBPs from cells, known as “interactome capture” was developed independently by the Hentze22 and Landthaler23 labs (Figure 2a). In their respective approaches, RBPs are first UV crosslinked to RNA and polyadenylated RNA-protein complexes are enriched from cells by hybridization to oligo-dT beads. After enzymatic digestion of RNA, mass spectrometry-based proteomics is used to characterize the identities of the isolated RBPs. Since nucleic acid hybridization is tolerant to high salt and ionic detergents, oligo-dT enrichment can be performed under stringent, denaturing conditions, allowing the clean isolation of RNA-crosslinked proteins from free proteins in the cellular milieu. The interactome capture method has been widely used to study RBPs interacting with mRNA22–29, and can be employed with 254 nm UV crosslinking or longer wavelength irradiation combined with 4-SU-labeled RNA. In the original reports22,23, ~800 RBPs were identified in HeLa and HEK293 cells, many of which were novel RBPs. An alternative strategy that is not restricted to polyadenylated RNA is the RICK30/CARIC31 approach that can be applied to profile RBPs associated with RNA transcripts irrespective of their polyadenylation status (Figure 2b). This methodology relies upon 5-ethynyluridine (5-EU) labeling of RNA and click chemistry with biotin-azide in order to enrich RBPs crosslinked to nascent transcripts, and provides orthogonal results to oligo-dT-based enrichment.
Figure 2.
Methods to profile RNA-binding proteins. (a) RBPs that bind to polyadenylated RNA are captured by hybridization of UV-crosslinked protein-RNA to oligo-dT beads. The isolated complexes are digested with RNAse to release proteins for MS analysis. Variations of this method utilizing photocrosslinkable nucleosides such as 4-thiouridine (4-SU) can improve the efficiency of crosslinking. (b) RICK/CARIC approaches rely on labeling of nascent RNA transcripts with 5-ethynyluridine (5-EU). After UV treatment, labeled transcripts with crosslinked RBPs can be modified by click chemistry with biotin-azide to enable affinity purification. (c) In RBR-ID, RNA binding regions in RBPs are identified by differences in MS signal intensity resulting from UV crosslinking. (d) Phase separation techniques use aqueous-organic partitioning to purify RNA-protein complexes based on their unique physicochemical properties. The XRNAX and OOPS methods use acidic guanidinium thiocyanate/phenol/chloroform (Trizol) to capture UV-crosslinked RNA-protein complexes in the interphase between the aqueous and organic layers. In PTex, a preliminary neutral phenol-toluol extraction removes DNA and lipids which would otherwise contaminate the Trizol interphase. (e) TRAPP relies on the strong interaction between nucleic acids and silica beads to purify RNA-bound proteins under strongly denaturing conditions. The method can be further applied to map peptide-RNA crosslinks with amino acid resolution by titanium dioxide (TiO2) enrichment of peptide-nucleotide species for tandem MS analysis.
Further efforts to profile RBPs have focused on approaches that afford greater generality and are not restricted to the analysis of RBPs that associate with polyadenylated RNA, as well as those that provide insight into protein domains and amino acid residues involved in RNA binding29,32,33. One such approach, RBR-ID32, was developed by Bonasio, Garcia and colleagues (Figure 2c). In their strategy, RBP binding regions are identified by analysis of crosslinked tryptic peptides. Rather than seeking to measure the specific crosslinked peptide-oligonucleotide species, RBR-ID takes advantage of the concomitant decrease in signal intensity for the parent (non-crosslinked) tryptic peptide. Using this strategy on the nuclear proteome of embryonic stem cells resulted in the identification of ~800 RBP binding regions at peptide level resolution, including several previously unknown RNA-binding domains in chromatin regulators. Moreover, the RBR-ID strategy does not require RNA enrichment and therefore provides an unbiased snapshot of the RNA-binding proteome.
