Abstract
Objectives:
Intraoperative photodynamic therapy (IO-PDT) is typically administered by a hand held light source. This can result in uncontrolled distribution of light irradiance that impacts tissue and tumor response to photodynamic therapy. The objective of this work was to characterize a novel optical surface applicator (OSA) designed to administer controlled light irradiance in IO-PDT.
Materials and Methods:
An OSA was constructed from a flexible silicone mesh applicator with multiple cylindrically diffusing optical fibers (CDF) placed into channels of the silicone. Light irradiance distribution, at 665 nm, was evaluated on the OSA surface and after passage through solid tissue-mimicking optical phantoms by measurements from a multi-channel dosimetry system. As a proof of concept, the light administration of the OSA was tested in a pilot study by conducting a feasibility and performance test with 665-nm laser light to activate 2-(1’-hexyloxyethyl) pyropheophorbide-a (HPPH) in the thoracic cavity of adult swine.
Results:
At the OSA surface, the irradiance distribution was non-uniform, ranging from 128 – 346 mW/cm2. However, in the tissue mimicking phantoms, beam uniformity improved markedly, with irradiance ranges of 39 – 153 mW/cm2, 33 – 87 mW/cm2, and 12 – 28 mW/cm2 measured at phantom thicknesses of 3 mm, 5 mm, and 10 mm, respectively. The OSA safely delivered the prescribed light dose to the thoracic cavities of four swine.
Conclusion:
The OSA can provide predictable light irradiances for administering a well-defined and potentially effective therapeutic light in IO-PDT.
Keywords: intraoperative photodynamic therapy, non-small cell lung cancer, optical tissue-mimicking phantom
INTRODUCTION
Lung cancer is the leading cause of cancer-related death in the United States1 with 80–85% of these cases diagnosed as non-small cell lung cancer (NSCLC).2 Surgery remains the primary method of treatment for patients with NSCLC. However, locoregional recurrence occurs relatively often even after complete resection of the bulk tumor.3,4 This is most likely due to residual disease. Recent studies have shown that that intraoperative photodynamic therapy (IO-PDT) following surgery is a promising adjuvant therapy. Simone and Cengel reported that IO-PDT after surgery prolonged survival in patients with NSCLC with pleural dissemination.5 In addition, IO-PDT delivered after surgical resection of malignant pleural mesothelioma has been reported to prolong patients’ overall survival and improve local control.6
In current IO-PDT, a photosensitizer (PS) is administered about 24 to 48 hours prior to the light delivery. The light is delivered through a single cylindrical diffuser fiber placed in a modified endotracheal tube (ET-tube).7 The ET-tube and thoracic cavity are filled with a dilute 0.01% intralipid solution, a fat emulsion often used as a scattering agent, to improve homogeneity of the irradiance. About eight light detectors are sewn in at specified anatomical locations to monitor the light dose (i.e. radiant exposure, J/cm2) to the thoracic cavity.8 The treating physician moves the ET-tube light applicator within the liquid filled thoracic cavity, until all light detectors read the target radiant exposure (e.g., 30–60 J/cm2). In this light delivery method, the radiant exposure (that is cumulative) can be recorded fairly accurately, and can be reproducible. However, the light irradiance (mW/cm2) will vary throughout the treatment, because it is a function of the light source-to-surface distance, which is difficult to control with manual manipulation of the ET-tube light applicator. Additionally, the light detectors are relatively far apart and hence; do not ensure uniform light delivery at locations outside their detection range. In particular, there is a natural tendency of the surgeon to direct the ET-tube light applicator toward the detector locations to achieve the recommended light dose. Together, these two factors result in unknown and heterogeneous light irradiance within the target tissue. Many studies have demonstrated that light irradiance will impact the response to PDT.9–12 Our goal is to improve the delivery of the light irradiance with a surface applicator that is scalable and can conform to complex anatomy within the thoracic cavity.
