Abstract
Prenatal alcohol exposure (PAE) causes developmental abnormalities known as fetal alcohol spectrum disorder (FASD). Maternal iron status modulates the severity of these defects in the offspring. Because placenta is central in supporting fetal development, we investigated if maternal iron status similarly modulates alcohol’s effects in placenta. We hypothesized that PAE causes placental insufficiency by decreasing placental weight and efficiency, and these are worsened by maternal iron deficiency (ID) and alleviated by dietary iron-fortification (IF). We also determined whether altered placental iron flux and inflammatory balance contribute to placental insufficiency. Pregnant Long-Evans rats consumed an ID (2-6 ppm), iron-sufficient (IS; 100 ppm), or IF (500 ppm) diet. Alcohol (5g/kg body weight) or isocaloric maltodextrin (MD) was gavaged daily from gestational day (GD) 13.5-19.5. Placental outcomes were evaluated on GD20.5. PAE reduced fetal weight (P < 0.0001), placental weight (P = 0.0324), and placental efficiency (P = 0.0043). PAE downregulated placental transferrin receptor (P = 0.0032); it also altered placental Il1b and Tnf expression and Il6:Il10 ratio (P = 0.0337, 0.0300 and 0.0034, respectively) to generate a response favoring inflammation. ID-PAE further reduced fetal growth and placental efficiency and induced a heightened pro-inflammatory placental profile. IF did not rescue the alcohol-reduced fetal weight, but it normalized placental efficiency and decreased placental inflammation. These placental cytokines correlated with fetal and placental growth, and explained 45% of variability in fetal weight and 20% of variability in placental efficiency. In summary, alcohol induces placental insufficiency and is associated with a pro-inflammatory cytokine profile exacerbated by maternal ID and mitigated by maternal IF. Because placenta is closely linked to intrauterine growth, the placental insufficiency reported here may correlate with the lower birth weights in a subgroup of individuals who experienced PAE.
Keywords: prenatal alcohol exposure, iron, placental insufficiency, inflammatory cytokines, fetal development
Introduction
Despite public health efforts to increase awareness of alcohol’s teratogenic effects and reduce alcohol consumption among pregnant women, 11.5% report drinking alcohol and 3.9% report binge drinking in the past month (Denny et al., 2019). Prenatal alcohol exposure (PAE) is the leading preventable cause of a range of developmental defects, which include prenatal and/or postnatal growth deficits, facial dysmorphology, cognitive deficits and neurobehavioral problems; these are collectively referred to as fetal alcohol spectrum disorder (FASD; (Williams et al., 2015)). These defects are not completely independent of each other; for example, severity of intrauterine growth restriction (IUGR) correlates with severity of cognitive dysfunction (Carter et al., 2016). A recent study (May et al., 2018) in four US regions estimates that FASD prevalence ranges 1%-5%. Among those children diagnosed with FASD, a broad spectrum of severity is observed despite similar exposure, supporting the importance of other factors involved in modulating alcohol’s actions.
Maternal nutritional status is a modifier of alcohol’s teratogenicity (Young et al., 2014). Due to the high iron demand from the rapidly growing fetus and the expanding maternal blood volume, pregnant women are particularly vulnerable to iron deficiency (ID). ID affects approximately 16% of pregnant women in the US (Brannon et al., 2017). ID adversely impacts offspring development, particularly in the brain where abnormal brain structure leads to long-lasting cognitive and behavioral dysfunction (Georgieff, 2017; McArdle et al., 2014). Using a rat model of FASD, we showed that PAE in the presence of ID (ID-PAE) causes greater impairment in the offsprings’ brain development and cognitive learning than does PAE or ID alone (Huebner et al., 2015; Rufer et al., 2012). The presence of ID also worsens alcohol’s effects on growth, as clinical studies report that individuals with both FASD and ID exhibit a delay in postnatal growth and a growth deficiency that persists into late childhood (Carter et al., 2012; Carter et al., 2007). Compared to PAE or ID alone, ID-PAE also disrupts maternal-fetal iron metabolism to a greater extent (Huebner et al., 2016), and induces stronger inflammatory responses in both the maternal and fetal compartments (Saini et al., 2019). These adverse outcomes are reversible with better maternal iron status. In this regard, our work in this FASD rat model reveals that a maternal diet fortified with iron (IF) normalizes the PAE-altered maternal-fetal iron metabolism, improves the hematological indices in the PAE fetuses, and attenuates inflammation in maternal and fetal tissues (Huebner et al., 2018; Saini et al., 2019).
The placenta, an interface between mother and fetus, performs various functions essential to the success of the pregnancy and the normal growth and healthy development of the offspring (Gude et al., 2004). One such function is to supply the fetus with adequate nutrients, such as iron. Placental nutrient supply largely depends upon its morphological development, metabolic activity and transporter availability (Lager et al., 2012). When these determinants are altered, which is described as placental insufficiency (Chaddha et al., 2004; Salavati et al., 2018), placental nutrient supply to the fetus is compromised and IUGR ensues. Although the placenta serves as a barrier for some toxic substances, teratogens including alcohol freely cross the placenta and inhibit its transport capability (Gude et al., 2004). Indeed, other studies indicate that PAE impairs nutrient flux and upregulates the production of placental pro-inflammatory cytokines including IL-1β and TNF-α (Burd et al., 2007; Ramadoss et al., 2008; Terasaki et al., 2016; Washburn et al., 2013), thereby inducing placental insufficiency. Similar changes are also reported in ID pregnancy (Gambling et al., 2002; Gambling et al., 2001).
Although the consequences of PAE and maternal iron status on fetal development, fetal iron metabolism, and inflammatory balance in fetal organs have been studied, their effects and potential interactions on these corresponding processes in the placenta remain poorly understood. Here, we report that PAE and maternal ID decrease placental weight and efficiency (markers of placental development and nutrient supply function, respectively), and these reductions are related to an altered iron flux and inflammatory balance present in the placenta. Further, we show that these adverse placental outcomes are normalized by maternal IF.
Material and Methods
Animals, diets and alcohol exposure
All animal protocols and procedures were approved by the Institutional Animal Care and Use Committees at University of Wisconsin-Madison and conducted in accordance with the Guide for the Care and Use of Laboratory Animals. The placental tissues used in the present study were previously collected from our FASD rat model subjected to different iron interventions during gestation (Huebner et al., 2016; Huebner et al., 2018). In that study, 8-week-old nulliparous Long-Evans rats were housed in a temperature-controlled room with a 12-hour light/dark cycle (light on at 7 a.m. and off at 7 p.m.). They consumed an iron sufficient (IS) diet containing 100 ppm iron (TD.06016; Teklad, Madison, WI) until mating at 10 weeks old. The morning when a vaginal plug was found was designated as gestational day (GD) 0.5. On this day, dams were randomized into IS, IF, or ID groups (n=16 dams/diet group). The IS dams continued to consume the IS diet until GD20.5. The IF dams consumed a diet containing 500 ppm iron (TD.110880; Teklad, Madison, WI) until GD20.5, this intake is similar to that found in commercial rodent chows (Huebner et al., 2018; National Research Council, 1995; Saini et al., 2019). Dams in the ID group consumed the IS diet until GD5.5, then consumed a diet containing 20 ppm iron (TD.06013; Teklad, Madison, WI) until GD13.5, and then consumed a diet containing 2-6 ppm iron (TD.80396; Teklad, Madison, WI) until GD20.5. This gradual reduction in the iron content of the maternal ID diet limits maternal hepatic iron stores but does not result in maternal anemia (Beard et al., 2006; Huebner et al., 2015; Rufer et al., 2012). No differences in food intake among dams were found (Huebner et al., 2016).
In this model of chronic binge alcohol exposure, beginning on GD13.5, half of the dams from the IS, IF and ID groups were randomized to receive 5g alcohol/kg body weight (PAE; 10 mL alcohol/kg body weight; 200 proof alcohol, USP grade; Decon Labs, King of Prussia, PA; given as 40% alcohol in water), and the remaining dams were given isocalorically-equivalent maltodextrin (MD). The alcohol and maltodextrin were given daily from GD13.5 to GD19.5 as 2 half-doses via oral gavage. The first dose was given at 9 a.m., and the second dose was given at 11 a.m. A total of 8 dams per each dietary iron-alcohol exposure group were included in this study. A separate group of dams was used to obtain blood alcohol concentration (BAC) 30 minutes after the second gavage on GD13.5. Maternal iron interventions had no effects on maternal BAC, which ranged from 364 ± 24 mg/dL to 376 ± 19 mg/dL (Huebner et al., 2016). On GD20.5, dams were euthanized, and tissues were collected and stored at −80°C until analyses.
