Abstract
Epidemiological studies have found that diabetes and cognitive dysfunction are closely related. Quercetin has been certified with the effect on improving diabetes mellitus (DM) and cognitive impairment. However, the effect and related mechanism of quercetin on diabetic encephalopathy (DE) are still ambiguous. In this study, we used the db/db mice (diabetic model) to discover whether quercetin could improve DE through the Sirtuin1/NLRP3 (NOD‐, LRR‐ and pyrin domain‐containing 3) pathway. Behavioural results (Morris water maze and new object recognition tests) showed that quercetin (70 mg/kg) improved the learning and memory. Furthermore, quercetin alleviated insulin resistance and the level of fasting blood glucose. Besides, Western blot analysis also showed that quercetin increased the protein expressions of nerve‐ and synapse‐related protein, including postsynapticdensity 93 (PSD93), postsynapticdensity 95 (PSD95), brain‐derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in the brain of db/db mice. Quercetin also increased the protein expression of SIRT1 and decreased the expression of NLRP3 inflammation‐related proteins, including NLRP3, the adaptor protein ASC and cleaved Caspase‐1, the pro‐inflammatory cytokines IL‐1β and IL‐18. In conclusion, the present results indicate that the SIRT1/NLRP3 pathway may be a crucial mechanism for the neuroprotective effect of quercetin against DE.
Keywords: diabetic encephalopathy, NLRP3 inflammasome, quercetin, SIRT1
1. INTRODUCTION
Diabetes mellitus (DM) is one of the most prevalent systemic metabolic diseases, which can damage many organs in body.1 In worldwide, more than 415 million adults with DM, including 6 million new cases, were counted annually.2, 3 Many complications caused by diabetes have become the focus of widespread attention. A growing body of evidence suggests that both type 1 diabetes (T1DM) and type 2 diabetes (T2DM) patients exhibit a variety of neuropathological and neurobehavioural changes, including cerebrovascular changes,4 insulin signalling systems impairments in cerebral,5 poor visual space construction, planning and visual memory injury.6, 7 Diabetic encephalopathy (DE) is a series of neuropathological changes caused by diabetes, which common symptoms are paraesthesia, numbness and impaired cognition.8 The pathogenesis of DE is not completely clear. Persistent inflammation caused by the large secretion of pro‐inflammatory factors is a possible mechanism for DE.9 In addition, the insulin signalling pathway is another potential mechanism of DE, which is not only involved in the deposition of amyloid in the brain, but also a neurotrophic factor of nerve cells.10 DE has become an important direction in the current research and prevention of diabetes. However, it is still unclear about effective treatment methods and drugs of DE. Therefore, it is urgent to study the pathogenesis of DE and explore new drugs.
Quercetin (3,3′,4′,5,7‐pentahydroxyflavone) is a typical representative flavonoid. Quercetin widely exists in various fruits, vegetables and traditional Chinese medicine plants6, 11, 12; and whose daily intake is about 3‐38 mg/d.13 To be a therapeutic agent, quercetin is used to alleviate various diseases, including hepatotoxicity, cardiotoxicity, neurotoxicity and nephrotoxicity.14, 15 The great effects of quercetin are attributed to its antioxidant16 and anti‐inflammatory capacity.17 Recently, the combination of dasatinib and quercetin is reported to extend the lives of older people.18 Quercetin is an SIRT1 activator.19, 20, 21 Studies have found that quercetin increases monoamine synthesis in aged rats by activating SIRT1 and improves cognitive function in aged rats.19 In a diabetic rat model, quercetin can activate SIRT1 and promote glucose and lipid metabolism.22 Can quercetin relieve DE through SIRT1? These experimental results provide a reasonable basis for the assumptions of our experiments.19, 22
SIRT1 (Sirtuin type 1), one of the main members of the sirtuin family, is a deacetylase that targets and regulates the function and activity of the corresponding protein by deacetylation.21, 22 SIRT1 is thought to play a major role in cell proliferation, differentiation, senescence and apoptosis.23, 24 Many researches have shown that SIRT1 inhibits the occurrence of inflammatory responses by negatively regulating the NLRP3 inflammasome in vascular endothelial cells.25, 26 In C57BL/6 mice, quercetin rutin alleviates acute endotoxin‐induced renal injury by inhibiting inflammation and up‐regulating the expression of SIRT1.27 In addition, our previous findings suggest that SIRT1 may be the key to improving cognitive function in diabetic mice.7 However, whether NLRP3 participates in SIRT1 to improve cognitive function in diabetic encephalopathy mice remains to be further studied.
The nucleotide‐binding domain‐like receptor protein 3 (NLRP3) inflammasome is a multiprotein complex, and it includes the oligomerization of NLRP3, ASC (adaptor protein) and caspase‐1.28 And it also plays a very important role in many diseases, including autologous inflammatory disease (CAPS),29 multiple sclerosis and lupus,30, 31 diabetes, acute kidney injury, chronic kidney disease.32, 33 NLRP3 catalyzes the transformation from inactive pro‐caspase‐1 protein to active caspase‐1protein, and then pro‐IL‐1β and pro‐IL‐18 mature and secrete IL‐1β and IL‐18 under the action of activated caspase‐1 protein.34 NLRP3 inflammasome promotes diabetes‐induced endothelial inflammation and atherosclerosis.35 Studies have reported that quercetin inhibits NLRP3 inflammatory activation in a rat spinal cord injury model.36 Targeting adjustment of the NLRP3 inflammasome protect the nerve damage in spinal cord injury rats.37 Previous studies have demonstrated that quercetin can up‐regulate SIRT1 leading to neuroprotection and improve glycolipid metabolism.19, 22 However, in the DE model, what is the mechanism of action of quercetin? Whether it is related to SIRT1/NLRP3 remains to be verified.