Several surface light applicators have been investigated in an effort to control light delivery in the thoracic cavity.13–16 These include light blankets that offer a flexible light source that conforms to curved surfaces and uniformity in light distribution from the applicator source. In addition, woven or textile light diffusers have been used to illuminate curved surfaces. The primary limitation of these blankets is the inability to alter light irradiance to enhance the PDT or to protect certain regions (to decrease PDT for minimal toxicity) within the treatment field.
We developed an optical surface applicator (OSA) where we can adjust the light irradiance to enhance the PDT reaction, and scale the shape and irradiance of the applicator to protect regions within the treatment field. The OSA is constructed using a flexible silicone mesh (Freiburg Flap, Elekta, Atlanta, GA), a clinically approved device in high dose rate (HDR) brachytherapy, which will aide in future clinical implementation. Here, we studied the light irradiance distribution of the OSA using optical phantoms. As a proof of concept, we also demonstrate the use of the OSA to administer light in the thoracic cavity of adult swine, using clinically relevant surgical techniques.
MATERIALS AND METHODS
Terminology
In this paper, we refer to the energy received by a surface per unit area as the radiant exposure, with units of J/cm2. Luminance will refer to the pixel intensity observed in the high-resolution digital images. The radiant flux on a surface, or power, per unit area is referred to as irradiance, with units of mW/cm2.
The Optical Surface Applicator (OSA)
The prototype OSA was comprised of a 10 cm × 10 cm section of a silicone mesh applicator used for HDR brachytherapy (Freiburg Flap, Elekta, Atlanta, GA). The mesh applicator consists of 1-cm diameter silicone rubber spheres. Channels with a 2.5 mm diameter run parallel through the center of the spheres, making them 1 cm apart and 0.5 cm from the surface of the mesh. Cylindrical diffuser fibers (CDFs) of about 1 mm in diameter and 3 m long (Radial diffuser, RD, Medlight SA, Ecublens, Switzerland) are inserted with their diffuser end into chosen channels of the applicator. On the other end, the CDFs were connected to laser diodes emitting 665-nm light (Modulight, Inc., Tampere, Finland).
Transmittance of light was measured in an OSA consisting of 1 cm × 5 cm beaded-mesh and a single CDF with a 2-cm long cylindrical light diffuser end (RD20, Medlight, Ecublens, Switzerland). The CDF was connected to a laser diode set to deliver 600 mW of 665-nm light. The percent change in laser power was calculated as (PRD20 – POSA)/PRD20 × 100%, where PRD20 is the power measured from the RD20 alone and the POSA is the power measured for the RD20 incorporated in the beaded-mesh. Power measurements were performed three times for both configurations by placing them at the center of an integrating sphere (sphere model 221/2500; radiometer model XB0402, UDT Instruments, San Diego, CA). All fiber powers for the following studies were set by placing each fiber in the center of the integrating sphere.
Fabrication of Gel Phantoms