Quantification of placental protein expression
The placentae were homogenized in a buffer containing 5 mmol EDTA/L, 1 mmol DTT/L, 1 mmol Na3VO4/L, 1% Triton-X100, 2 μmol pepstatin/L, 2 μmol leupeptin/L, 10 kIU aprotinin/mL and 400 μmol PMSF/L. Protein concentration was determined by BCA assay, and western blotting was done as described previously (Flentke et al., 2014; Huebner et al., 2016) to quantify the abundance of key proteins involved in placental iron transport (McArdle et al., 2014). The following primary antibodies were used: transferrin receptor 1 (TfRc; #13-6890, Life Technologies, 1:2000), divalent metal transporter 1 (DMT1; #sc-30120, Santa Cruz Biotechnology, 1:2000), ferritin (FTN; #sc-25617, Santa Cruz Biotechnology, 1:2000), ferroportin (FPN; #MTP-11A, Alpha Diagnostic International, 1:2000) and GAPDH (#G8795, Sigma-Aldrich, 1:10,000). Secondary antibodies (#A-21109, Life Technologies, 1:15,000; #926-68022, LI-COR, 1:15,000; #610-132-007, Rockland Immunochemicals, 1:30,000) were then applied. The protein bands were visualized using the Odyssey imaging system (LI-COR) and quantified using the Image Studio software (LI-COR). The targeted proteins were normalized to GAPDH within the same sample and then compared between samples. Alcohol treatments or maternal iron interventions did not alter GAPDH abundance (Huebner et al., 2016; Huebner et al., 2018).
Quantification of placental gene expression
All qPCR procedures followed the Minimum Information for Publication of Quantitative Real-Time Experiments (MIQE) guidelines (Bustin et al., 2009). Total RNA from the placentae was isolated following the TRIzol-chloroform protocol (Invitrogen), and its purity was confirmed by spectrophotometry (Nanodrop) and agarose gel. cDNA synthesis was performed using 1 μg RNA, random primers and ImProm-II reverse transcriptase (Promega). Real-time qPCR was performed in CFX384 Real-Time PCR Detection System (Bio-Rad) using the SYBR Green PCR Master Mix (Applied Biosystems), along with 2 μmol forward and reverse primers, cDNA template and DEPC-water in a final volume of 15 μL. Primers (Integrated DNA Technologies, Coralville, IA) for the targeted genes were designed using Primer-BLAST available on NCBI (Table 1). All primers had an efficiency between 88%-107%. The qPCR reaction conditions were as follows: 95 °C for 3 min, followed by 45 cycles of 15 sec at 95 °C, 15 sec at the annealing temperatures listed in Table 1, and 30 sec at 72 °C. A melting curve analysis was done after the last amplification cycle to confirm the specificity of the PCR products. Each sample was run in triplicate. The expression of the targeted gene was normalized to the expression of Gapdh, and the mean relative expression change was determined using 2−ΔΔCT method (Livak et al., 2001). Alcohol treatments or maternal iron interventions did not alter Gapdh abundance (Huebner et al., 2016; Huebner et al., 2018).
Table 1:
Primers information
| Gene Name | Accession Number | Primer Sequence | Amplicon Length | Annealing Temperature |
|---|---|---|---|---|
| Interleukin 1β (Il1b) | NM_031512.2 | F: 5’ – TGTGATGAAAGACGGCACAC – 3’ R: 5’ – TGTGCTCTGCTTGAGAGGTGCT – 3’ |
176 | 60.2 ºC |
| Tumor necrosis factor (Tnf) | NM_012675.3 | F: 5’ – CCGACTCTGACCCCCATTAC – 3’ R: 5’ – CCCAGAGCCACAATTCCCTT – 3’ |
98 | 60.2 ºC |
| Interleukin 6 (Il6) | NM_012589.2 | F: 5’ – ATGAGGTCTACTCGGCAAAC – 3’ R: 5’ – TCTGACCACAGTGAGGAATG – 3’ |
75 | 60.2 ºC |
| Interferon gamma (Ifng) | NM_138880.2 | F: 5’ – AGTCTGAAGAACTATTTTAACTCAAGTAGCAT – 3’ R: 5’ – CTGGCTCTCAAGTATTTTCGTGTTAC – 3’ |
117 | 60.2 ºC |
| Interleukin 10 (Il10) | NM_012854.2 | F: 5’ – TGCGACGCTGTCATCGATTT – 3’ R: 5’ – AGACACCTTTGTCTTGGAGCTT – 3’ |
96 | 61.8 ºC |
| Interleukin 4 (Il4) | NM_201270.1 | F: 5’ – AACAAGGAACACCACGGAGAA – 3’ R: 5’ – TCTTCAAGCACGGAGGTACA – 3’ |
95 | 62.2 ºC |
| Glyceraldehyde-3-Phosphate Dehydrogenase (Gapdh) | NM_017008.4 | F: 5’ – TGACAAAGTGGACATTGTTGC – 3’ R: 5’ – CTTGCCGTGGGTAGAGTCAT – 3’ |
91 | 60.2 ºC, 61.8 ºC, 62.2 ºC |
Statistical analysis
For the placental weight, placental efficiency (the ratio of fetal body weight to placental weight), and fetal body weight (Table 2), all samples within the litter were included in the analyses. Data were checked for normality and equal variance assumptions, and then analyzed using mixed linear model (MLM) in SPSS, Version 25 (SPSS Inc, Chicago, IL). The MLM included iron status, PAE, and the interaction of iron and PAE as independent fixed effect variables, and litter as independent random effect variable, because placentas or fetuses within the same litter are not independent.
For the placental protein and gene quantification (Figures 1–3 and Table 3), the litter was analyzed as an experimental unit, sampling 2 placentae per litter per endpoint while holding uterine position constant. Data were checked for normality and equal variance assumptions. For data on iron transporters, Il1b, Il10, Il4 and Il6:Il10, the assumptions were met and a 2-way analysis of variance (ANOVA) was used to determine the effects of iron status and/or PAE. The model included iron status, PAE, and the interaction of iron and PAE as independent fixed effect variables. Because both alcohol and iron status are already known to modify fetal outcomes in this model, when the ANOVA result indicated that there was a significant (P < 0.05) effect of iron status, PAE, and/or their interaction, Tukey’s honestly significant difference (HSD) test was performed for a priori comparisons of the treatments to one another in a planned, pairwise manner (e.g.: IS-MD vs. IS-PAE etc). If the normality and/or equal variance assumptions were not met, as for Il1b:Il10, Tnf:Il10 and Ifng:Il4, natural log transformation was performed prior to analyses by 2-way ANOVA and Tukey’s HSD test as detailed above. For Tnf, Ifng and Il6, assumptions were not met even after data transformation, so Kruskal-Wallis test was used along with Wilcoxon rank sum test for pairwise comparisons and Benjamini-Hochberg procedure for multiple testing correction. The protein data were analyzed using SAS (SAS Institute, Cary, NC), and the gene data were analyzed with the car package under the R software, Version 3.4.3 (Fox et al., 2012).
To determine the association of the placental cytokines with fetal and placental growth outcomes (Table 4), Pearson correlation analyses were conducted using the ggpubr package under the R software, Version 3.4.3 (Kassambara, 2017). Benjamini-Hochberg correction was applied to adjust for multiple comparisons. To identify the cytokine(s) that significantly predicted fetal and placental growth outcomes (Table 5), stepwise linear regression analyses were performed using SPSS, Version 25 (SPSS Inc, Chicago, IL).
All data were checked for outliers using Grubbs’ Test found on the GraphPad QuickCalcs Web site (https://www.graphpad.com/quickcalcs/Grubbs1.cfm) prior to the above analyses. Data are presented as means ± SEMs. P < 0.05 was defined as statistical significance, and 0.05 ≤ P < 0.10 was indicative of trends.