While the above approaches for RBP analysis largely rely upon specific recognition of nucleic acid sequences or affinity reagents for the isolation of crosslinked RBP-RNA complexes, the physicochemical properties of covalent protein-RNA conjugates can be directly exploited in order to enrich these species from free RNA and free protein (Figure 2d). Indeed, the differential partitioning of RNA from DNA and protein upon extraction with acidic guanidinium thiocyanate/phenol/chloroform (i.e. “Trizol”) is a common technique for RNA isolation from cellular samples. With the rationale that covalent RNA-protein complexes may exhibit hybrid phase separation properties distinct from either individual component, several groups decided to investigate whether these crosslinked species could be enriched by isolation of the interphase layer, which is typically discarded. In three independent reports published this year34–36, phase separation was described as an effective strategy to isolate RNA-crosslinked RBPs. In two approaches, named XRNAX35 and OOPS36 (Figure 2d), the standard Trizol formulation was used; the XRNAX method also incorporates an additional silica-based purification of crosslinked peptide-RNA fragments prior to mass spectrometry analysis. The third method, PTex34, utilizes a modified phenol-toluol organic layer, and sequential extractions at neutral and acidic pH (Figure 2d). Application of all 3 approaches to mammalian cells consistently identified greater numbers of RBPs than were found by oligo-dT based interactome capture, reflecting the more general nature of these strategies for isolating crosslinked RNA-RBP complexes. In addition, OOPS and PTex were applied to study bacterial RBPs, which are not accessible through oligo-dT interactome capture since bacterial RNA lacks polyadenylation. Tollervey and co-workers37 have also developed an approach based on silica-bead purification (named “TRAPP”) in order to enrich RNA-RBP complexes from cells that is not restricted to polyA-RNA interactome analysis (Figure 2e).
Approaches to study RNA-modification-associated proteins
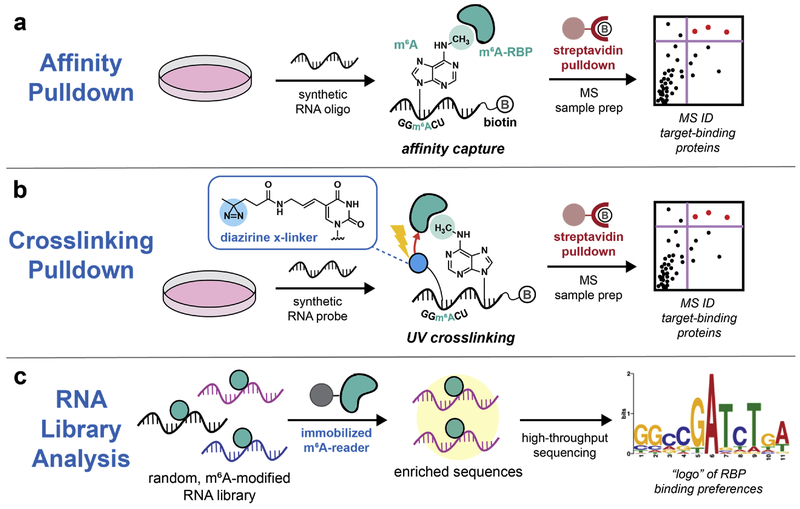
CLIP and RNA interactome capture strategies are not readily adaptable to study RNA modification-dependent RBPs (or “readers”) since modification levels are typically low and we lack approaches to bias photocrosslinking events in the proximity of modification sites in cells. Therefore, strategies for identifying readers have largely relied upon affinity pulldown with synthetic biotinylated oligonucleotides containing the modified base of interest within a particular sequence context. When combined with quantitative proteomics, this approach has been used successfully to identify readers of N6-methyladenosine (m6A)38–42 and 5-methylcytidine (m5C)43,44. Since RBP readers often bind to modified oligonucleotides with low affinity, additional steps may be necessary in order to efficiently capture these proteins. Notably, Vermeulen and co-workers38 utilized a synthetic oligonucleotide containing four tandem repeats of the GG(m6A)CU consensus sequence and SILAC-based quantitative proteomics to profile the m6A interactome in mammalian cells (Figure 3a). Our lab has developed a photocrosslinking-based chemical proteomics approach39 relying upon synthetic oligonucleotides modified with a diazirine-containing uridine residue flanking the modification of interest (Figure 3b). For the purpose of discovering new readers, this strategy combines the advantages of photocrosslinking (e.g. stabilization of low-affinity binders and stringent purification conditions) with the versatility of chemical synthesis, enabling the interrogation of any synthetically accessible nucleotide. Both our approach and traditional affinity pulldown strategies rely upon a comparative analysis between enrichment with modified and unmodified oligonucleotides, and therefore reveal both positive and negative effects of modified nucleotides on RNA-protein interaction affinity. In the case of m6A, for example, the stress granule protein G3BP1 has been characterized as a protein that is repelled by the m6A modification38,39.