Solid tissue-mimicking phantoms were constructed with agar powder, intralipid as a scatterer,17 and India ink as an absorber.18 Select Agar (Cat. No. 30391–023, Invitrogen, Carlsbad, CA) was added at a concentration of 1% (w/v) to de-ionized water and heated to 90–95°C on a hot plate while stirring. While stirring continued, the agar solution was allowed to cool to 75–80°C, at which point intralipid (Liposyn® 487 III 30%, Hospira, Inc., Lake Forest, IL) and India ink (Higgins 44201, Chartpak, Inc., Leeds, MA) were added. Once the temperature was below 60° C, the solution was poured into a plastic frame (Martellato Srl, Vigonza, Italy) of either 3 mm or 5 mm thickness, sandwiched between two sheets of tempered safety glass (Aberdeen Plate Glass Co., Buffalo, NY). The molds were refrigerated at 5°C to solidify agar. The concentration of intralipid and India ink were chosen to produce a reduced scattering coefficient, μs’ of 7 cm−1 and an absorption coefficient, μa of 0.26 cm−1.19–22
Characterization of Light Distribution
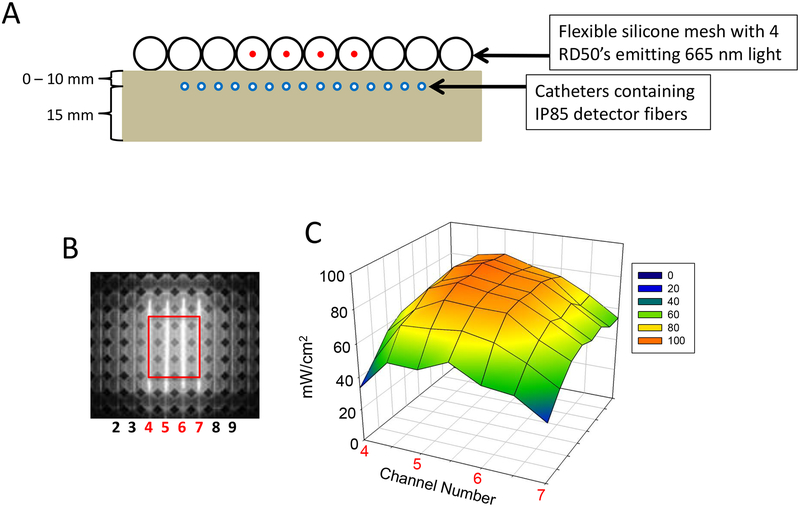
Experiments were performed to characterize irradiance distribution at the surface of the OSA and through optical tissue-mimicking phantoms of multiple thicknesses. Here, the OSA contained four 5-cm cylindrical diffuser fibers (RD50) connected to a diode laser delivering 500 mW 665-nm light per fiber. A custom-designed, real-time light dosimetry system was used to measure light irradiance.23,24 The dosimetry system includes fiber-coupled isotropic probes (IP85; Medlight SA, Ecublens, Switzerland) to collect light from the surface of the OSA and the underlying phantoms. An array of IP85 isotropic probes were translated under the phantoms or OSA surface through optically translucent catheters (Fig. 1A),25 resulting in a matrix of 256 collected data points. A 15 mm thick phantom of the same optical properties was placed under the catheters to mimic backscatter that would occur due to surrounding tissue at each depth.
Fig. 1.
Irradiance profiles for the OSA and a 5-mm thick optical phantom obtained using calibrated IP85 isotropic light detectors. Four CDFs with 5-cm long cylindrical light diffusers were inserted in adjacent channels 4, 5, 6, and 7 (labeled in red) of a 10 cm × 10 cm OSA. Each CDF was connected to a laser diode emitting 500 mW of 665-nm light. The transmitted 665-nm light was collected by a linear array of IP85 isotropic light detectors below the phantom, which was manually translated at 5-mm intervals through optically transparent catheters. IP85s were positioned between the OSA or tissue-mimicking phantom of defined thickness and a thicker tissue-mimicking phantom below (A). The IP85 detectors were previously calibrated to generate irradiance (mW/cm2) values. For a 3-cm × 3.5-cm area of the OSA (B) irradiance measurements over the distal surface of a 5-mm thick optical phantom were plotted (C).
Evaluation of OSA in Swine
The safety and feasibility of IO-PDT with the OSA was evaluated in 4 adult Yorkshire pigs (~70 kg body weight). All protocols adhered to the guidelines and approval of the Institutional Animal Care and Use Committee (IACUC) at Roswell Park Comprehensive Cancer Center, the US National Research Council’s Guide for the Care and Use of Laboratory Animals, and the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. Animals were injected with 2-(1’-Hexyloxyethyl)-2-devinyl pyropheophorbide-a PS (HPPH), at a dose of 0.1 mg/kg, 24 h before thoracotomy. This dose is equivalent to the standard dose used in patients.26 An OSA containing four RD50 CDFs and four IP85 isotropic probes was placed between the lung and pleural surface. A multi-diode laser system (ML6500; Modulight, Inc. Tampere, Finland) was used to deliver a prescribed radiant exposure of 60 J/cm2 of 665-nm light. Treatment conditions for this study are summarized in Table 2.
Table 2.