Results
Gestational outcomes
Starting and final maternal body weight, gestational weight gain, and maternal hematological values were previously reported (Huebner et al., 2016; Huebner et al., 2018) and reproduced here in Supplemental Tables 1–4. Neither PAE nor maternal iron intake had an effect on litter size (Table 2). As expected, PAE significantly reduced (P < 0.0001) fetal body weight (Table 2). Additionally, ID-PAE led to lower fetal body weight compared to IS-MD (−18%, P = 0.0337), ID-MD (−26%, P = 0.0012), and IF-MD (−21%, P = 0.0123). A similar PAE-induced reduction in body weight was also found even when maternal iron intake was sufficient (IS-PAE vs. ID-MD, −22%, P = 0.0066; IS-PAE vs. IF-MD, −17%, P = 0.0480) or improved (IF-PAE vs. IS-MD, −17%, P = 0.0500; IF-PAE vs. ID-MD, −25%, P = 0.0020; IF-PAE vs. IF-MD, −20%, P = 0.0191). Thus, improving maternal iron nutriture did not normalize the PAE-reduced fetal body weight to a level comparable to the MD groups.
Table 2:
Effect of prenatal alcohol exposure and maternal iron intake on litter size, fetal and placental growth outcomes on gestational day 20.5. a
| IS-MD | IS-PAE | ID-MD | ID-PAE | IF-MD | IF-PAE | ||
|---|---|---|---|---|---|---|---|
| Litter size | 12.50 ± 0.93 | 10.86 ± 1.22 | 12.25 ± 2.12 | 11.63 ± 1.51 | 11.38 ± 2.39 | 12.50 ± 1.93 | Iron group: P = 0.8968 EtOH group: P = 0.4648 Iron x EtOH: P = 0.0998 |
| Fetal weight (g) | 3.15 ± 0.05 | 2.71 ± 0.05 | 3.48 ± 0.05# | 2.57 ± 0.10*^† | 3.26 ± 0.04# | 2.62 ± 0.04*^† | Iron group: P = 0.8422 EtOH group: P < 0.0001 Iron x EtOH: P = 0.4674 |
| Placental weight (g) | 0.40 ± 0.01 | 0.37 ± 0.01 | 0.43 ± 0.01 | 0.38 ± 0.01 | 0.40 ± 0.01 | 0.36 ± 0.01^ | Iron group: P = 0.4318 EtOH group: P = 0.0324 Iron x EtOH: P = 0.7449 |
| Placental efficiency b | 8.10 ± 0.15 | 7.32 ± 0.14 | 8.41 ± 0.19 | 6.89 ± 0.18*^† | 8.32 ± 0.12 | 7.46 ± 0.15 | Iron group: P = 0.8395 EtOH group: P = 0.0043 Iron x EtOH: P = 0.6436 |
Values are means ± SDs for litter size, and means ± SEMs for fetal weight, placental weight, and placental efficiency. n = 8 litters/treatment, in which all fetuses or placentae from the same litter were included in the analyses. Data were analyzed by mixed linear model.
indicates statistical significance from IS-MD (P < 0.05);
indicates statistical significance from IS-PAE (P < 0.05);
indicates statistical significance from ID-MD (P < 0.05);
indicates statistical significance from IF-MD (P < 0.05).
Abbreviations: ID, iron deficient; IF, iron fortified; IS, iron sufficient; MD, maltodextrin; PAE, prenatal alcohol exposure.
Placental efficiency was defined as the ratio of fetal body weight to placental weight.
Similarly, PAE significantly reduced placental weight (P = 0.0324) and placental efficiency (P = 0.0043), a proxy indicator of placental function (Hayward et al., 2016). ID-PAE had the lowest placental efficiency among all groups, and it significantly differed from IS-MD (−15%, P = 0.0486), ID-MD (−18%, P = 0.0151), and IF-MD (−17%, P = 0.0212). Although not statistically significant, IF-PAE placentas had the highest efficiency among all PAE placentas, and their efficiency did not differ from any of the MD placentas (IF-PAE vs. IS-MD, P = 0.2843; IF-PAE vs. ID-MD, P = 0.1193; IF-PAE vs. IF-MD, P = 0.1543). These data suggest that poor maternal iron status may be a risk factor for reduced placental efficiency in alcohol-exposed pregnancy, and this risk can be minimized by improving maternal iron status.
Abundance of proteins involved in placental iron transport and storage
Because iron transport and storage proteins in the maternal and fetal compartments were altered in response to PAE and maternal iron intake (Huebner et al., 2016; Huebner et al., 2018), we sought to determine if similar changes were also detected in the placenta by quantifying the abundance of these proteins. PAE significantly decreased (P = 0.0032) placental TfRc abundance (Figure 1A), suggesting a reduced capacity for iron uptake from maternal circulation into the placenta under the PAE condition. Maternal ID was associated with a non-significant increase in placental TfRc, and ID-PAE had lower placental TfRc abundance compared to ID-MD (−76%, P = 0.0020) and IS-MD (−64%, P = 0.0415).
Figure 1:
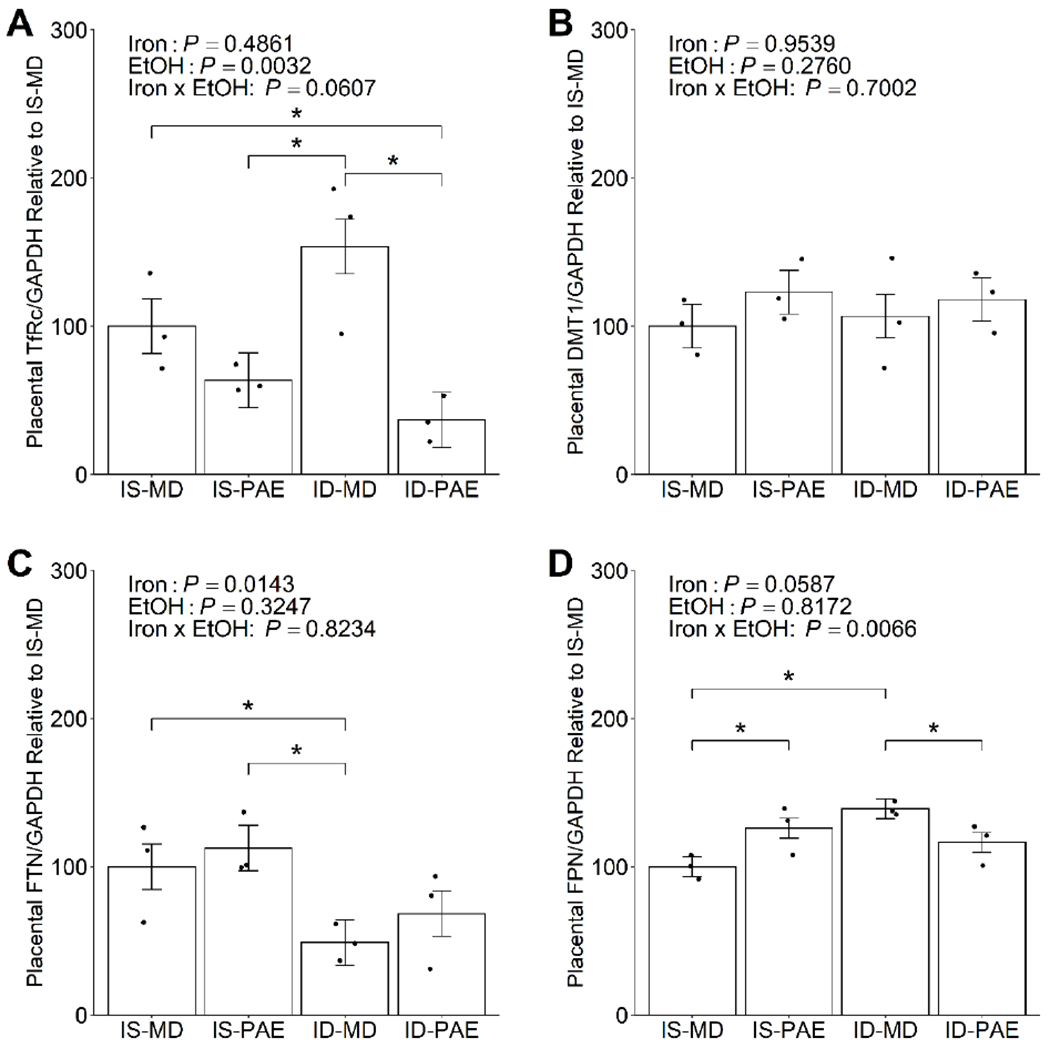
Effect of prenatal alcohol exposure and maternal iron intake on the placental abundance of (A) transferrin receptor, (B) divalent metal transporter 1, (C) ferritin, and (D) ferroportin on gestational day 20.5. Placental TfRc abundance was significantly reduced in IS-PAE compared to ID-MD, and was significantly reduced in ID-PAE compared to both IS-MD and ID-MD. Placental FTN abundance was lower in ID-MD than in IS-MD or IS-PAE. Placental FPN abundance was higher in both IS-PAE and ID-MD compared to IS-MD, and was reduced in ID-PAE compared to ID-MD. Placental DMT1 abundance was unaffected by PAE or maternal iron intake. Data were analyzed by 2-way ANOVA, followed by Tukey HSD post hoc test. Values are presented as means ± SEMs of TfRc, DMT1, FTN or FPN protein content normalized to GAPDH protein content, and are expressed relative to IS-MD. n=3 litters/treatment, 2 placentae/litter. Each dot represents an individual placenta. * indicates statistical significance (P < 0.05). Abbreviations: DMT1, divalent metal transporter 1; FPN, ferroportin; FTN, ferritin; ID, iron deficient; IF, iron fortified; IS, iron sufficient; MD, maltodextrin; PAE, prenatal alcohol exposure; TfRc, transferrin receptor.
