Abstract
Mechanical stimulation is an important factor regulating mesenchymal stem cell (MSC) functions such as proliferation. The Ca2+‐activated K+ channel, KCa3.1, is critically engaged in MSC proliferation but its role in mechanical regulation of MSC proliferation remains unknown. Here, we examined the KCa3.1 channel expression and its role in rat bone marrow‐derived MSC (BMSC) proliferation in response to mechanical stretch. Application of mechanical stretch stimulated BMSC proliferation via promoting cell cycle progression. Such mechanical stimulation up‐regulated the KCa3.1 channel expression and pharmacological or genetic inhibition of the KCa3.1 channel strongly suppressed stretch‐induced increase in cell proliferation and cell cycle progression. These results support that the KCa3.1 channel plays an important role in transducing mechanical forces to MSC proliferation. Our finding provides new mechanistic insights into how mechanical stimuli regulate MSC proliferation and also a viable bioengineering approach to improve MSC proliferation.
Keywords: bone marrow‐derived mesenchymal stem cells, cell proliferation, KCa3.1 channel, mechanical stretch
1. INTRODUCTION
Mesenchymal stem cells (MSCs) have many promising applications in regenerative medicine.1, 2 The capability of MSC proliferation however declines upon in vitro expansion.3, 4 Identification of practical methods to maintain or increase MSC proliferation to increase their availability is helpful to their clinical applications. Compelling evidence shows that mechanical stimulation is an important factor regulating MSC functions, including proliferation, but the underlying mechanism is far from understood.5, 6, 7 KCa3.1 channel, also known as KCNN4, IKCa and SK4, is an intermediate‐conductance member of the Ca2+‐activated K+ channel family and is widely expressed in both excitable and non‐excitable cells, where it plays an important role in various cell functions.8 Interestingly, there is increasing evidence to support the KCa3.1 channel is engaged in MSC proliferation.9, 10, 11 Previous studies showed that the expression of the KCa3.1 channel in human umbilical vein endothelium cells was up‐regulated by fluid flow‐induced shear stress, or its channel activity in vascular smooth muscle cells was enhanced by hypotonic solution‐induced membrane stretch.12, 13 These findings led us to hypothesize a role of the KCa3.1 channel in mechanical stretch‐induced regulation of MSC proliferation. Here, we showed that application of mechanical stretch to rat bone marrow‐derived MSCs (BMSCs) significantly increased cell proliferation, mainly via altering cell cycle progression. Such mechanical stretch also up‐regulated the KCa3.1 expression at mRNA, protein and functional levels. Importantly, mechanical stretch‐induced stimulation of BMSC proliferation and alteration in cell cycle progression were prevented by pharmacological inhibition of the KCa3.1 channel or siRNA‐mediated knockdown of the KCa3.1 expression. Taken together, our results provide compelling evidence to support an important role of the KCa3.1 channel in mechanical stimuli‐induced stimulation of MSC proliferation, thus revealing a new molecular mechanism in transducing mechanical forces to regulate MSC proliferation and identifying a viable bioengineering approach to improve MSC proliferation.
2. MATERIALS AND METHODS
2.1. Cell isolation, culture and siRNA transfection
Isolation of BMSCs from 30‐day‐old male Sprague‐Dawley rats, BMSC characterization and culture were previously described.14 All experiments were approved by the Animal Research Ethics Committee of Beihang University. Passage 3‐5 BMSCs were used. Transfection with KCa3.1‐specific siRNA (siKCa3.1) or control siRNA (siCTL) was described previously,15 and the efficiency of knockdown was confirmed by Western blotting (Figure S1).
2.2. Application of mechanical stretch
Cells were seeded at density of 1 × 105 on silicone chambers pre‐coated with collagen I (Becton Dickinson). When cells reached 80% confluence, the silicone chambers were mounted to a stretch device (STREX, Japan) and were exposed to stretch by 2.5%, 5%, 10% and 15% for 6, 12 and 24 hours. Cells cultured without stretch were used as static control (SC). To block the KCa3.1 channel, 100 nmol/L TRAM‐34 (Alomone) was added into the culture medium.
2.3. Reverse transcription‐polymerase chain reaction (RT‐PCR)
Total RNA extraction and RT‐PCR were described in the Supplementary File. The primers used were listed in Table S1.