Summary of OSA swine study treatments.
| Swine ID | Laser Power† (W) | Light Intensity‡ (mW/cm) | Power per CDF (W) | Treatment time§ (min) | Mean irradiance¶ (mW/cm2) |
|---|---|---|---|---|---|
| 2018SW-02 | 1.0 | 50 | 0.25 | 19.5 | 79.7 [42.2, 105.9] |
| 2017SW-03 | 2.0 | 100 | 0.50 | 7.8 | 180.5 [114.51, 233.7] |
| 2017SW-02 | 4.0 | 200 | 1.00 | 3.4 | 341.6 [127.6, 578.65] |
| 2018SW-01 | 6.0 | 300 | 1.50 | 2.8 | 491.2 [339.3, 709.4] |
Total power delivered into the OSA containing four 5-cm cylindrical diffuser fibers (CDF). Light source was 665-nm multi-diode laser. All swine received intravenous 0.1 mg/kg HPPH 24h before intraoperative placement and activation of the OSA.
Light intensity per cm length of each CDF.
Time to deliver prescribed radiant exposure of 60 J/cm2 light, as measured by integrated isotropic light detectors.
Average irradiance. Square brackets denote irradiance upper and lower bounds. All dosimetry was done with the real-time light dosimetry system.
RESULTS
OSA Optical Transparency at 665 nm
We first determined the change in power from inserting a 2-cm CDF into the 1 cm × 5 cm beaded-mesh. The emitted power was reduced by 4.07 ± 0.44%, in comparison to the CDF alone, for 600 mW and 665 ± 3 nm.
OSA Beam Dosimetry
Irradiance measurements of the light emitted by the OSA, before and after transmission through tissue-mimicking phantoms (optical properties: μs’ = 7 cm−1 and μa = 0.26 cm−1) were taken to evaluate light distribution. We measured the irradiance by manually scanning a linear array of calibrated IP85 light detection fibers in optically translucent catheters that were laid underneath a 10 cm × 10 cm OSA containing four RD50 CDFs and tissue mimicking phantoms of either 3, 5, or 10 mm thickness (Fig. 1A). Figure 1 shows a 3D plot of irradiances of the target 3 cm × 3.5 cm region after passage through a 5-mm thick phantom (Fig. 1B, C).
In the 3D irradiance plots, we observe highest irradiance along the length of the CDFs at the surface of the OSA, with a range of values from 128 – 346 mW/cm2 in the target region (Table 1). High scattering of tissue-mimicking phantoms produced uniformity of the central beam both at the surface of the OSA and after passage through tissue mimicking phantoms. Specifically, irradiance values ranged from 39 –153 mW/cm2, 33 –87 mW/cm2, and 12 – 28 mW/cm2 at phantom thicknesses of 3 mm, 5 mm, and 10 mm, respectively. As expected, light attenuation decreased exponentially with respect to phantom thickness (data not shown).
Table 1.
Mean irradiance values at OSA surface and through solid optical phantoms.
| Phantom thickness† (mm) | Mean irradiance over field‡ (mW/cm2) | Ratio of upper/lower bound irradiance§ |
|---|---|---|
| 0 | 234.21 [128.59, 346.64] | 2.70 |
| 3 | 112.08 [39.42, 153.43] | 3.89 |
| 5 | 67.90 [33.31, 87.72] | 2.63 |
| 10 | 21.79 [12.53, 28.08] | 2.24 |
Solid agar-based optical phantoms were made using precision molds with 3-mm and 5-mm depths.
The OSA was comprised of four RD50 5-cm cylindrical diffuser fibers connected to a diode laser delivering 500 mW 665-nm light per fiber. Light intensity was measured every 5 mm across the plane of the OSA, using a linear array of IP85 isotropic light detectors, to create a matrix of irradiance values. The mean, upper and lower bound irradiance values (in brackets) of the target region were calculated at the surface of the OSA or through solid optical phantoms of various thicknesses and optical properties of μs’ of 7 cm−1, μa of 0.26 cm−1.
Ratio was computed from the highest and lowest irradiance values of the target region, reported in column 2, mean irradiance over field.