Placental DMT1 protein abundance, which transports iron within the placental syncytiotrophoblast, was unaltered by PAE or maternal iron intake (Figure 1B). Maternal ID, as expected, significantly reduced (P = 0.0143) the protein abundance of FTN, the placental iron storage protein (Figure 1C). Iron is exported from the syncytiotrophoblast cells into fetal circulation by placental FPN, which increased (+39%, P = 0.0033) in maternal ID alone compared to maternal IS alone (Figure 1D). Additionally, PAE and maternal iron intake interacted to alter placental FPN protein abundance, and IS-PAE increased (+26%, P = 0.0253) placental FPN protein abundance compared to IS-MD, and ID-PAE reduced (−17%, P = 0.0427) placental FPN protein abundance compared to ID-MD. Nevertheless, effects of PAE on these proteins were relatively modest; hence, they were unlikely to be a key factor driving the differences observed in fetal and placental growth outcomes. In support of this, we previously showed that PAE does not affect the total iron content in the fetus, but rather dysregulates the iron distribution between fetal brain and fetal liver, suggesting that it does not impair placental iron transport per se (Huebner et al., 2016; Huebner et al., 2018). Thus, we did not examine the effect of maternal IF on the abundance of these iron proteins in the PAE placentae.
Expression of inflammatory cytokines in the placenta
Because placental pro-inflammatory responses adversely affect placental development, and are implicated in the pathology of placental insufficiency and IUGR (Bowen, Chamley, Keelan, et al., 2002; Chaddha et al., 2004; Challis et al., 2009; Gelber et al., 2015; Li et al., 2009; Wang et al., 2003), we performed qPCR experiments to evaluate placental cytokine gene expression from animals exposed to PAE and various iron treatments. We found that placental cytokine gene expression was affected by PAE and/or maternal iron intake. There was a significant main effect of PAE (P = 0.0337) on placental Il1b expression (Figure 2A). There was also a significant main effect of iron (P = 0.0084), which was largely driven by the downregulation (−40%, P = 0.0328) of placental Il1b expression in IF-MD vs. ID-MD. In addition, PAE and iron interacted to modulate placental Il1b expression such that PAE had a higher (+65%, P = 0.0355) placental expression of Il1b than MD when the mother received the IF diet during gestation. Placental Tnf expression was also similarly impacted (P = 0.0300; Figure 2B). It was downregulated in the IS-PAE placentae compared to the IS-MD placentae (−25%, P = 0.037), and in the IF-MD placentae compared to the ID-MD placentae (−28%, P = 0.037). These data support an effect of PAE and maternal iron intake, both independently and synergistically, on the expression of these placental cytokines. However, placental Il6 and Ifng expression remained unchanged in response to PAE or maternal iron intake (Figures 2C and 2D), suggesting a selective effect of PAE and/or iron on placental cytokines.
Figure 2:
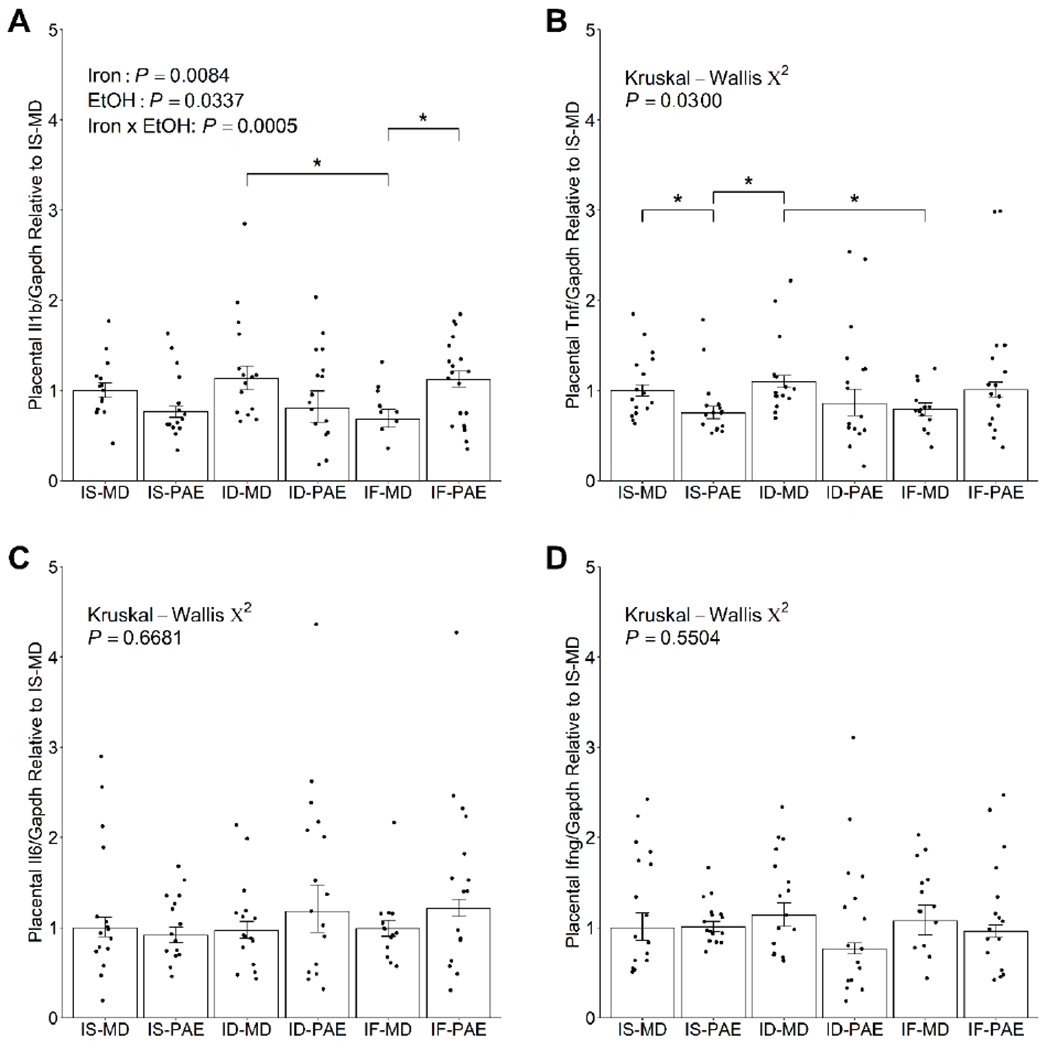
Effect of prenatal alcohol exposure and maternal iron intake on placental expression of pro-inflammatory cytokines (A) Il1b, (B) Tnf, (C) Il6, and (D) Ifng on gestational day 20.5. IF-MD downregulated placental Il1b expression compared to ID-MD. Within the IF groups, PAE significantly increased placental Il1b expression compared to MD. Placental Tnf expression in IS-PAE was significantly lower than in IS-MD or ID-MD. IF-MD also downregulated placental Tnf expression compared to ID-MD. Placental Il6 and Ifng expression were unaffected by PAE or maternal iron intake. Data on Il1b were analyzed by 2-way ANOVA, followed by Tukey HSD post hoc test. Data on Tnf, Il6 and Ifng were analyzed by Kruskal-Wallis test, followed by Wilcoxon rank sum test with Benjamini-Hochberg correction for post hoc pairwise comparisons. Values are presented as means ± SEMs of Il1b, Tnf, Il6 or Ifng transcript content normalized to Gapdh transcript content, and are expressed relative to IS-MD. n=8 litters/treatment, 2 placentae/litter. Each dot represents the mean value for an individual placenta across the triplicates run for the qPCR experiments. * indicates statistical significance (P < 0.05). Abbreviations: ID, iron deficient; IF, iron fortified; Ifng, interferon gamma; Il1b, interleukin 1 beta; Il6, interleukin 6; IS, iron sufficient; MD, maltodextrin; PAE, prenatal alcohol exposure; Tnf, tumor necrosis factor.