2.4. Protein expression determination
The cell surface KCa3.1 protein expression was determined using fluorescence‐activated cell sorting (FACS) as previously described.16 Cells were fixed without permeabilization and incubated with FITC‐conjugated antibody recognizing the extracellular domain of IKCa3.1 (Alomone). Isotype control IgG was used as control. The fluorescence intensity was determined by FACSCalibur (Becton Dickinson) and analysed using CellQuest software.
2.5. Cell proliferation
Cell proliferation was examined by determining the number of living cells using CCK‐8 kits according to the manufacturer's instructions (Dojindo). The absorbance at 450 nm was measured using a microplate reader (Thermo Scientific).
2.6. Cell cycle analysis
Cells, after being synchronized for 24 hours in serum‐free medium, were subjected to mechanical stretch for further 24 hours in media containing 10% foetal bovine serum. The cell cycle distribution was determined by FACSCalibur (Becton Dickinson) and analysed by ModFit software as previously described.16
2.7. Electrophysiology
Whole‐cell patch‐clamp recording of the K+ currents was performed using a HEKA amplifier (Lambrecht) as described previously9 and detailed in the Supplementary File. Membrane stretch was induced by applying hypotonic solution as described previously.13 The KCa3.1 channel currents were derived from TRAM‐34‐sensitive current components.
2.8. Statistical analysis
Data are presented as mean ± standard deviation, where appropriate. Statistical analysis was conducted using Student's test or one‐way ANOVA and post hoc Fisher's test as indicated. P < .05 was considered statistically significant.
3. RESULTS
We started with examining the effects of mechanical stretch on BMSC proliferation. We chose mechanical stretch from 2.5% to 15%, the most commonly used range in study of mechanical stretch‐induced regulation of MSC functions.5, 6, 7, 17, 18 As shown in Figure 1A, exposure to mechanical stretch for 6 hours, regardless of mechanical stretch strength, had no effect, and prolonged exposure for 12 and 24 hours of 5%, 10% and 15%, but not 2.5%, resulted in a significant increase in living cell number (Figure 1A). Taken together, these results show that mechanical stretch stimulates BMSC proliferation in a time‐ and strength‐dependent manner. To further understand how mechanical stretch accelerated BMSC proliferation, we analysed cells in different phases of the cell cycle after exposure to 5%‐15% stretch for 24 hours (Figure 1B‐C). Exposure to mechanical stretch increased the percentage of cells in the S phase and reduced the percentage of cells in the G0/G1 phase (Figure 1C), suggesting that mechanical stretch stimulates cell proliferation via promoting cell cycle progression.
Figure 1.
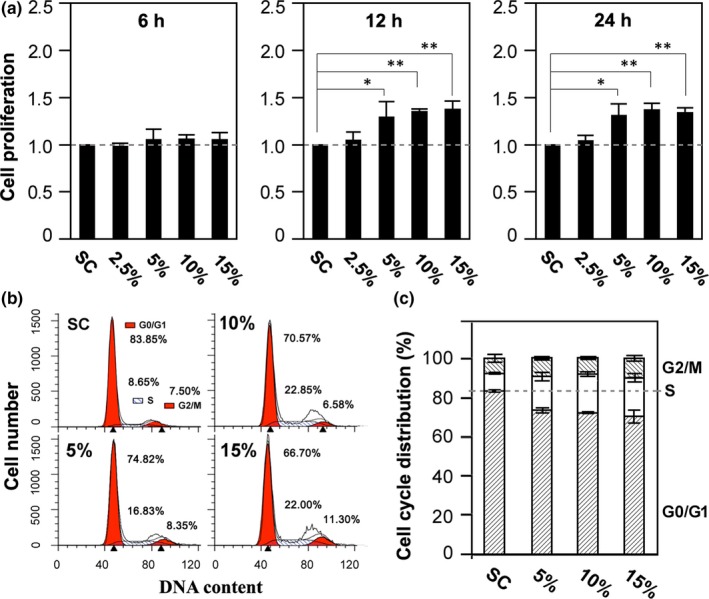
Effects of mechanical stretch on BMSC proliferation. A, summary of the effects of exposing BMSC to 2.5%‐15% mechanical stretch for 6, 12 and 24 h on cell proliferation relative to static control (SC). The mean data are from five independent experiments. *P < .05 and **P < .01 compared to SC using one‐way ANOVA and post hoc Fisher's test. B‐C, representative analysis of cell cycle distribution in cells under indicated conditions (B), and summary of the mean data from 4 independent experiments (C). Cells were fixed with 70% ethanol overnight, incubated in PBS staining solution (20 μg/mL propidium iodide, 100 μg/mL RNase A, and 0.1% Triton X‐100) at 37°C for 30 min and analysed by FACS on FL‐2 channel. The data were analysed using ModFit software