Swine Studies
Adult swine, ~70 kg bodyweight, underwent placement of an OSA by means of thoracotomy, where the applicator was positioned between the lung and pleural surface (Fig. 2). A total of 5 swine were entered into this study. However, early in the study, one animal expired shortly after an HPPH infusion of around 5-minute duration. Swine receiving intravenous HPPH over 60 minutes showed no post-infusion complications. The short infusion time appeared toxic, but we did not investigate this further, since a typical HPPH infusion for patients is 60 minutes.27 The IO-PDT treatment included dosimetry measurements to monitor and control the delivered radiant exposure, where the laser light was turned off when all the IP85 isotropic detectors reported that the radiant exposure reached or exceeded the target of 60 J/cm2. Table 2 reports the mean irradiance and the elapsed time (range 2.8 – 19.5 min) to deliver this light radiant exposure as a function of power delivered to the OSA. The mean duration of general anesthesia and procedure for administering IO-PDT was 2.5 h (range 2 h – 3.5 h). There were no postoperative complications. All animals recovered well from surgery and were euthanized 7 days from surgery, as planned.
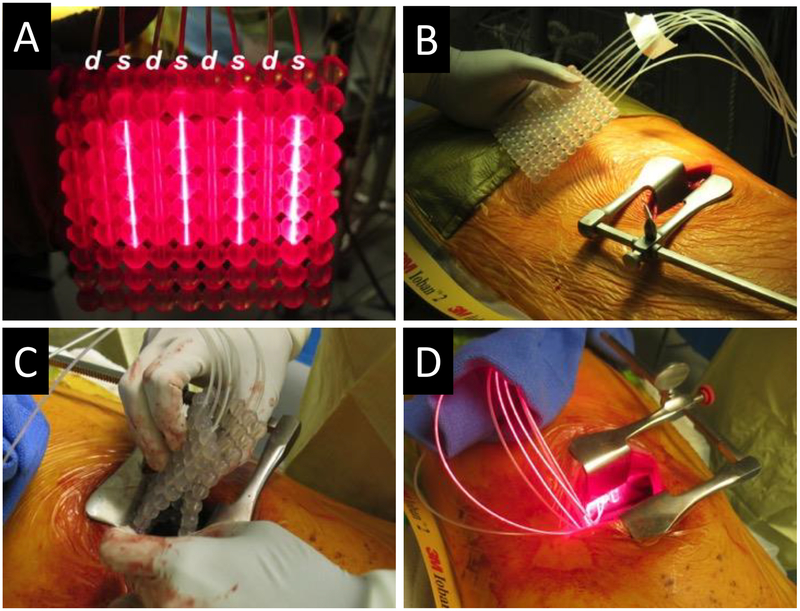
Fig. 2.
OSA for swine studies. The OSA for intraoperative PDT (IO-PDT) with four RD50 CDFs attached to a 4-channel laser diode emitting 665-nm light and four IP85 isotropic light detectors attached to our real-time dosimetry system. CDF light sources (labeled s) were spaced 20 mm apart, with isotropic detectors (labeled d) sutured to alternating channels (A). OSA before insertion into the animal (B). Flexible OSA folded for insertion through the left lateral thoracotomy incision (C). Working OSA following placement between the lung and pleural surface (D).
DISCUSSION
We have described an optical surface applicator made of a flexible silicone mesh and multiple optical fibers to deliver and collect light. The results in this study support the notion that the OSA can be used to control irradiance for IO-PDT treatments within the thoracic cavity.
The effectiveness of PDT depends on multiple variables, including PS concentration, tissue oxygenation, and light delivered. Properties such as the PS in the tissue and oxygen presence are not easily controllable, but light delivery can be monitored throughout treatment. A target radiant exposure is currently used as the parameter for light dose delivery to the thoracic cavity. However, it has been shown that irradiance has significant importance, since oxygen depletion occurs if the rate of oxygen consumption by PDT exceeds the rate of oxygen supplied.28 The handheld light applicator currently used in IO-PDT of the thoracic cavity makes it difficult to control light irradiance. Here, our OSA may offer a key advantage because it has a defined source-to-surface distance, resulting in more predictable irradiance.