A trend (+70%, P = 0.056) for higher placental expression of Il10, an anti-inflammatory cytokine, in IF-PAE relative to IS-PAE was also found (Figure 3A), suggesting that it may be upregulated in PAE if maternal iron intake is improved. In contrast, PAE and maternal iron intake did not affect placental Il4 expression (Figure 3B). This again affirms that PAE and/or iron selectively modulate placental cytokines.
Figure 3:
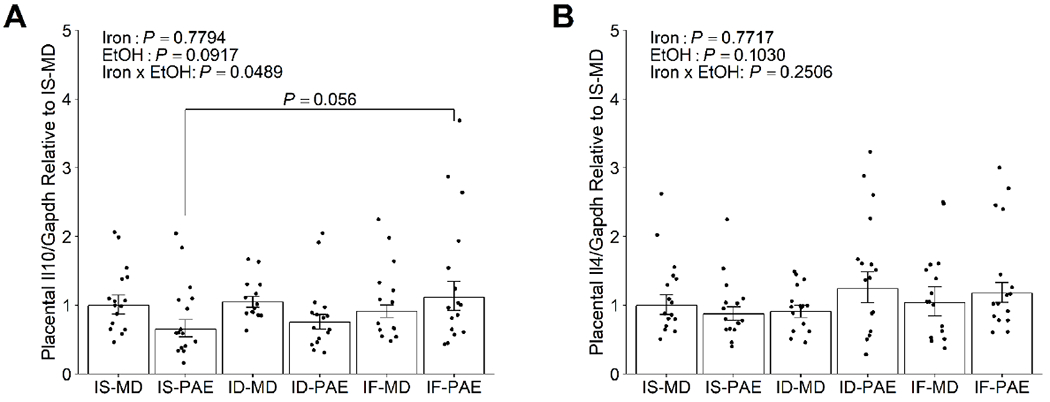
Effect of prenatal alcohol exposure and maternal iron intake on placental expression of anti-inflammatory cytokines (A) Il10, and (B) Il4 on gestational day 20.5. IF-PAE trended to have a higher placental Il10 expression than IS-PAE. Both PAE and maternal iron intake had no effects on placental Il4 expression. Data were analyzed by 2-way ANOVA, followed by Tukey HSD post hoc test. Values are presented as means ± SEMs of Il10 or Il4 transcript content normalized to Gapdh transcript content, and are expressed relative to IS-MD. n=8 litters/treatment, 2 placentae/litter. Each dot represents the mean value for an individual placenta across the triplicates run for the qPCR experiments. Abbreviations: ID, iron deficient; IF, iron fortified; Il4, interleukin 4; Il10, interleukin 10; IS, iron sufficient; MD, maltodextrin; PAE, prenatal alcohol exposure.
Pro-inflammatory cytokines and anti-inflammatory cytokines are dependent upon each other, jointly regulating immune response through a feedback mechanism (Mosser et al., 2008; Paludan, 1998). Indeed, specific interactions have been shown among IL-1β, TNF-α, IL-6 and IL-10, as well as IFN-γ and IL-4, and an imbalance of these cytokines has been associated with adverse pregnancy outcomes (Calleja-Agius et al., 2012; Dong et al., 2005; Nan et al., 2007; Scott et al., 2009; Weel et al., 2016). To determine if PAE and/or iron also similarly disrupt the inflammatory balance in the placenta, we evaluated these known interactions by calculating a ratio of each pro-inflammatory cytokine to the functionally-relevant anti-inflammatory cytokine.
Il1b:Il10 and Tnf:Il10 ratios were not significantly affected by PAE or maternal iron intake (Table 3). In contrast, the placental Il6:Il10 ratio was increased (P = 0.0034) whereas the Ifng:Il4 ratio was decreased (P = 0.0109) in response to PAE. Follow-up pairwise analyses revealed that PAE doubled (P = 0.0385) the Il6:Il10 ratio compared to MD if the mother was also ID. Compared to IS-MD, the ID-PAE placentae also tended to exhibit a higher Il6:Il10 ratio (P = 0.0662). Maternal IF reduced the Il6:Il10 ratio to a level comparable to the IS groups. Together, these data indicate a shift of inflammatory response to favor a pro-inflammatory response that converged upon IL-6 under the ID-PAE condition, and this was mitigated with an improved maternal iron status.
Table 3:
Effect of prenatal alcohol exposure and maternal iron intake on the pro- vs. anti- inflammatory cytokine ratios in gestational day 20.5 placentae. a
| IS-MD | IS-PAE | ID-MD | ID-PAE | IF-MD | IF-PAE | ||
|---|---|---|---|---|---|---|---|
| Il1b:Il10 | 35.62 ± 3.92 | 47.93 ± 6.03 | 42.86 ± 5.52 | 49.14 ± 7.73 | 31.04 ± 4.00 | 41.82 ± 5.99 | Iron group: P = 0.3290 EtOH group: P = 0.6725 Iron x EtOH: P = 0.7691 |
| Tnf:Il10 | 5.42 ± 0.83 | 6.16 ± 0.76 | 5.50 ± 0.74 | 5.83 ± 0.90 | 4.35 ± 0.72 | 4.35 ± 0.45 | Iron group: P = 0.2953 EtOH group: P = 0.9522 Iron x EtOH: P = 0.9119 |
| Il6:Il10 | 10.18 ± 1.68 | 16.34 ± 2.63 | 9.62 ± 1.33 | 19.99 ± 4.12* | 11.08 ± 2.22 | 10.89 ± 1.39 | Iron group: P = 0.9133 EtOH group: P = 0.0034 Iron x EtOH: P = 0.1027 |
| Ifng:Il4 | 2.64 ± 0.46 | 2.76 ± 0.37 | 3.07 ± 0.41 | 1.77 ± 0.45# | 3.51 ± 0.54 | 2.27 ± 0.50 | Iron group: P = 0.4035 EtOH group: P = 0.0109 Iron x EtOH: P = 0.0840 |
Values are means ± SEMs. n = 8 litters/treatment, sampling 2 placentae/litter.
indicates statistical significance from ID-MD (P < 0.05);
indicates statistical significance from IF-MD (P < 0.05).
Abbreviations: ID, iron deficient; IF, iron fortified; IS, iron sufficient; MD, maltodextrin; PAE, prenatal alcohol exposure.
Correlation of placental cytokines and growth indices
To determine if these placental cytokines were associated with fetal and placental growth, we performed Pearson correlation analyses (Table 4). The results indicated that select cytokines were moderately associated with fetal weight. Specifically, placental Il6 expression (R = −0.43, P = 0.0003) and Il6:Il10 ratio (R = −0.37, P = 0.0014) were both inversely associated with fetal body weight. Placental Ifng (R = 0.28, P = 0.0158) and Il4 (R = −0.38, P = 0.0014) expression as well as Ifng:Il4 ratio (R = 0.36, P = 0.0018) were also related to fetal body weight.
For the placental outcomes, the correlation analyses showed that placental Il6 expression was inversely (R = −0.35, P = 0.010) related to placental weight (Table 4). No other cytokines had a significant relationship with placental weight. Similarly, after multiple testing adjustment, none of the placental cytokines examined in the present study remained significantly correlated with placental efficiency.