In our study, the db/db mice, a model of T2DM,7 were used for study. We explored whether quercetin could improve cognitive dysfunction through NLRP3 signal pathway in db/db mice. We treated our mice with two different doses (35 and 70 mg/kg/d) of quercetin. We finally found the potential mechanism of quercetin of alleviating DE might be through SIRT1/NLRP3 signal pathway.
2. MATERIALS AND METHODS
2.1. Chemical reagents
Quercetin (98%, Figure 1A) was purchased from Sigma‐Aldrich. Primary antibodies including Postsynapticdensity 93 (PSD93), Postsynapticdensity 95 (PSD95), Nerve growth factor (NGF), SIRT1, ASC, NLRP3 and IL‐1β were purchased from Cell Signaling Technology, Inc. Anti–brain‐derived neurotrophic factor (BDNF), anti‐NLRP3, anti–β‐actin were purchased from Abcam, Inc. Anti–IL‐18 and anti–cysteinyl‐aspartate‐specific proteinase‐1 (Cleaved Caspase‐1) were purchased from Affinity Biosciences. Secondary antibodies (antimouse IgG and anti‐rabbit IgG) were also from CST, Inc.
Figure 1.
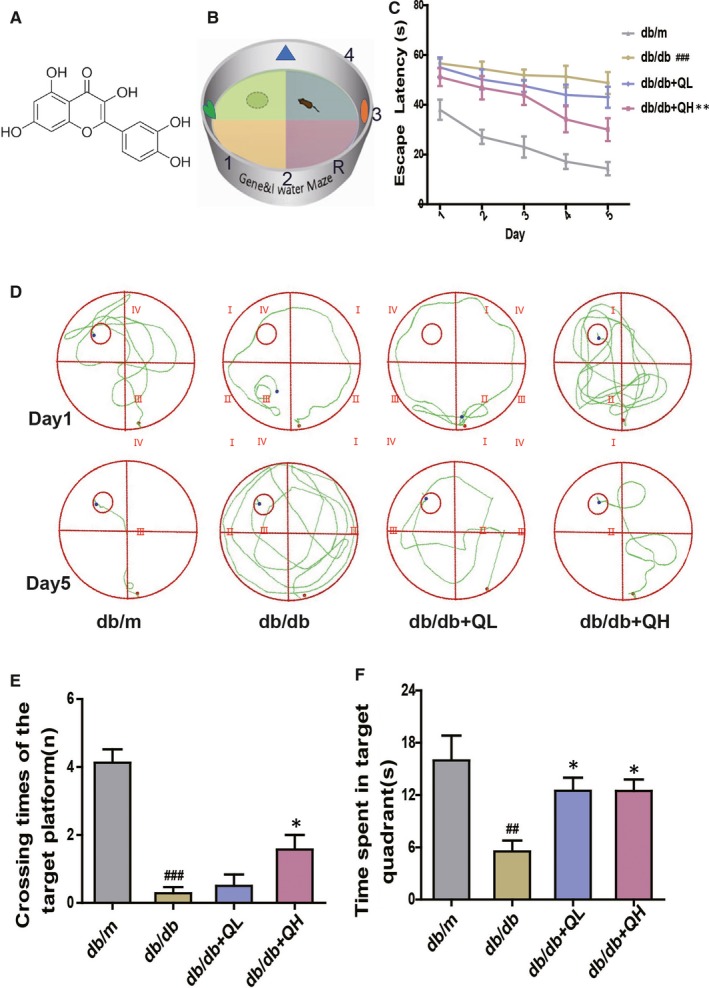
Quercetin ameliorates diabetes‐induced cognitive dysfunction shown by the Morris water maze test in db/db mice. A, The chemical structure of Quercetin. B, Schematic plan of water maze experimental device. C, Escape latency of five consecutive daily tests. D, Swimming paths of the respective groups on the first and fifth day. E, Crossing times of the target platform in the probe trial. F, Time spent in the target quadrant in the probe trial. db/db + QL: Quercetin (35 mg/kg/d); db/db + QH: Quercetin (70 mg/kg/d). Data represent mean ± SEM (n = 8 per group). # P < .05, ## P < .01, ### P < .001 vs db/m; *P < .05, **P < .01, ***P < .001 vs db/db
2.2. Animals and treatment
The db/db mice and age‐matched wild‐type C57BL/6J‐db/m mice were purchased from Nanjing Biomedical Research Institute of Nanjing University, Nanjing, China (8 weeks, female). Animals were maintained in SPF animal room, where was provided a 12‐hour light‐12‐hour dark cycle with a relative humidity of 40%‐60% and temperature 22 ± 2°C. The animals were all fed with standard pellet food and freshwater. The animals were randomly arranged into four groups: db/m (0.9% saline, n = 8), db/db (0.9% saline, n = 8), db/db + low dosage of quercetin (QL, 35 mg/kg/d, n = 8) and db/db + high dosage of quercetin (QH, 70 mg/kg/d, n = 8). The treatment cycle is 12 weeks by gavage. The experimental methods applied in our study were conformed to the guide which was promulgated and adopted by the NIH.
2.3. Morris water maze test
After 12 weeks of drug treatment, the spatial memory was detected by the Morris water maze test (similar to Morris).7, 38 Experimental equipment consisted of a black platform, a black circular pool and a record system. The circular pool with a diameter of 120 cm and filled with white and opaque water (30 cm in depth; temperature: 22‐26°C). Furthermore, the pool was divided into 4 symmetrical quadrants. And the escape platform was placed in the central area of appointed quadrant. Animals were conducted an orientation navigation tests for five successive days, and all mice were trained to look for the centre platform before the test.
Furthermore, there were four training trials a day, and the drop location would be changed randomly, recording the time for finding the platform. In this phase, we set up the platform in quadrant IV. The test of time was set as 60 seconds for each trial. When the mouse failed to find the target, it would be there for 10 seconds. Hence, escape latency would be 60 seconds. After the acquisition phase, the centre platform was removed subsequently and mice could swim allodially for 60 seconds to seek for the platform. In this phase, the times of crossing through the centre platform position and all the time spent in the target quadrant suggested the ability of memory retention after learning.