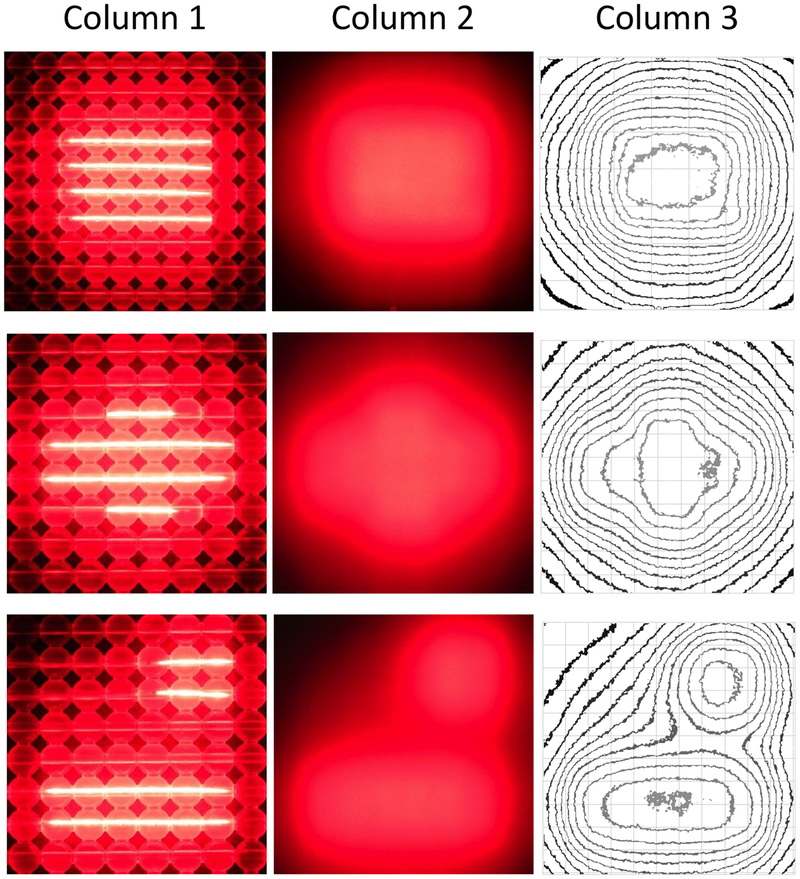
Furthermore, with our OSA, we can generate multiple light source configurations that can alter the shape of the isodose of the irradiance in tissue-mimicking phantoms (Fig. 3). It is demonstrated that by the use of multiple CDFs of various lengths that the OSA can deliver a light field which can be shaped to a region of interest. We can further modify the OSA light intensity during treatment by changing the laser power to each of the CDFs. This can be exploited to minimize light delivery to sensitive regions and enhance light irradiance to regions that require additional PDT.
Fig. 3.
High-resolution digital images of a 10 cm × 10 cm OSA using a variety of CDF lengths and positions to provide custom shaping of the light beam. Light from the OSA alone (column 1) and through a 5-mm thick solid tissue-mimicking optical phantoms (column 2). Luminance isointensity curves were plotted using ImageJ software (column 3).
For the majority of the phantom studies, our OSA was comprised of a 10 cm × 10 cm beaded mesh, with four 5-cm long CDFs inserted into channels 1 cm apart. In this configuration, light scatter was observed in the adjacent beads (Fig. 3, row 1, column 1). However, the beaded-mesh can also be easily cut and shaped to any desired dimensions, allowing for removal of excess beads. The ability to shape the beaded mesh can further improve light field edges.
We employed gel optical tissue-mimicking phantom to assess the light beam penetration and irradiance distribution. Our OSA configuration of four 5-cm long CDFs delivering a total of 2W 665-nm light yielded a mean central field irradiance of around 234 mW/cm2 at the OSA surface. This is comparable with irradiances used in external beam PDT of malignant tumors in patients. In addition, our results showed that the high scattering coefficient of the optical phantoms diffuses the 665-nm light and results in well-defined light irradiance distribution. Dimofte et. al. reported in-vivo optical properties in HPPH-mediated pleural PDT patients with measurements of optical properties of lung tissue having ranges of μa, = [0.18, 10.67] cm−1, and μs’ = [5.74, 112.81] cm−1.21 Similar to our phantoms, human tissue of the pleural cavity and lungs have relatively high scattering coefficient at 665-nm, hence we expect that the light irradiance distribution in human tissue will be similar to the one we measured in the phantoms. The fixed field of light will allow a known source-to-surface distance, creating a predictable light irradiance at the surface. Thus, we expect that the OSA can be used to administer effective, prescribed light dose during IO-PDT. Future studies will include developing a three dimensional model of the OSA for light simulation studies. Here, we will simulate light delivery in flat and curved tissue phantoms mimicking various optical properties of the thoracic cavity.