As introduced above, the KCa3.1 channel is critically engaged in MSC proliferation.9, 10, 11 We were therefore interested in the effects of mechanical stretch on the KCa3.1 channel expression. Exposure to mechanical stretch of 5%‐15%, but not 2.5%, for 24 hours increased the expression of KCa3.1 at the mRNA level shown by RT‐PCR (Figure 2A‐B) and also at the protein level shown by FACS (Figure 2C‐D). In addition, whole‐cell recording showed that the amplitude of TRAM34‐sensitive K+ currents was enhanced by membrane stretch induced using hypotonic solution (Figure 2E). Taken together, these results indicate that mechanical stimulation significantly enhances the KCa3.1 channel expression and activity. We finally investigated whether the KCa3.1 channel plays a role in mechanical stretch‐induced stimulation of BMSC proliferation. Treatment with TRAM34, a KCa3.1 channel‐specific inhibitor, prevented mechanical stretch‐induced increase in cell proliferation, without effect on cell proliferation under normal control condition (Figure 2F). Similarly, siRNA‐mediated knockdown of the KCa3.1 expression (Figure S1) suppressed mechanical stretch‐induced stimulation of cell proliferation (Figure 2G). Analysis of cell cycle further revealed that pharmacological inhibition of the KCa3.1 channel or genetic depletion of the KCa3.1 expression prohibited mechanical stretch‐induced arrest of cell cycle in the G0/G1 phase (Figure 2H‐K). Collectively, these results consistently support a critical role of the KCa3.1 channel in mediating mechanical stretch‐induced stimulation of BMSC proliferation.
Figure 2.
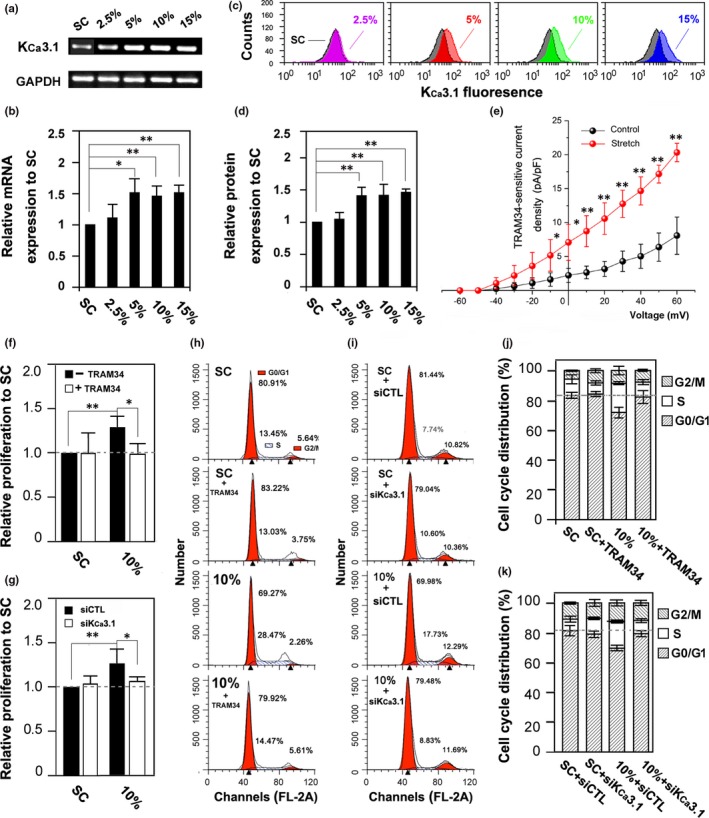
Effects of mechanical stretch on KCa3.1 expression and activity and the role of KCa3.1 channel in mechanical stimulation of BMSC proliferation. A‐D, effects of exposing BMSC to 2.5%‐15% mechanical stretch for 24 h on the KCa3.1 expression levels. A and C, representative results showing the KCa3.1 mRNA expression using RT‐PCR and KCa3.1 cell surface protein expression using flow cytometry. B and D, summary of the mean data as shown in (A) and (C), respectively, from six independent experiments. *P < .05 and **P < .01, using one‐way ANOVA and post hoc Fisher's test. E, summary of the I‐V relationship curves of the mean TRAM‐34 sensitive K+ current densities recorded from seven cells for each condition. Control, isotonic solution; Stretch, hypotonic solution. *P < .05 and **P < .01. Student's t test was used to compare the current density between control and stretch at the same potential. F‐K, summary of BMSC proliferation and cell cycle under indicated conditions after treatment with 100 nmol/L TRAM34 (F, H, J) or siRNA‐mediated knockdown of the KCa3.1 expression (G, I, K), from four independent experiments. *P < .05 and **P < .01, using one‐way ANOVA and post hoc Fisher's test
4. DISCUSSION