2.4. Novel object recognition test
Novel object recognition test was a method for learning and memory test, based on the principle that animals have instinct to explore new objects.39 The experimental method was composed of three stages: adaptive phase, orientation phase and finally test phase. The experimental installation was composed of rectangular white box (50 × 25 × 50 cm) and three objects (named A, B and C), of which A was same to B, while the object of C was completely diverse (shape, colour) from the A and B. The test is based on a previous research with minor modification.40 On the day 1, the stage of familiarity, mice were acclimated to our test zone (including A and B) for 10 min, then returned to mouse cage. After 24 hours, the mice were placed in the empty box together with the objects of A and B and they would explore for 5 minutes in the test zone. After 24 hours, the B was replaced with C, and animals were also placed back to the box for 5 minutes. In addition, the test zone and objects were washed with 70% ethanol to avoid being affected by the smell after each test. The computer equipment, respectively, recorded the time spent by exploring the novel object (TN) and the familiar object (TF). The time which the mouse spent distinguishing novel and similar objects could be calculated using the identify index (TNI) = (TN − TF)/(TN + TF).41, 42
2.5. Oral glucose tolerance test and insulin tolerance test
Blood glucose levels were measured using the ACCU‐CHEK Advantage glucose analyzer (Roche Diagnostics). The test is based on a previous research with slight modification.7 All mice were weighed, and then OGTT was tested after 16 hours fasting. A 2 g/kg glucose solution was given orally by weight; then, glucose levels were tested at 0, 30, 60, 90 and 120 minutes after giving glucose solution by gavage. ITT (insulin tolerance test) was proceeded after three days. The animals were given 0.5 U/kg insulin (Eli Lilly and Co.) in saline by intraperitoneal injection after 4‐hour fasting. Blood glucose levels obtained from the tail vein at appointed time (0, 30, 60, 90 and 120 minutes) Furthermore, AUC, an index of whole glucose excursion after glucose loading, was calculated in according to a previous study.43
2.6. Nissl's staining
After ITT experiments, all mice were anaesthetized with chloral hydrate (0.04 mL/10 g, Intraperitoneal injection), then they were killed by cervical dislocation. Three paraffin sections of each group were dewaxed in xylene and passed through a series of gradient ethanol and double distilled water rehydrated. Staining was performed according to the Nissl staining kit (Nanjing Jiancheng Bioengineering Research Institute, Nanjing). Images were analysed using an optical microscope and LEICA QWin Plus (Leica Microsystems).
2.7. Immunohistochemistry
Three paraffin sections of each group were taken for dewaxing and rehydration. The sections were placed in sodium citrate buffer for antigenic repair for 30 minutes (microwave heating). Blocking with 5% normal goat serum in PBS (37°C, 30 minutes), then incubating with anti‐SIRT1 (1:400; CST) overnight at 4°C. It was washed three times with PBS (10 minutes/time) after rewarming for 30 minutes, and the secondary antibody was added dropwise for 1 hour at 37°C.
2.8. Western blot analysis
The tissues of brain were lysed and homogenized in lysis buffer for 15 minutes. The lysed cocktail was centrifuged for 12 minutes (12 000 g, 4°C), and then we measured the protein concentrations using BCA protein assay kit. The total protein (30ug) were separated by SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) analysis gel and transferred to PVDF membranes. Then, membranes were immersed in 5% skim milk (or BSA) for 1.5 hours at 25°C. The membrane was incubated, respectively, with anti‐NLRP3, anti‐ASC, anti–Caspase‐1, anti–IL‐1β, anti–IL‐18, anti‐PSD93, anti‐PSD95, anti‐BDNF, anti‐NGF and anti‐SIRT1, and mouse anti–β‐actin overnight at 4°C. Then, the membrane was incubated with secondary antibodies for 2 hours. Routinely, a reagent of super‐enhanced chemiluminescence (ECL) made the membrane visualized.
2.9. Statistical analysis
Our experimental values were all presented as mean ± SEM. Statistical analyses were all performed using SPSS 19.0 program (IBM). The sample as a whole is normally distributed, and statistical differences in data between groups were performed with one‐way ANOVA, and followed by a post hoc test (Dunnett). P < .05 was presented as statistically significant.
3. RESULTS
3.1. Quercetin relieves cognitive impairment in db/db mice
To investigate whether quercetin could relieve memory and learning impairments, we performed the tests of Morris maze and novel object recognition. In the Morris water maze test, the time for mice to find central platform was decreased gradually in the five testing days (Figure 1B,C). The time for finding the central platform signally prolonged in the db/db group, compared with db/m group. After treated with quercetin (db/db + QL, 35 mg/kg; db/db + QH, 70 mg/kg), animals showed a marked shortness of the escape latency performance, especially for the high‐dose group (Figure 1B).
The swimming path (Figure 1C): Animals swimmed irregularly in designated areas on the first day. After five days of training, the trajectory of db/db showed a long and disorderly swimming path, which was improved by quercetin treatment, especially in high‐dose group (Figure 1C). After the removement of the platform on the sixth day, the group of db/db had shorter crossing times and target quadrant dwelling time than the group of db/m (Figure 1D,E). The groups of quercetin had longer platform crossing times and target quadrant dwell time than those in the db/db group, notably for db/db + QH group (Figure 1D,E).
In the novel object recognition test (Figure 2A), the db/db group showed signally lower level of the TNI than db/m group. After treated with quercetin, db/db + QH group show significantly a higher level than db/db group (Figure 2B).
Figure 2.