To evaluate systemic toxicity and if the OSA is sufficiently conformable to be advanced into the pleural space of a patient via thoracotomy, we used it to administer light in 70-kg swine with thoracic cavities approximately the same size as an average adult human. Using surgical techniques similar to those employed clinically, the surgeon (SY) was able to place the 10 cm by 10 cm OSA, containing four CDFs and four detector fibers connected to a dosimetry system, on the pleural surface via thoracotomy (Fig. 2) without complication. The OSA was conformable to the thoracic cavity, and it was observed to have good contact with tissue. It should be noted that if contact with tissue is not obtained the OSA can be sutured into to tissue for light delivery. A total of 60 J/cm2 was delivered to the pleural cavity of each of the four swine. Adverse events, including systemic toxicity of photosensitizer activated by light during or after treatment and difficulty in placement or removal of OSA, were not observed during or following surgery. Irradiance measurements were successfully collected throughout treatment (summarized, Table 2). While irradiance values varied widely between the detector positions as seen by the minimum and maximum irradiance values reported, irradiance measurements at each position were consistent throughout treatment (data not shown). This variation was in part due to respiration of the animal and detector positions, where one fiber was located at the edge of the CDF light field while other detectors were located more directly in the light field. These in vivo studies demonstrate the feasibility and safety of the OSA in the thoracic cavity. Future studies in a tumor-bearing model should be performed to study PDT efficacy and normal tissue toxicities post-treatment with the OSA.
CONCLUSION
The OSA enables control of light irradiances for IO-PDT. The beam shape and irradiance can be altered to deliver a therapeutic light irradiance in specific targeted regions. The OSA is scalable and flexible to fit curved surfaces, thus facilitating its use in the thoracic cavity and curved anatomy.
Acknowledgements
This work was supported in part by the Roswell Park Alliance Foundation, by National Cancer Institute of the National Institutes of Health (NCI/NIH) under Award Number R01 CA193610 to G. Shafirstein, and by Roswell Park Cancer Institute Support Grant NCI/NIH P30 CA16056. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCI/NIH or Roswell Park Cancer Institute. We thank the staff of the Laboratory Animal Shared Resource at Roswell Park Cancer Institute. We also thank Dr. Sherri McFarland (University of North Carolina at Greensboro) for comments on the manuscript.
Sarah Chamberlain received a travel award from the American Society for Laser Medicine and Surgery (ASLMS) to attend and present the concept of the work disclosed in this paper at the 39th Annual Conference of the ASLMS, Denver, Co, March 27–31, 2019.