Table 4:
Correlation between placental inflammatory cytokine expression and their ratios, with fetal and placental growth indices on gestational day 20.5. a
| Fetal weight | Placental weight | Placental efficiency | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R | Punadjusted | PBH-adjusted | R | Punadjusted | PBH-adjusted | R | Punadjusted | PBH-adjusted | |
| Il1b | 0.015 | 0.89 | 0.94 | −0.22 | 0.043 | 0.086 | 0.19 | 0.08 | 0.20 |
| Tnf | 0.008 | 0.94 | 0.94 | −0.20 | 0.059 | 0.098 | 0.23 | 0.035 | 0.20 |
| Il6 | −0.430 | < 0.001 | 0.0003 * | −0.35 | 0.001 | 0.0100 * | −0.13 | 0.23 | 0.29 |
| Ifng | 0.280 | 0.0079 | 0.0158 * | 0.14 | 0.20 | 0.25 | 0.15 | 0.17 | 0.24 |
| Il10 | 0.140 | 0.21 | 0.35 | −0.02 | 0.87 | 0.87 | 0.22 | 0.040 | 0.20 |
| Il4 | −0.380 | 0.0003 | 0.0014 * | −0.25 | 0.019 | 0.063 | −0.19 | 0.08 | 0.20 |
| Il1b:Il10 | −0.097 | 0.37 | 0.48 | −0.18 | 0.09 | 0.13 | 0.007 | 0.95 | 0.95 |
| Tnf:Il10 | −0.095 | 0.38 | 0.48 | −0.12 | 0.27 | 0.30 | −0.027 | 0.80 | 0.89 |
| Il6:Il10 | −0.370 | 0.0004 | 0.0014 * | −0.26 | 0.015 | 0.063 | −0.17 | 0.12 | 0.20 |
| Ifng:Il4 | 0.360 | 0.0007 | 0.0018 * | 0.23 | 0.030 | 0.075 | 0.17 | 0.11 | 0.20 |
Litters from all 6 treatment groups (n = 8 litters/treatment, 2 fetuses or placentae/litter) were included in the analyses. P values before multiple testing adjustment, and after multiple testing adjustment using Benjamini-Hochberg method are presented.
indicates statistical significance (P < 0.05).
Predictive values of placental cytokines on the growth outcomes
Because our correlation analyses suggested relationships between the placental cytokines examined in this study and fetal and placental growth, we sought to determine the predictive values of these placental cytokines upon the growth outcomes using stepwise linear regression models. The results (Table 5) revealed that placental Il6 expression was the best predictor of fetal weight in our FASD model, explaining 18.6% of the variability in fetal weight. Placental Tnf expression entered the regression model at the second step and was the second best predictor of fetal weight in our FASD model, explaining an additional 19.9% of the variability. Placental Ifng expression entered the regression model at the third step and was the third best predictor of fetal weight in our FASD model, explaining another 6.3% of the variability. Together, placental Il6, Tnf, and Ifng expression explained more than 44% of the variability observed in fetal weight in our FASD model.
Table 5:
Stepwise linear regression analyses identify the placental cytokines that best predict fetal and placental growth outcomes, and the amount of variance explained by these cytokines. a
| Predictor(s) included in the model | % variability explained by the model | P value of the model | |
|---|---|---|---|
| Fetal weight: | |||
| Model 1 | Il6 | 18.6 | < 0.001 |
| Model 2 | Il6, Tnf | 38.5 | < 0.001 |
| Model 3 | Il6, Tnf, Ifng | 44.8 | < 0.001 |
| Equation of regression line: Fetal weight = 2.780 – 47.064 (Il6) + 85.773 (Tnf) + 77.755 (Ifng) | |||
| Placental weight: | |||
| Model 1 | Il6 | 10.4 | 0.002 |
| Model 2 | Il6, Ifng | 14.4 | 0.001 |
| Equation of regression line: Placental weight = 0.398 – 2.777 (Il6) + 7.618 (Ifng) | |||
| Placental efficiency: | |||
| Model 1 | Tnf | 5.6 | 0.023 |
| Model 2 | Tnf, Il6 | 19.2 | < 0.001 |
| Equation of regression line: Placental efficiency = 7.366 + 280.359 (Tnf) – 88.278 (Il6) | |||
Litters from all 6 treatment groups (n = 8 litters/treatment, 2 fetuses or placentae/litter) were included in the analyses.
Placental Il6 expression was also the best predictor of placental weight in our FASD model, as it explained 10.4% of the variability in placental weight (Table 5). Placental Ifng expression entered the regression model at the second step and was the second best predictor of placental weight, accounting for an additional 4.0% of the variability. Collectively, placental Il6 and Ifng expression explained a total of 14.4% of the variability found in placental weight in our FASD model.
The best predictor of placental efficiency in our FASD model was placental Tnf expression, which explained 5.6% of the variability (Table 5). Placental Il6 expression also predicted placental efficiency, as it entered the regression model at the second step and accounted for another 13.6% of the variability. Together, placental Tnf and Il6 expression explained a total of 19.2% of the variability observed in placental efficiency in our FASD model.
Discussion
The etiology of intrauterine growth restriction (IUGR) is multifactorial, with placental insufficiency accounting for a majority of the cases (“ACOG practice bulletin no. 134: Fetal growth restriction,” 2013). IUGR is one of the diagnostic criteria of FAS, the most severe end of the FASD spectrum (Williams et al., 2015), and previous reports have described the presence of placental insufficiency in FASD pregnancies (Burd et al., 2007; Gundogan et al., 2008; Keegan et al., 2010; Tai et al., 2017). In this study, we found a significant effect of PAE on the placental efficiency ratio. More importantly, we found that this effect of alcohol on placental efficiency is driven largely by poor maternal iron status, in which the lowest placental efficiency ratio is detected. This finding suggests that maternal ID is a risk factor that may exacerbate the placental insufficiency previously reported in FASD pregnancies.
Upregulation of placental pro-inflammatory cytokines and disruption of normal inflammatory balance are implicated in placental insufficiency (Bowen, Chamley, Keelan, et al., 2002; Chaddha et al., 2004; Challis et al., 2009; Gelber et al., 2015; Li et al., 2009; Wang et al., 2003). Pro-inflammatory cytokines regulate placental angiogenesis and perfusion through modulating the expression and activities of angiogenic proteins (Bowen, Chamley, Mitchell, et al., 2002; Cotechini et al., 2014; Rizov et al., 2017; Shah et al., 2015). They also activate apoptosis to increase the death of endothelial cells (Bowen, Chamley, Keelan, et al., 2002; Huppertz et al., 2006; Sharp et al., 2010) and trophoblast cells (Atia, 2017; Huppertz et al., 2004; Ribeiro et al., 2018; Sharp et al., 2010; Straszewski-Chavez et al., 2005). In contrast, placental anti-inflammatory cytokines counteract the actions of pro-inflammatory cytokines to promote normal placental morphology and sufficient perfusion (Naldini et al., 2003; Orange et al., 2005). PAE and ID are both pro-inflammatory stimuli capable of independently inducing pro-inflammatory cytokine production (Ahluwalia et al., 2000; Svinarich et al., 1998; Terasaki et al., 2016) that subsequently alter placental vascularization (Biswas et al., 2014; Gundogan et al., 2008; Gundogan et al., 2015; Lewis et al., 2001; Lo et al., 2017; Sharma et al., 2017; Tai et al., 2017). Here, we identify both PAE and iron effects on the placental expression of the pro-inflammatory cytokines Il1b and Tnf, as well as the anti-inflammatory cytokine Il10. These changes produce a cytokine imbalance that collectively generates a pro-inflammatory placental response, consistent with the higher placental pro-inflammatory cytokine to anti-inflammatory cytokine ratio. Notably, this placental inflammatory imbalance is higher in PAE, and is more pronounced in ID-PAE. Although additional studies are needed, we hypothesize that the observed placental pro-inflammatory response in PAE will damage normal placental vasculature and morphology, which will contribute to the lower placental efficiency ratio. We further predict that the presence of an intensified placental pro-inflammatory response in ID-PAE will produce more severe placental vascular and morphological abnormalities, further reducing the placental efficiency ratio. Regardless of the underlying mechanisms, placental pro-inflammatory cytokines are related and predictive of fetal and placental growth in our FASD model.
Carter and colleagues (2007) found a lower body weight at birth and early postnatal life in infants who are prenatally exposed to alcohol. Moreover, ID exacerbated alcohol’s effects, and the body weight reductions were greatest in infants who also had iron deficiency anemia. Their follow-up study (Carter et al., 2016) found that postnatal growth inversely correlates with the severity of cognitive dysfunction. Our preclinical rat model similarly found that the ID-PAE fetuses had the lowest body weight and severe anemia (Huebner et al., 2016). Unfortunately, our study only measured body weight at a single time point, just prior to birth, and we do not know if the ID-PAE fetuses continue their growth restriction after birth, and whether this extends to worsened cognitive problems. However, the placentae of these ID-PAE fetuses exhibit the most intensified inflammatory response and have the lowest placental efficiency ratio. Given that normal placental functioning is a crucial determinant of fetal body weight and the offspring’s future health (Thornburg et al., 2016), we speculate that improved placental function, perhaps through improved iron status, might reduce placental inflammation and enhance placental efficiency, and thus improve the growth outcomes of these individuals.