Quercetin prevents learning and memory impairments by the novel object discrimination in db/db mice. A, Schematic diagram of new object recognition experimental device. B, Recognition index (TNI) = (TN − TF)/(TN + TF). db/db + QL: Quercetin (35 mg/kg/d); db/db + QH: Quercetin (70 mg/kg/d). Data represent mean ± SEM (n = 8 per group). # P < .05, ## P < .01, ### P < .001 vs db/m; *P < .05, **P < .01, ***P < .001 vs db/db
3.2. Quercetin reduces fasting glucose and insulin resistance in db/db mice
In OGTT test, the db/db group showed a higher peak of glucose rise and a slower reduction of blood glucose concentration than db/m group. The db/db group manifested glucose intolerance because of the obviously high glucose excursion, and it is AUC during the OGTT (Figure 3A,B). After treated with quercetin (db/db + QL, 35 mg/kg; db/db + QH, 70 mg/kg), the rising peak of mice glucose became lower and the decreasing concentration of blood glucose was faster than db/db group, especially for high‐dose group (Figure 3A).
Figure 3.
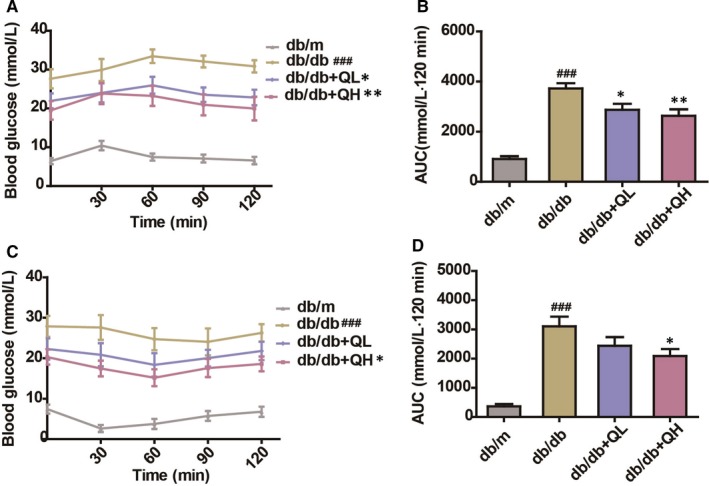
Quercetin decreases fasting glucose in db/db mice. A, OGTT. B, AUC (OGTT). C, ITT. D, AUC (ITT). db/db + QL: Quercetin (35 mg/kg/d); db/db + QH: Quercetin (70 mg/kg/d). Data represent mean ± SEM (n = 8 per group). # P < .05, ## P < .01, ### P < .001 vs db/m; *P < .05, **P < .01, ***P < .001 vs db/db
In ITT test, the group of db/db mice exhibited insulin resistance, compared with db/m group. The db/db group showed a slower rate of blood glucose concentration decline than db/m (Figure 3C). After treated with quercetin, the blood glucose concentration was significantly lower and insulin resistance was significantly improved compared with db/db group, notably for db/db + QH group (Figure 3C,D).
3.3. Quercetin improves neurodegeneration in db/db mice
As illustrated in Figure 4, the protein expressions of the neurotrophic factors, including PSD93, PSD95, NGF and BDNF, were sharply decreased in db/db group (Figure 4A‐E). After treatment with quercetin, especially for high‐dose group, the levels of PSD93, PSD95, BDNF and NGF were increased in the brain (Figure 4A‐E). In addition, the results of Nissl staining further confirmed the above changes (Figure 4F). The number of Nissl bodies in the db/db mice was significantly reduced compared with the db/m mice, and the staining was observably shallow. After treated with quercetin, the number and colour of Nissl bodies were significantly improved. These results indicate that quercetin could improve neurodegeneration in db/db mice.
Figure 4.