Footnotes
Conflicts of Interest see attached ICMJE forms.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Lung Cancer - Non-Small Cell: Statistics. 2018; https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics [Google Scholar]
- 3.Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer. 2009;115(22):5218–5227. [DOI] [PubMed] [Google Scholar]
- 4.Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3(4):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simone CB, 2nd, Cengel KA. Definitive surgery and intraoperative photodynamic therapy: a prospective study of local control and survival for patients with pleural dissemination of non-small cell lung cancer. Proc SPIE Int Soc Opt Eng. 2014;8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedberg JS, Simone CB, 2nd, Culligan MJ, et al. Extended pleurectomy-decortication-based treatment for advanced stage epithelial mesothelioma yielding a median survival of nearly three years. Ann Thorac Surg. 2017;103(3):912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munck C, Mordon S, Betrouni N. Illumination profile characterization of a light device for the dosimetry of intra-pleural photodynamic therapy for mesothelioma. Photodiagnosis Photodyn Ther. 2016;16:23–26. [DOI] [PubMed] [Google Scholar]
- 8.Friedberg JS, Mick R, Stevenson JP, et al. Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J Clin Oncol. 2004;22(11):2192–2201. [DOI] [PubMed] [Google Scholar]
- 9.Shafirstein G, Bellnier DA, Oakley E, et al. Irradiance controls photodynamic efficacy and tissue heating in experimental tumours: implication for interstitial PDT of locally advanced cancer. Br J Cancer. 2018;119(10):1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson BW, Busch TM, Vaughan LA, et al. Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer research. 2000;60(3):525–529. [PubMed] [Google Scholar]
- 11.Henderson BW, Gollnick SO, Snyder JW, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer research. 2004;64(6):2120–2126. [DOI] [PubMed] [Google Scholar]
- 12.Henderson BW, Busch TM, Snyder JW. Fluence rate as a modulator of PDT mechanisms. Lasers Surg Med. 2006;38:489–493. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Wang K, Zhu TC. A light blanket for intraoperative photodynamic therapy. Proc SPIE Int Soc Opt Eng. 2013;7380:73801W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan T, Unternahrer M, Buchholz J, et al. Performance of a contact textile-based light diffuser for photodynamic therapy. Photodiagnosis Photodyn Ther. 2006;3:51–60. [DOI] [PubMed] [Google Scholar]
- 15.Selm B, Rothmaier M, Camenzind M, Khan T, Walt H. Novel flexible light diffuser and irradiation properties for photodynamic therapy. J Biomed Opt. 2007;12(3):034024. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Wang K, Zhu TC. Pre-clinic study of uniformity of light blanket for intraoperative photodynamic therapy. Proc SPIE Int Soc Opt Eng. 2010;7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimofte A, Finlay JC, Zhu TC. A method for determination of the absorption and scattering properties interstitially in turbid media. Phys Med Biol. 2005;50(10):2291–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Ninni P, Martelli F, Zaccanti G. The use of India ink in tissue-simulating phantoms. Opt Express. 2010;18:26854–26865. [DOI] [PubMed] [Google Scholar]
- 19.Sandell JL, Zhu TC. A review of in-vivo optical properties of human tissues and its impact on PDT. J Biophotonics. 2011;11–12(4):773–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu TC, Friedberg JS, Dimofte A, et al. The ratio of the spherical and flat detectors at tissue surfaces during pleural photodynamic therapy. Proc SPIE Int Soc Opt Eng. 2002;4612:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimofte A, Zhu TC, Finlay JC, et al. In vivo light dosimetry for HPPH-mediated pleural PDT. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XIX; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacques SL. Corrigendum: Optical properties of biological tissues: a review. Physics in Medicine and Biology. 2013;58(14):5007–5008. [DOI] [PubMed] [Google Scholar]
- 23.Shafirstein G, Battoo A, Harris K, et al. Photodynamic Therapy of Non-Small Cell Lung Cancer. Narrative Review and Future Directions. Ann Am Thorac Soc. 2016;13(2):265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oakley E, Bellnier DA, Hutson A, et al. Surface markers for guiding cylindrical diffuser fiber insertion in interstitial photodynamic therapy of head and neck cancer. Lasers Surg Med. 2017;49(6):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakley E, Wrazen B, Bellnier DA, Syed Y, Arshad H, Shafirstein G. A new finite element approach for near real-time simulation of light propagation in locally advanced head and neck tumors. Lasers Surg Med. 2015;47(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigual N, Shafirstein G, Cooper MT, et al. Photodynamic therapy with 3-(1’-hexyloxyethyl) pyropheophorbide a for cancer of the oral cavity. Clin Cancer Res. 2013;19(23):6605–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellnier DA, Greco WR, Nava H, Loewen GM, Oseroff AR, Dougherty TJ. Mild skin photosensitivity in cancer patients following injection of Photochlor (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemother Pharmacol. 2006;57(1):40–45. [DOI] [PubMed] [Google Scholar]
- 28.Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, Hilf R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiation Research. 1991;126(3):296–303. [DOI] [PubMed] [Google Scholar]