Figure 3.
Methods to study RNA modification-dependent RBPs. (a) Affinity pulldown using synthetic modified oligonucleotides. RBP-oligonucleotide complexes are captured by streptavidin and RBPs are identified by mass spectrometry-based proteomics. (b) Photocrosslinking pulldown to identify RNA modification readers. To enhance capture of low-affinity binders and facilitate stringent affinity purification, a photocrosslinkable nucleotide can be incorporated near the site of modification in a synthetic oligonucleotide. (c) In vitro selection of a site-specifically modified RNA library followed by next-generation RNA sequencing enables the identification of the sequence binding preferences of m6A-binding RBPs.
As a complementary method for RNA modification analysis, we have developed an in vitro selection platform for interrogating the sequence binding preferences of epitranscriptomic reader proteins (Figure 3c)45. Our strategy relies on the chemical synthesis of a site-specifically modified random-sequence RNA library, affinity selection, and next-generation RNA sequencing. We applied this approach to profile the binding preferences of YTH-domain proteins, the major class of m6A readers, revealing distinct biochemical preferences for m6A-modified sequence motifs. This strategy should be readily generalizable to study other RNA modification-protein interactions.
Conclusion and future outlook
The discovery and characterization of new gene regulatory mechanisms operating on the transcriptomic and post-transcriptomic level have spurred increased interest in RNA-centric biology. These advances in our basic understanding of cellular biology have coincided with (and in part were made possible by) transformative technological advances in RNA and DNA sequencing and mass spectrometry-based proteomics. Taking advantage of these technologies, researchers have developed robust approaches for mapping RNA-protein interactions in cellular populations, thereby laying the groundwork for a holistic understanding of the physical associations between the proteome and transcriptome. Moving forward, several areas present promising directions for further study and methodological development. First, the principles underlying the trafficking and subcellular localization of RNA are still poorly understood, and would benefit from the application and development of tools to study these dynamic processes at subcellular resolution – towards this end, RNA-focused proximity labeling strategies46–50 have emerged as a promising strategy to address these questions. Second, the effect of RNA post-transcriptional modifications on RNA-protein interactions and cellular processes is still largely unexplored, and we lack general approaches to probe and study modifications on individual transcripts in living cells. Thirdly, transcriptomic and proteomic analyses need to be applied to single cells51, rather than in bulk, in order to capture the heterogeneity present among cellular populations. Finally, it is important to consider that the approaches described in this review are largely observational and serve as tools for hypothesis generation. Therefore, with the explosion in the number and size of available transcriptomic and proteomic datasets, an important goal is to develop robust strategies for selecting high-confidence interactions that are of functional significance and warrant further biological examination.
Acknowledgements
Financial support was provided by the NIH (R01 GM132189) and Princeton University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
References
• of special interest
•• of outstanding interest
- 1.Gerstberger S, Hafner M & Tuschl T A census of human RNA-binding proteins. Nat Rev Genet 15, 829–845, doi: 10.1038/nrg3813 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roundtree IA, Evans ME, Pan T & He C Dynamic RNA Modifications in Gene Expression Regulation. Cell 169, 1187–1200, doi: 10.1016/j.cell.2017.05.045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoemaker HJ & Schimmel PR Photo-induced joining of a transfer RNA with its cognate aminoacyl-transfer RNA synthetase. J Mol Biol 84, 503–513, doi: 10.1016/0022-2836(74)90112-0 (1974). [DOI] [PubMed] [Google Scholar]
- 4.Mayrand S, Setyono B, Greenberg JR & Pederson T Structure of nuclear ribonucleoprotein: identification of proteins in contact with poly(A)+ heterogeneous nuclear RNA in living HeLa cells. J Cell Biol 90, 380–384, doi: 10.1083/jcb.90.2.380 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayrand S & Pederson T Nuclear ribonucleoprotein particles probed in living cells. Proc Natl Acad Sci U S A 78, 2208–2212, doi: 10.1073/pnas.78.4.2208 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellington AD & Szostak JW In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822, doi: 10.1038/346818a0 (1990). [DOI] [PubMed] [Google Scholar]