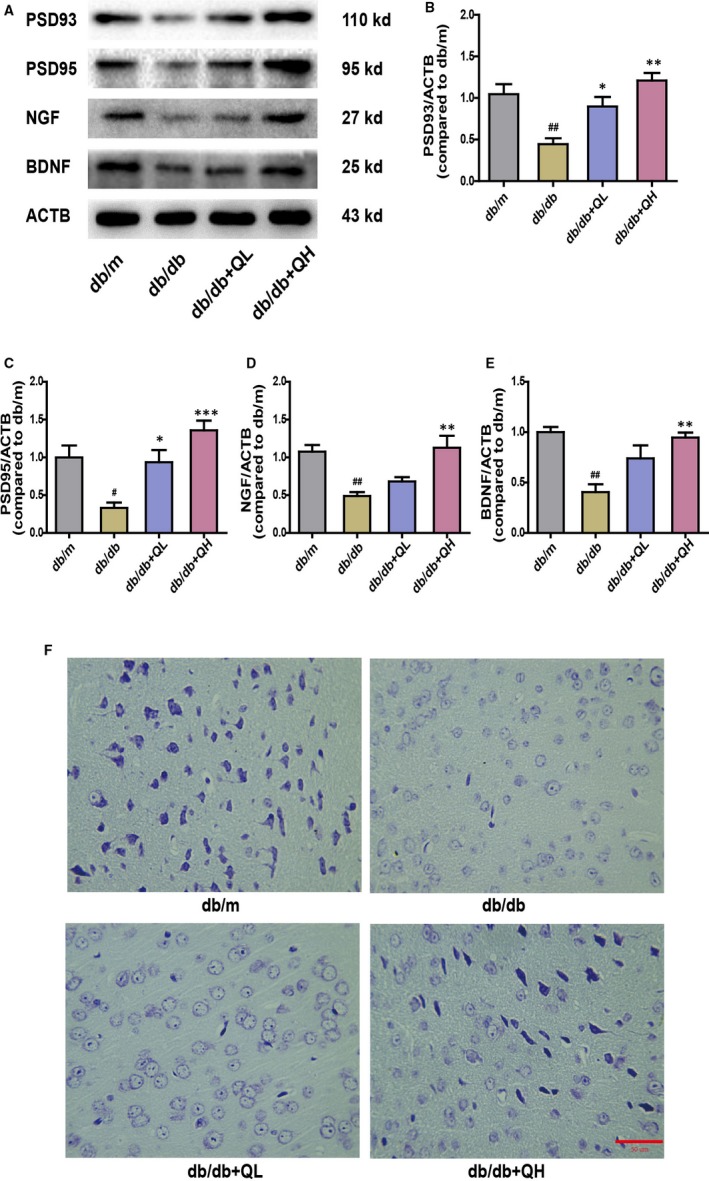
Quercetin increases neurotrophic factor levels in the brain of db/db mice. Representative Western blot results A, of protein expression in the brain of db/db mice. B, PSD93. C, PSD95. D, NGF. E, BDNF. F, Nissl's staining in cortex. db/db + QL: Quercetin (35 mg/kg/d); db/db + QH: Quercetin (70 mg/kg/d). Data represent mean ± SEM (n = 8 per group). # P < .05, ## P < .01, ### P < .001 vs db/m; *P < .05, **P < .01, ***P < .001 vs db/db. Bar: 50 μm
3.4. Quercetin activates SIRT1 and inhibits NLRP3 inflammasome activation
As illustrated in Figures 5 and 6, the db/db group showed a lower protein expression of SIRT1 than db/m (Figures 5 and 6A,B). After treatment with quercetin, especially for high‐dose group, the protein expression of SIRT1 was increased (Figures 5A and 6B). Subsequently, we measured the expression levels of NLRP3 inflammation‐related proteins, including NLRP3, cleaved Caspase‐1(p20), ASC, IL‐1β and IL‐18, which were evident different between groups of db/m and db/db (Figure 6C‐G). After treatment with quercetin, the expression of these proteins expression was sharply decreased, contradistinguished with db/db group (Figure 6C‐G). The data indicated quercetin could activate SIRT1 and inhibit NLRP3 inflammasome activation to protect DE.
Figure 5.
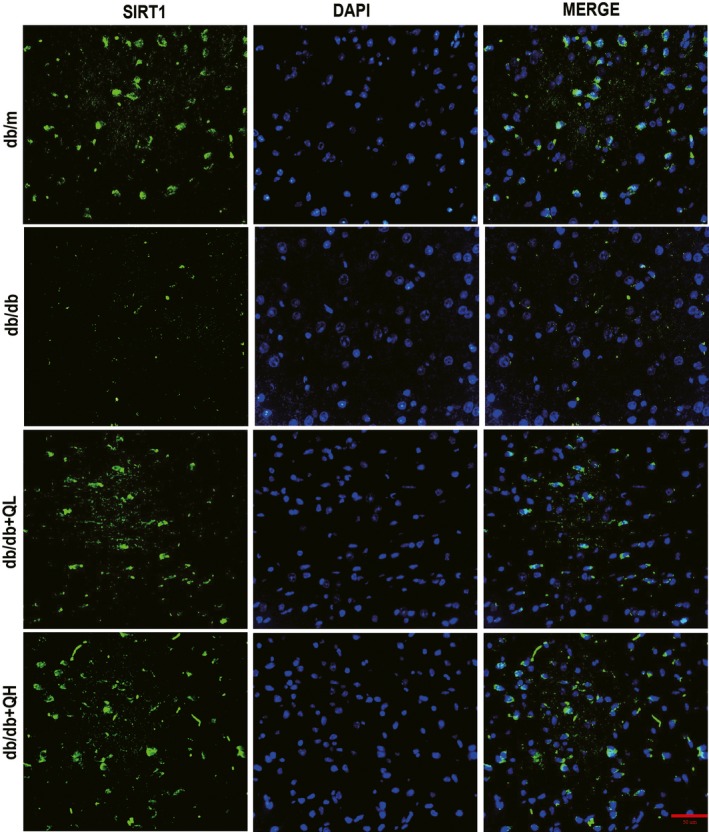
Quercetin activates SIRT1 in the brain of db/db mice. Immunofluorescence of SIRT1 in cortex. Bar: 50 μm
Figure 6.

Quercetin activates SIRT1 and inhibits NLRP3 inflammasome activation in the brain of db/db mice. Representative Western blots A, results of protein expression in the brain of db/db mice. B, SIRT1. C, NLRP3. D, ASC. E, IL‐18. F, Cleaved Caspase‐1. G, IL‐1β. db/db + QL: Quercetin (35 mg/kg/d); db/db + QH: Quercetin (70 mg/kg/d). Data represent mean ± SEM (n = 8 per group). # P < .05, ## P < .01, ### P < .001 vs db/m; *P < .05, **P < .01, ***P < .001 vs db/db
4. DISCUSSION
In our study, we proved that quercetin could relieve diabetes‐associated cognitive impairment in db/db mice. The experimental results found that quercetin preserved learning and memory, alleviated insulin resistance and decreased blood glucose in db/db mice. For mechanism exploring, quercetin increased nerve and synapse‐related protein expression and reduced the protein expression of neuroinflammatory factors in the brain of db/db mice. Furthermore, quercetin activated SIRT1 and inhibited the expressions of NLRP3‐regulated inflammation‐related proteins, which might be the key mechanisms of the neuroprotective effect of quercetin.