Supporting this is our finding that alcohol’s deleterious effects in the placenta are reversible by maternal IF. Maternal IF normalized the PAE-reduced placental efficiency, possibly in part by upregulating placental Il10 to dampen the PAE-triggered placental pro-inflammatory response to level comparable to IS-MD. We do not know if maternal IF also improves placental vascularization and morphology, which are also modified by inflammation. Pharmaceutical interventions that induce anti-inflammatory cytokine production have been shown to normalize placental inflammation and vascularization and improve placental efficiency (Amash et al., 2012; Tinsley et al., 2010; Xu et al., 2007). It will be important to test whether IF in this model confers a similar benefit. Improving maternal nutriture through dietary iron fortification does not increase cellular oxidation or alter the bioavailability of related minerals (Huebner et al., 2018), and lacks the undesirable side effects such as gastrointestinal distress commonly caused by oral iron supplementation (Tolkien et al., 2015). Collectively, these data suggest that dietary iron fortification may be an acceptable option for mitigating the placental insufficiency associated with PAE, thereby improving pregnancy outcomes.
We recognize that the placenta responds to gestational insults including prenatal alcohol exposure and poor maternal iron intake in a sex-specific manner; however, limited sample availability precludes our ability to determine the sex of the fetuses used in this study. Consequently, this information is not included in our current analyses. It is also important to note that our alcohol regimen models a chronic binge exposure, and cannot inform the consequence of low-moderate intake. Because 3.9% of pregnant women reported binge drinking in the past month, our model is of high clinical relevance and addresses an important public health problem.
In conclusion, these data demonstrate that placental efficiency is adversely affected by PAE, and this correlates with a cytokine dysregulation which promotes an intensified placental inflammatory response. Moreover, maternal iron status is a critical and previously unappreciated modulator of placental responses to PAE. Improvement of iron status may be a novel avenue for improving gestational outcomes in alcohol-exposed pregnancy, in part through its effects on placenta. Such a mechanism may partially explain how maternal multivitamin/mineral supplementation improves measures of cognition and memory in infants exposed to PAE (Coles et al., 2015; Kable et al., 2015).
Supplementary Material
Highlights.
PAE causes placental insufficiency, as shown by lower weight and efficiency ratio.
Maternal ID worsens, whereas IF improves, PAE-induced placental insufficiency.
Placental inflammatory imbalance may contribute to PAE’s placental insufficiency.
Placental cytokine profiles predict growth outcomes in this FASD model.
Acknowledgments
Grant Funding:
This work was supported by NIH/NIAAA R01 Award to S.M.S. (awards #R01AA22999 and #R01AA11085), NIH/NIAAA Fellowship Award to S.T.C.K. (award #F32AA027121), NIH/NIDDK Training Grant to K.K.H. (award #T32DK007686), and NIH/NIAAA Fellowship Award to S.M.H. (award #F32AA21311). The funder had no role in the study design, data collection, data analyses, data interpretation, and manuscript preparation.
Footnotes
Declaration of Interest: None
Reference
- ACOG practice bulletin no. 134: Fetal growth restriction. (2013). Obstet Gynecol, 121(5), 1122–1133. [DOI] [PubMed] [Google Scholar]
- Ahluwalia B, Wesley B, Adeyiga O, Smith DM, Da-Silva A, & Rajguru S (2000). Alcohol modulates cytokine secretion and synthesis in human fetus: an in vivo and in vitro study. Alcohol, 21(3), 207–213. [DOI] [PubMed] [Google Scholar]
- Amash A, Holcberg G, Sapir O, & Huleihel M (2012). Placental secretion of interleukin-1 and interleukin-1 receptor antagonist in preeclampsia: Effect of magnesium sulfate. J Interferon Cytokine Res, 32(9), 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atia TA (2017). Placental apoptosis in recurrent miscarriage. Kaohsiung J Med Sci, 33(9), 449–452. [DOI] [PubMed] [Google Scholar]
- Beard JL, Felt B, Schallert T, Burhans M, Connor JR, & Georgieff MK (2006). Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res, 170(2), 224–232. [DOI] [PubMed] [Google Scholar]
- Biswas S, Meyur R, Adhikari A, Bose K, & Kundu P (2014). Placental changes associated with maternal anaemia. Eur J Anat, 18(3), 165–169. [Google Scholar]
- Bowen JM, Chamley L, Keelan JA, & Mitchell MD (2002). Cytokines of the placenta and extra-placental membranes: Roles and regulation during human pregnancy and parturition. Placenta, 23(4), 257–273. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Chamley L, Mitchell MD, & Keelan JA (2002). Cytokines of the placenta and extra-placental membranes: Biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta, 23(4), 239–256. [DOI] [PubMed] [Google Scholar]
- Brannon PM, & Taylor CL (2017). Iron supplementation during pregnancy and infancy: Uncertainties and implications for research and policy. Nutrients, 9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd L, Roberts D, Olson M, & Odendaal H (2007). Ethanol and the placenta: A review. J Matern Fetal Neonatal Med, 20(5), 361–375. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (2009). The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem, 55(4), 611–622. [DOI] [PubMed] [Google Scholar]
- Calleja-Agius J, Jauniaux E, & Muttukrishna S (2012). Inflammatory cytokines in maternal circulation and placenta of chromosomally abnormal first trimester miscarriages. Clin Dev Immunol, 2012, 175041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, & Jacobson SW (2016). Fetal alcohol growth restriction and cognitive impairment. Pediatrics, 138(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Jiang H, Meintjes EM, Jacobson SW, et al. (2012). Effects of heavy prenatal alcohol exposure and iron deficiency anemia on child growth and body composition through age 9 years. Alcohol Clin Exp Res, 36(11), 1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Jacobson SW, Molteno CD, & Jacobson JL (2007). Fetal alcohol exposure, iron-deficiency anemia, and infant growth. Pediatrics, 120(3), 559–567. [DOI] [PubMed] [Google Scholar]
- Chaddha V, Viero S, Huppertz B, & Kingdom J (2004). Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med, 9(5), 357–369. [DOI] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, & Petraglia F (2009). Inflammation and pregnancy. Reprod Sci, 16(2), 206–215. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, et al. (2015). Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Matern Child Health J, 19(12), 2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, & Graham CH (2014). Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med, 211(1), 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CH, Acero CS, Naimi TS, & Kim SY (2019). Consumption of alcohol beverages and binge drinking among pregnant women aged 18-44 years - United States, 2015-2017. MMWR Morb Mortal Wkly Rep, 68(16), 365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, He J, Wang Z, Xie X, & Wang H (2005). Placental imbalance of Th1- and Th2-type cytokines in preeclampsia. Acta Obstet Gynecol Scand, 84(8), 788–793. [DOI] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Hernandez M, & Smith SM (2014). CaMKII represses transcriptionally active beta-catenin to mediate acute ethanol neurodegeneration and can phosphorylate beta-catenin. J Neurochem, 128(4), 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S, Bates D, & Fox MJ (2012). Package “car”. https://cran.r-project.org/web/packages/car/car.pdf.