- 7.Tuerk C & Gold L Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Licatalosi DD et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 456, 464–469, doi: 10.1038/nature07488 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreyfuss G, Choi YD & Adam SA Characterization of heterogeneous nuclear RNA-protein complexes in vivo with monoclonal antibodies. Mol Cell Biol 4, 1104–1114, doi: 10.1128/mcb.4.6.1104 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ule J et al. CLIP identifies Nova-regulated RNA networks in the brain. Science 302, 1212–1215, doi: 10.1126/science.1090095 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Granneman S, Kudla G, Petfalski E & Tollervey D Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proc Natl Acad Sci U S A 106, 9613–9618, doi: 10.1073/pnas.0901997106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafner M et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141, doi: 10.1016/j.cell.2010.03.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konig J et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat Struct Mol Biol 17, 909–915, doi: 10.1038/nsmb.1838 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Nostrand EL et al. Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nat Methods 13, 508–514, doi: 10.1038/nmeth.3810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C & Darnell RB Mapping in vivo protein-RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nat Biotechnol 29, 607–614, doi: 10.1038/nbt.1873 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohnsack KE, Hobartner C & Bohnsack MT Eukaryotic 5-methylcytosine (m(5)C) RNA Methyltransferases: Mechanisms, Cellular Functions, and Links to Disease. Genes (Basel) 10, doi: 10.3390/genes10020102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoddami V & Cairns BR Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat Biotechnol 31, 458–464, doi: 10.1038/nbt.2566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain S et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep 4, 255–261, doi: 10.1016/j.celrep.2013.06.029 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon AC et al. TRIBE: Hijacking an RNA-Editing Enzyme to Identify Cell-Specific Targets of RNA-Binding Proteins. Cell 165, 742–753, doi: 10.1016/j.cell.2016.03.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper describes an enzymatic strategy for identifying RBP-associated transcripts using ADAR-mediated A:I editing.
- 20.Xu W, Rahman R & Rosbash M Mechanistic implications of enhanced editing by a HyperTRIBE RNA-binding protein. RNA 24, 173–182, doi: 10.1261/rna.064691.117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapointe CP, Wilinski D, Saunders HA & Wickens M Protein-RNA networks revealed through covalent RNA marks. Nat Methods 12, 1163–1170, doi: 10.1038/nmeth.3651 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castello A et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406, doi: 10.1016/j.cell.2012.04.031 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Baltz AG et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell 46, 674–690, doi: 10.1016/j.molcel.2012.05.021 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Beckmann BM et al. The RNA-binding proteomes from yeast to man harbor conserved enigmRBPs. Nat Commun 6, 10127, doi: 10.1038/ncomms10127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrad T et al. Serial interactome capture of the human cell nucleus. Nat Commun 7, 11212, doi: 10.1038/ncomms11212 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Perri JI et al. Discovery of RNA-binding proteins and characterization of their dynamic responses by enhanced RNA interactome capture. Nat Commun 9, 4408, doi: 10.1038/s41467-018-06557-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This report describes an improved strategy for oligo-dT interactome capture using a modified LNA containing probe.
- 27.Reichel M et al. In Planta Determination of the mRNA-Binding Proteome of Arabidopsis Etiolated Seedlings. Plant Cell 28, 2435–2452, doi: 10.1105/tpc.16.00562 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sysoev VO et al. Global changes of the RNA-bound proteome during the maternal-to-zygotic transition in Drosophila. Nat Commun 7, 12128, doi: 10.1038/ncomms12128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castello A et al. Comprehensive Identification of RNA-Binding Domains in Human Cells. Mol Cell 63, 696–710, doi: 10.1016/j.molcel.2016.06.029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao X et al. Capturing the interactome of newly transcribed RNA. Nat Methods 15, 213–220, doi: 10.1038/nmeth.4595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper describes an approach for profiling RBPs associated with nascent RNAs based upon metabolic labeling with 5-EU and click chemistry enrichment.
- 31.Huang R, Han M, Meng L & Chen X Transcriptome-wide discovery of coding and noncoding RNA-binding proteins. Proc Natl Acad Sci U S A 115, E3879–E3887, doi: 10.1073/pnas.1718406115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He C et al. High-Resolution Mapping of RNA-Binding Regions in the Nuclear Proteome of Embryonic Stem Cells. Mol Cell 64, 416–430, doi: 10.1016/j.molcel.2016.09.034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This method provides a general strategy for mapping RNA-binding regions based upon the decrease in signal intensity of tryptic peptides resulting from RNA photocrosslinking.