Diabetes is a key cause of cardiovascular disease, retinal disease, and the development and progression of neurological diseases.44 DE is a series of behavioural and pathological changes caused by constitutive hyperglycaemia, including cognitive decline, neuronal loss and disorders of glycolipid metabolism.45, 46, 47 DE decreased the patient's ability of the learning and memory.48 In previous studies, quercetin has been shown to lower blood lipid levels and increase glucose tolerance.49 Recently, studies have reported that quercetin has neuroprotective effects on cognitive impairment caused by diabetes.50 In our study, using db/db mice showed the neuroprotective mechanisms of quercetin. Behavioural results (Morris water maze test, new object recognition test) showed that quercetin (70 mg/kg) significantly improved learning and memory levels. At the same time, quercetin reduces insulin resistance and promotes glucose metabolism by reducing the susceptibility to T2D/IR. The therapeutic effect of quercetin on DE is clearly consistent with previous studies.49, 51, 52
The activation of NLRP3 inflammatory bodies is closely related to the pathogenesis of DM.53, 54 In a previous study, NLRP3 inflammasome is activated in the neurons of hippocampus in db/db mice.55 NLRP3 catalyzes the activation of caspase‐1, thereby promoting the maturation and secretion of IL‐1β and IL‐18.7, 56 Studies have found that inhibiting NLRP3 inflammasome activation can reduce IL‐1β levels in hippocampus of DM rats,57 and the expression level of hippocampal IL‐1β is related to cognitive function in DM mice.55 Quercetin has been shown to increase microglial activation and potently inhibit pro‐inflammatory factors.58 In addition, quercetin attenuates NLRP3 inflammatory activation and lipid accumulation in diabetic rats.59, 60, 61 In our study, we found that the NLRP3 pathway is activated in the brain of db/db mice and that synapses and trophic factors are significantly reduced. This effect of quercetin on neuroinflammation is based on previous studies.62, 63, 64, 65
SIRT1 regulates intracellular signalling molecules, inhibits apoptosis, regulates inflammation and resists oxidative stress.66, 67 SIRT1 can be involved in regulating the formation of Alzheimer's disease amyloid and maintaining the stability of the neuronal genome.68, 69 Studies have reported that quercetin improve lipid, glucose metabolism and inhibits neurodegeneration through SIRT1 signalling pathway.22, 70 These results suggest SIRT1 might play a pivotal role of quercetin on DE in db/db mice. In addition, SIRT1 is closely related to the activation of NLRP3 inflammasome.71 In rat cerebral ischaemia/reperfusion models and ventilation‐induced lung injury models, SIRT1‐dependent inhibition of NLRP3 inflammatory body activation.72, 73 Therefore, it may be assumed that quercetin effects on DM in db/db mice through SIRT1/NLRP3 signalling pathway. In our study, we found that SIRT1 were observably decreased when the NLRP3 increased in the brain of db/db mice. This effect of quercetin on SIRT1/NLRP3 signalling pathway is according with previous studies.19, 22, 74
5. CONCLUSION
Quercetin may ameliorate DE by decreasing fasting glucose, up‐regulating activity and protein level of SIRT1, and inhibiting the expressions of NLRP3‐regulated inflammation‐related proteins. Quercetin shows the potential for the prevention and therapy of DE. However, further evidence is still needed to confirm this phenomenon. These data could be useful for explaining the underlying neuroprotective mechanisms of quercetin on DE.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
S.‐JZ designed the study. HT, J.‐JS and X.‐QL conducted the experiment. S.‐JZ and HT contributed to initial data analysis and interpretation and drafted the initial manuscript. X.‐Y.L and Q.‐B.C helped revised the manuscript. QW, S.‐JZ and Y.‐BC supervised all aspects of the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 81674040) and the Guangzhou Science Technology and Innovation Commission Technology Research Projects (No. 201805010005)
Hu T, Lu X‐Y, Shi J‐J, et al. Quercetin protects against diabetic encephalopathy via SIRT1/NLRP3 pathway in db/db mice. J Cell Mol Med. 2020;24:3449–3459. 10.1111/jcmm.15026
Tian Hu and Xin‐Yi Lu contributed equally to this work.
[Correction Statement: Correction added on 04 April 2020 after first online publication: The affiliation of Shi‐Jie Zhang has been updated in this version.]
Contributor Information
Yun‐Bo Chen, Email: ybchengz@gzucm.edu.cn.
Shi‐Jie Zhang, Email: zsj19891122@gmail.com.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Zhao Y, Ye W, Boye KS, Holcombe JH, Hall JA, Swindle R. Prevalence of other diabetes‐associated complications and comorbidities and its impact on health care charges among patients with diabetic neuropathy. J Diabetes Complications. 2010;24(1):9‐19. [DOI] [PubMed] [Google Scholar]
- 2. Cole AR, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev. 2007;31(7):1046‐1063. [DOI] [PubMed] [Google Scholar]
- 3. Roriz‐Filho JS, Sá‐Roriz TM, Rosset I, et al. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792(5):432‐443. [DOI] [PubMed] [Google Scholar]
- 4. Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle‐aged adults. Neurology. 2001;56(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 5. Hoyer S. Is sporadic Alzheimer disease the brain type of non‐insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm. 1998;105(4–5):415‐422. [DOI] [PubMed] [Google Scholar]
- 6. Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33(12):1061‐1080. [DOI] [PubMed] [Google Scholar]
- 7. Li HY, Wang XC, Xu YM, et al. Berberine improves diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Rejuvenation Res. 2018;21:200‐209. [DOI] [PubMed] [Google Scholar]
- 8. Schemmel KE, Padiyara RS, D'Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications. 2010;24(5):354‐360. [DOI] [PubMed] [Google Scholar]