- Gambling L, Charania Z, Hannah L, Antipatis C, Lea RG, & McArdle HJ (2002). Effect of iron deficiency on placental cytokine expression and fetal growth in the pregnant rat. Biol Reprod, 66(2), 516–523. [DOI] [PubMed] [Google Scholar]
- Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, et al. (2001). Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J, 356(Pt 3), 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber SE, Brent E, Redecha P, Perino G, Tomlinson S, Davisson RL, et al. (2015). Prevention of defective placentation and pregnancy loss by blocking innate immune pathways in a syngeneic model of placental insufficiency. J Immunol, 195(3), 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff MK (2017). Iron assessment to protect the developing brain. Am J Clin Nutr, 106(Suppl 6), 1588s–1593s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gude NM, Roberts CT, Kalionis B, & King RG (2004). Growth and function of the normal human placenta. Thromb Res, 114(5–6), 397–407. [DOI] [PubMed] [Google Scholar]
- Gundogan F, Elwood G, Longato L, Tong M, Feijoo A, Carlson RI, et al. (2008). Impaired placentation in fetal alcohol syndrome. Placenta, 29(2), 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogan F, Gilligan J, Qi W, Chen E, Naram R, & de la Monte SM (2015). Dose effect of gestational ethanol exposure on placentation and fetal growth. Placenta, 36(5), 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, et al. (2016). Placental adaptation: What can we learn from birthweight:placental weight ratio? Front Physiol, 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner SM, Blohowiak SE, Kling PJ, & Smith SM (2016). Prenatal alcohol exposure alters fetal iron distribution and elevates hepatic hepcidin in a rat model of fetal alcohol spectrum disorders. J Nutr, 146(6), 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner SM, Helfrich KK, Saini N, Blohowiak SE, Cheng AA, Kling PJ, et al. (2018). Dietary iron fortification normalizes fetal hematology, hepcidin, and iron distribution in a rat model of prenatal alcohol exposure. Alcohol Clin Exp Res, 42(6), 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner SM, Tran TD, Rufer ES, Crump PM, & Smith SM (2015). Maternal iron deficiency worsens the associative learning deficits and hippocampal and cerebellar losses in a rat model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res, 39(11), 2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B, Kadyrov M, & Kingdom JC (2006). Apoptosis and its role in the trophoblast. Am J Obstet Gynecol, 195(1), 29–39. [DOI] [PubMed] [Google Scholar]
- Huppertz B, & Kingdom JC (2004). Apoptosis in the trophoblast--Role of apoptosis in placental morphogenesis. J Soc Gynecol Investig, 11(6), 353–362. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok L, et al. (2015). The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol, 49(7), 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A (2017). ggpubr:’ggplot2’based publication ready plots. https://cran.r-project.org/web/packages/ggpubr/ggpubr.pdf. [Google Scholar]
- Keegan J, Parva M, Finnegan M, Gerson A, & Belden M (2010). Addiction in pregnancy. J Addict Dis, 29(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Lager S, & Powell TL (2012). Regulation of nutrient transport across the placenta. J Pregnancy, 2012, 179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RM, Doherty CB, James LA, Burton GJ, & Hales CN (2001). Effects of maternal iron restriction on placental vascularization in the rat. Placenta, 22(6), 534–539. [DOI] [PubMed] [Google Scholar]
- Li M, & Huang SJ (2009). Innate immunity, coagulation and placenta-related adverse pregnancy outcomes. Thromb Res, 124(6), 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods, 25(4), 402–408. [DOI] [PubMed] [Google Scholar]
- Lo JO, Schabel MC, Roberts VH, Wang X, Lewandowski KS, Grant KA, et al. (2017). First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am J Obstet Gynecol, 216(3), 302.e301–302.e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, et al. (2018). Prevalence of fetal alcohol spectrum disorders in 4 US communities. Jama, 319(5), 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle HJ, Gambling L, & Kennedy C (2014). Iron deficiency during pregnancy: the consequences for placental function and fetal outcome. Proc Nutr Soc, 73(1), 9–15. [DOI] [PubMed] [Google Scholar]
- Mosser DM, & Zhang X (2008). Interleukin-10: New perspectives on an old cytokine. Immunol Rev, 226, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini A, Pucci A, Bernini C, & Carraro F (2003). Regulation of angiogenesis by Th1- and Th2-type cytokines. Curr Pharm Des, 9(7), 511–519. [DOI] [PubMed] [Google Scholar]
- Nan CL, Lei ZL, Zhao ZJ, Shi LH, Ouyang YC, Song XF, et al. (2007). Increased Th1/Th2 (IFN-gamma/IL-4) cytokine mRNA ratio of rat embryos in the pregnant mouse uterus. J Reprod Dev, 53(2), 219–228. [DOI] [PubMed] [Google Scholar]
- National Research Council. (1995). Nutrient requirements of laboratory animals,: fourth revised edition, 1995 Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Orange S, Rasko JE, Thompson JF, Vaughan J, Olive E, Pedler M, et al. (2005). Interleukin-10 regulates arterial pressure in early primate pregnancy. Cytokine, 29(4), 176–185. [DOI] [PubMed] [Google Scholar]
- Paludan SR (1998). Interleukin-4 and interferon-gamma: The quintessence of a mutual antagonistic relationship. Scand J Immunol, 48(5), 459–468. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Wu G, & Cudd TA (2008). Chronic binge ethanol-mediated acidemia reduces availability of glutamine and related amino acids in maternal plasma of pregnant sheep. Alcohol, 42(8), 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MR, Moreli JB, Marques RE, Papa MP, Meuren LM, Rahal P, et al. (2018). Zika-virus-infected human full-term placental explants display pro-inflammatory responses and undergo apoptosis. Arch Virol, 163(10), 2687–2699. [DOI] [PubMed] [Google Scholar]
- Rizov M, Andreeva P, & Dimova I (2017). Molecular regulation and role of angiogenesis in reproduction. Taiwan J Obstet Gynecol, 56(2), 127–132. [DOI] [PubMed] [Google Scholar]
- Rufer ES, Tran TD, Attridge MM, Andrzejewski ME, Flentke GR, & Smith SM (2012). Adequacy of maternal iron status protects against behavioral, neuroanatomical, and growth deficits in fetal alcohol spectrum disorders. PLoS One, 7(10), e47499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini N, Helfrich KK, Kwan STC, Huebner SM, Abazi J, Flentke GR, et al. (2019). Alcohol’s dysregulation of maternal-fetal IL-6 and p-STAT3 is a function of maternal iron status. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavati N, Smies M, Ganzevoort W, Charles AK, Erwich JJ, Plosch T, et al. (2018). The possible role of placental morphometry in the detection of fetal growth restriction. Front Physiol, 9, 1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott NM, Hodyl NA, Murphy VE, Osei-Kumah A, Wyper H, Hodgson DM, et al. (2009). Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol, 182(3), 1411–1420. [DOI] [PubMed] [Google Scholar]
- Shah DA, & Khalil RA (2015). Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol, 95(4), 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, & Mirza A (2017). Effect of maternal iron status on placenta, fetus and newborn. Global Journal of Food Science and Technology, 5(2), 146–150. [Google Scholar]
- Sharp AN, Heazell AE, Crocker IP, & Mor G (2010). Placental apoptosis in health and disease. Am J Reprod Immunol, 64(3), 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straszewski-Chavez SL, Abrahams VM, & Mor G (2005). The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev, 26(7), 877–897. [DOI] [PubMed] [Google Scholar]
- Svinarich DM, DiCerbo JA, Zaher FM, Yelian FD, & Gonik B (1998). Ethanol-induced expression of cytokines in a first-trimester trophoblast cell line. Am J Obstet Gynecol, 179(2), 470–475. [DOI] [PubMed] [Google Scholar]
- Tai M, Piskorski A, Kao JC, Hess LA, de la Monte S,M, & Gundogan F (2017). Placental morphology in fetal alcohol spectrum disorders. Alcohol Alcohol, 52(2), 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki LS, & Schwarz JM (2016). Effects of moderate prenatal alcohol exposure during early gestation in rats on inflammation across the maternal-fetal-immune interface and later-life immune function in the offspring. J Neuroimmune Pharmacol, 11(4), 680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg KL, Kolahi K, Pierce M, Valent A, Drake R, & Louey S (2016). Biological features of placental programming. Placenta, 48 Suppl 1, S47–s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JH, South S, Chiasson VL, & Mitchell BM (2010). Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol, 298(3), R713–719. [DOI] [PubMed] [Google Scholar]
- Tolkien Z, Stecher L, Mander AP, Pereira DI, & Powell JJ (2015). Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One, 10(2), e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Athayde N, & Trudinger B (2003). A proinflammatory cytokine response is present in the fetal placental vasculature in placental insufficiency. Am J Obstet Gynecol, 189(5), 1445–1451. [DOI] [PubMed] [Google Scholar]
- Washburn SE, Sawant OB, Lunde ER, Wu G, & Cudd TA (2013). Acute alcohol exposure, acidemia or glutamine administration impacts amino acid homeostasis in ovine maternal and fetal plasma. Amino Acids, 45(3), 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weel IC, Baergen RN, Romao-Veiga M, Borges VT, Ribeiro VR, Witkin SS, et al. (2016). Association between placental lesions, cytokines and angiogenic factors in pregnant women with preeclampsia. PLoS One, 11(6), e0157584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JF, & Smith VC (2015). Fetal alcohol spectrum disorders. Pediatrics, 136(5), e1395–1406. [DOI] [PubMed] [Google Scholar]
- Xu DX, Wang H, Ning H, Zhao L, & Chen YH (2007). Maternally administered melatonin differentially regulates lipopolysaccharide-induced proinflammatory and anti-inflammatory cytokines in maternal serum, amniotic fluid, fetal liver, and fetal brain. J Pineal Res, 43(1), 74–79. [DOI] [PubMed] [Google Scholar]
- Young JK, Giesbrecht HE, Eskin MN, Aliani M, & Suh M (2014). Nutrition implications for fetal alcohol spectrum disorder. Adv Nutr, 5(6), 675–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