- 33.Kramer K et al. Photo-cross-linking and high-resolution mass spectrometry for assignment of RNA-binding sites in RNA-binding proteins. Nat Methods 11, 1064–1070, doi: 10.1038/nmeth.3092 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urdaneta EC et al. Purification of cross-linked RNA-protein complexes by phenoltoluol extraction. Nat Commun 10, 990, doi: 10.1038/s41467-019-08942-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This report describes a phase separation-based strategy for isolating crosslinked RNA-protein complexes using a modified phenol-toluol organic phase.
- 35.Trendel J et al. The Human RNA-Binding Proteome and Its Dynamics during Translational Arrest. Cell 176, 391–403 e319, doi: 10.1016/j.cell.2018.11.004 (2019). [DOI] [PubMed] [Google Scholar]; •• This paper describes a phase separation-based approach for enriching crosslinked RNA-protein species using Trizol and silica-based based purification.
- 36.Queiroz RML et al. Comprehensive identification of RNA-protein interactions in any organism using orthogonal organic phase separation (OOPS). Nat Biotechnol 37, 169–178, doi: 10.1038/s41587-018-0001-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper describes a phase separation-based strategy for isolating crosslinked RNA-protein complexes from the interphase layer of Trizol extracted cellular lysate.
- 37.Shchepachev V et al. Defining the RNA interactome by total RNA-associated protein purification. Mol Syst Biol 15, e8689, doi: 10.15252/msb.20188689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edupuganti RR et al. N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 24, 870–878, doi: 10.1038/nsmb.3462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This report describes an affinity pulldown with synthetic, m6A-modified oligonucleotides providing a comprehensive view of the m6A interactome in mammalian cells.
- 39.Arguello AE, DeLiberto AN & Kleiner RE RNA Chemical Proteomics Reveals the N(6)-Methyladenosine (m(6)A)-Regulated Protein-RNA Interactome. J Am Chem Soc 139, 17249–17252, doi: 10.1021/jacs.7b09213 (2017). [DOI] [PubMed] [Google Scholar]; •• This paper presents a photocrosslinking-based pulldown approach with synthetic oligonucleotides and applies this strategy to profile the m6A interactome in HeLa cells.
- 40.Dominissini D et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206, doi: 10.1038/nature11112 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Huang H et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20, 285–295, doi: 10.1038/s41556-018-0045-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alarcon CR et al. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308, doi: 10.1016/j.cell.2015.08.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res 27, 606–625, doi: 10.1038/cr.2017.55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sajini AA et al. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat Commun 10, 2550, doi: 10.1038/s41467-019-10020-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arguello AE, Leach RW & Kleiner RE In Vitro Selection with a Site-Specifically Modified RNA Library Reveals the Binding Preferences of N(6)-Methyladenosine Reader Proteins. Biochemistry, doi: 10.1021/acs.biochem.9b00485 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An in vitro selection strategy with a chemically synthesized m6A-modified random sequence library to profile the sequence binding preferences of RNA modification reader proteins.
- 46.Fazal FM et al. Atlas of Subcellular RNA Localization Revealed by APEX-Seq. Cell 178, 473–490 e426, doi: 10.1016/j.cell.2019.05.027 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Application of the APEX proximity labeling strategy in order to sequence RNA transcripts in a spatially selective manner.
- 47.Padron A, Iwasaki S & Ingolia NT Proximity RNA labeling by APEX-Seq Reveals the Organization of Translation Initiation Complexes and Repressive RNA Granules. bioRxiv, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benhalevy D, Anastasakis DG & Hafner M Proximity-CLIP provides a snapshot of protein-occupied RNA elements in subcellular compartments. Nat Methods 15, 1074–1082, doi: 10.1038/s41592-018-0220-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaewsapsak P, Shechner DM, Mallard W, Rinn JL & Ting AY Live-cell mapping of organelle-associated RNAs via proximity biotinylation combined with protein-RNA crosslinking. Elife 6, doi: 10.7554/eLife.29224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramanathan M et al. RNA-protein interaction detection in living cells. Nat Methods 15, 207–212, doi: 10.1038/nmeth.4601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberwine J, Sul JY, Bartfai T & Kim J The promise of single-cell sequencing. Nat Methods 11, 25–27 (2014). [DOI] [PubMed] [Google Scholar]