- 9. Pabreja K, Dua K, Sharma S, Padi SSV, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti‐inflammatory and anti‐oxidant mechanisms. Eur J Pharmacol. 2011;661(1–3):15‐21. [DOI] [PubMed] [Google Scholar]
- 10. Chen R, Shi J, Yin Q, et al. Morphological and pathological characteristics of brain in diabetic encephalopathy. J Alzheimers Dis. 2018;65(1):15‐28. [DOI] [PubMed] [Google Scholar]
- 11. Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999;47(6):2274‐2279. [DOI] [PubMed] [Google Scholar]
- 12. Wiczkowski W, Romaszko J, Bucinski A, et al. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J Nutr. 2008;138(5):885. [DOI] [PubMed] [Google Scholar]
- 13. Hertog MG, Kromhout D, Aravanis C, et al. Flavonoid intake and long‐term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155(4):381‐386. [PubMed] [Google Scholar]
- 14. Haleagrahara N, Siew CJ, Ponnusamy K. Effect of quercetin and desferrioxamine on 6‐hydroxydopamine (6‐OHDA) induced neurotoxicity in striatum of rats. J Toxicol Sci. 2013;38(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 15. Sekaran S, Kandaswamy S, Gunasekaran K, et al. Protective role of quercetin on polychlorinated biphenyls (Aroclor‐1254) induced oxidative stress and apoptosis in liver of adult male rats. J Biochem Mol Toxicol. 2012;26(12):522‐532. [DOI] [PubMed] [Google Scholar]
- 16. Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride‐induced oxidative stress in the hepatic tissue. Food Chem. 2012;132(2):931‐935. [Google Scholar]
- 17. Wu Z, Zhao J, Xu H, et al. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur J Nutr. 2014;53(8):1669‐1683. [DOI] [PubMed] [Google Scholar]
- 18. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarubbo F, Ramis MR, Kienzer C, et al. Chronic silymarin, quercetin and naringenin treatments increase monoamines synthesis and hippocampal Sirt1 levels improving cognition in aged rats. J Neuroimmune Pharmacol. 2018;13(1):24‐38. [DOI] [PubMed] [Google Scholar]
- 20. Peredo‐Escárcega AE, Guarner‐Lans V, Pérez‐Torres I, et al. The combination of resveratrol and quercetin attenuates metabolic syndrome in rats by modifying the serum fatty acid composition and by upregulating SIRT 1 and SIRT 2 expression in white adipose tissue. Evid Based Complement Alternat Med. 2015;2015:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iskender H, Dokumacioglu E, Sen TM, Ince I, Kanbay Y, Saral S. The effect of hesperidin and quercetin on oxidative stress, NF‐κB and SIRT1 levels in a STZ‐induced experimental diabetes model. Biomed Pharmacother. 2017;90:500‐508. [DOI] [PubMed] [Google Scholar]
- 22. Peng J, Li Q, Li K, et al. Quercetin improves glucose and lipid metabolism of diabetic rats: involvement of Akt signaling and SIRT1. J Diabetes Res. 2017;2017:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804(8):1626‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nogueiras R, Habegger KM, Chaudhary N, et al. Sirtuin 1 and Sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zou P, Liu X, Li G, Wang Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol Med Rep. 2018;17(2):3212‐3217. [DOI] [PubMed] [Google Scholar]
- 26. Li Y, Wang P, Yang X, et al. SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Mol Immunol. 2016;77:148‐156. [DOI] [PubMed] [Google Scholar]
- 27. Khajevand‐Khazaei M‐R, Mohseni‐Moghaddam P, Hosseini M, Gholami L, Baluchnejadmojarad T, Roghani M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up‐regulation of antioxidants and SIRT1. Eur J Pharmacol. 2018;833:307‐313. [DOI] [PubMed] [Google Scholar]
- 28. Choe JY, Kim SK. Quercetin and ascorbic acid suppress fructose‐induced NLRP3 inflammasome activation by blocking intracellular shuttling of TXNIP in human macrophage cell lines. Inflammation. 2017;40(3):980‐994. [DOI] [PubMed] [Google Scholar]
- 29. Doria A, Zen M, Bettio S, et al. Autoinflammation and autoimmunity: bridging the divide. Autoimmun Rev. 2012;12(1):22‐30. [DOI] [PubMed] [Google Scholar]
- 30. Gris D, Ye Z, Iocca HA, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185(2):974‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kahlenberg JM, Kaplan MJ. The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis? Curr Opin Rheumatol. 2014;26(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hans‐Joachim A, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22(6):1007‐1018. [DOI] [PubMed] [Google Scholar]
- 33. Wen H, Ting J‐Y, O'Neill LAJ. A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat Immunol. 2012;13(4):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221. [DOI] [PubMed] [Google Scholar]
- 35. Wan Z, Fan Y, Liu X, et al. NLRP3 inflammasome promotes diabetes‐induced endothelial inflammation and atherosclerosis. Diabetes Metab Syndr Obes. 2019;12:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang W, Huang Y, Han N, et al. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord. 2016;54(8):592. [DOI] [PubMed] [Google Scholar]
- 37. Zendedel A, Johann S, Mehrabi S, et al. Activation and regulation of NLRP3 inflammasome by intrathecal application of SDF‐1a in a spinal cord injury model. Mol Neurobiol. 2016;53(5):3063‐3075. [DOI] [PubMed] [Google Scholar]
- 38. Eri H, Ohyagi Y, Ma L, et al. Apomorphine treatment in Alzheimer mice promoting amyloid‐β degradation. Ann Neurol. 2011;69(2):248‐256. [DOI] [PubMed] [Google Scholar]
- 39. Sakaguchi K, Takeda K, Maeda M, et al. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2015;7(1):53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohshima K, Mogi M, Jing F, et al. Roles of interleukin 17 in angiotensin II type 1 receptor‐mediated insulin resistance. Hypertension. 2012;59(2):493‐499. [DOI] [PubMed] [Google Scholar]
- 41. Legates TA, Altimus CM, Wang H, et al. Aberrant light directly impairs mood and learning through melanopsin‐expressing neurons. Nature. 2012;491(7425):594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tucci P, Mhillaj E, Morgese MG, et al. Memantine prevents memory consolidation failure induced by soluble beta amyloid in rats. Front Behav Neurosci. 2014;8(332):182‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lemini C, Estela Avila M, Medina M, et al. Proliferative properties of 17β‐aminoestrogens in MCF‐7 human breast cancer cells. Basic Clin Pharmacol Toxicol. 2017;120(3):235‐242. [DOI] [PubMed] [Google Scholar]
- 44. Giatti S, Mastrangelo R, D'Antonio M, et al. Neuroactive steroids and diabetic complications in the nervous system. Front Neuroendocrinol. 2018;48:58‐69. [DOI] [PubMed] [Google Scholar]
- 45. Simaabc AAF, Zhen GL. Insulin, C‐peptide, hyperglycemia, and central nervous system complications in diabetes. Eur J Pharmacol. 2004;490(1):187‐197. [DOI] [PubMed] [Google Scholar]
- 46. Strachan MWJ, Frier BM, Deary IJ. Type 2 diabetes and cognitive impairment. Diabet Med. 2010;20(1):1‐2. [DOI] [PubMed] [Google Scholar]
- 47. Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23(11):542‐549. [DOI] [PubMed] [Google Scholar]
- 48. Callisaya M, Moran C, Srikanth V. Type 2 diabetes mellitus as a causal factor for dementia – is there sufficient evidence from interventional studies? Australas Epidemiol. 2013;20(1):26‐28. [Google Scholar]
- 49. Shi X, Liao S, Mi H, et al. Hesperidin prevents retinal and plasma abnormalities in streptozotocin‐induced diabetic rats. Molecules. 2012;17(11):12868‐12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Son SM, Song H, Byun J, et al. Altered APP processing in insulin‐resistant conditions is mediated by autophagosome accumulation via the inhibition of mammalian target of rapamycin pathway. Diabetes. 2012;61(12):3126‐3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peng J, Li Q, Li K, et al. Quercetin improves glucose and lipid metabolism of diabetic rats: involvement of Akt signaling and SIRT1. J Diabetes Res. 2017;2017:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu X, Cheng Y‐Q, Lu Q, Du L, Yin X‐X, Liu Y‐W. Enhancement of glyoxalase 1, a polyfunctional defense enzyme, by quercetin in the brain in streptozotocin‐induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(11):1237‐1245. [DOI] [PubMed] [Google Scholar]
- 53. Kuhad A, Chopra K. Curcumin attenuates diabetic encephalopathy in rats: Behavioral and biochemical evidences. Eur J Pharmacol. 2007;576(1):34‐42. [DOI] [PubMed] [Google Scholar]
- 54. Vargas R, Rincón J, Pedreañez A, et al. Role of angiotensin II in the brain inflammatory events during experimental diabetes in rats. Brain Res. 2012;1453(1):64‐76. [DOI] [PubMed] [Google Scholar]
- 55. Zhai Y, Meng X, Luo Y, et al. Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Oncotarget. 2018;9(10):9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Song L, Pei L, Yao S, Wu Y, Shang Y. NLRP3 inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci. 2017;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun X, Li S, Xu L, et al. Paeoniflorin ameliorates cognitive dysfunction via regulating SOCS2/IRS‐1 pathway in diabetic rats. Physiol Behav. 2017;174:162‐169. [DOI] [PubMed] [Google Scholar]
- 58. Chinta SJ, Ganesan A, Reis‐Rodrigues P, Lithgow GJ, Andersen JK. Anti‐inflammatory role of the isoflavone diadzein in lipopolysaccharide‐stimulated microglia: implications for Parkinson's disease. Neurotox Res. 2013;23(2):145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Qiu Y‐Y, Tang L‐Q. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol Res. 2016;114:251‐264. [DOI] [PubMed] [Google Scholar]
- 60. Wang W, Wang C, Ding X‐Q, et al. Quercetin and allopurinol reduce liver thioredoxin‐interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br J Pharmacol. 2013;169(6):1352‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Q‐Y, Pan Y, Wang R, et al. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose‐fed rats. J Nutr Biochem. 2014;25(4):420‐428. [DOI] [PubMed] [Google Scholar]
- 62. Khan A, Ali T, Rehman SU, et al. Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front Pharmacol. 2018;9:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Domiciano TP, Wakita D, Jones HD, et al. Quercetin inhibits inflammasome activation by interfering with ASC oligomerization and prevents interleukin‐1 mediated mouse vasculitis. Sci Rep. 2017;7:41539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mehta V, Parashar A, Udayabanu M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol Behav. 2017;171:69‐78. [DOI] [PubMed] [Google Scholar]
- 65. Spagnuolo C, Moccia S, Russo GL. Anti‐inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. 2017;153:105. [DOI] [PubMed] [Google Scholar]
- 66. Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512. [DOI] [PubMed] [Google Scholar]
- 67. Wang YU, Bi Y, Chen XI, et al. Histone deacetylase SIRT1 negatively regulates the differentiation of interleukin‐9‐producing CD4 + T cells. Immunity. 2016;44(6):1337‐1349. [DOI] [PubMed] [Google Scholar]
- 68. Qin W, Yang T, Ho L, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281(31):21745‐21754. [DOI] [PubMed] [Google Scholar]
- 69. Dobbin MM, Madabhushi R, Pan L, et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat Neurosci. 2013;16(8):1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leyton L, Hott M, Acuña F, et al. Nutraceutical activators of AMPK/Sirt1 axis inhibit viral production and protect neurons from neurodegenerative events triggered during HSV‐1 infection. Virus Res. 2015;205:63‐72. [DOI] [PubMed] [Google Scholar]
- 71. Zou P, Liu X, Li G, Wang Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol Med Rep. 2018;17(2):3212. [DOI] [PubMed] [Google Scholar]
- 72. He QI, Li Z, Wang Y, Hou Y, Li L, Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1‐dependent autophagy induction. Int Immunopharmacol. 2017;50:208‐215. [DOI] [PubMed] [Google Scholar]
- 73. Wang Y, Xu C‐F, Liu Y‐J, et al. Salidroside attenuates ventilation induced lung injury via SIRT1‐dependent inhibition of NLRP3 inflammasome. Cell Physiol Biochem. 2017;42(1):34‐43. [DOI] [PubMed] [Google Scholar]
- 74. Peng Z, Li X, Xing D, et al. Nobiletin alleviates palmitic acid‐induced NLRP3 inflammasome activation in a sirtuin 1‐dependent manner in AML‐12 cells. Mol Med Rep. 2018;18(6):5815‐5822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