We here show that exposure to mechanical stretch stimulates BMSC proliferation (Figure 1A), in support of the notion that mechanical force regulates MSC proliferation.5, 6, 7 We further revealed mechanical stretch‐induced stimulation of BMSC proliferation via promoting cell cycle progression (Figure 1B‐C). Moreover, extended exposure to mechanical stretch strongly up‐regulated the KCa3.1 expression in BMSC (Figure 2A‐D) and, interestingly, acute exposure to hypotonic solution enhanced the KCa3.1 channel activity (Figure 2E). More importantly, inhibition of the KCa3.1 channel with TRAM‐34 (Figure 2F) or siRNA‐mediated knockdown of the KCa3.1 expression (Figure 2G) strongly suppressed mechanical stretch‐induced BMSC proliferation, and such pharmacological or genetic intervention of the KCa3.1 channel inhibited mechanical stretch‐induced alteration in cell cycle (Figure 2H‐K). Previous studies using mouse and dog BMSCs reported engagement of the KCa3.1 channel in cell proliferation.9, 11 However, under our static control condition, inhibition of the KCa3.1 channel in rat BMSC had no effect on cell proliferation (Figure 2F). It is noted that the expression level of Ca2+‐activated K+ channels, including KCa3.1, in BMSCs is strongly species‐dependent,19 which may contribute to the different observations in the present and previous studies, but the exact reason remains unknown. There is evidence that mechanical stimulation induces intracellular Ca2+ increase and cell proliferation in BMSCs.[19, 20, 21] This is consistent with our finding that mechanical stimulation via exposing to hypotonic solution enhanced the KCa3.1 channel activity (Figure 2E). Thus, it is tempting to hypothesize that the KCa3.1 channel plays a critical role in coupling mechanical stimulus‐induced Ca2+ signal to activation of the downstream signalling pathways to stimulate MSC proliferation. It has been also shown that the KCa3.1 channel can regulates extracellular Ca2+ entry and thereby MSC proliferation.11 Further investigations are required to better understand how the KCa3.1 channel is critically engaged in the transduction of mechanical forces to intracellular Ca2+ signalling and cell proliferation in MSCs.
In summary, this study shows a critical role of the KCa3.1 channel in mediating mechanical stretch‐induced stimulation of BMSC proliferation, thus revealing a new molecular mechanism in the transduction of mechanical forces to MSC proliferation. Such a finding should be helpful in developing new strategies to maintain and stimulate the capacity of MSC proliferation and increase the cell number required for MSC‐based applications in regenerative medicine.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
YF developed and organized this paper. XJ, HS, XC, YH and YZ carried out experimental work. XJ and HS mainly drafted the paper and created the figures. PJ, CG, XG and YH assisted in data analysis and interpretation. LH J critically reviewed the manuscript. This manuscript is not under review elsewhere, and all authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (No. 11872010, No. 11421202, No.11827803), National Key R&D Programme of China (No. 2017YFC0111104) and China Scholar Council (No. 201906025008).
Jia X, Su H, Chen X, et al. A critical role of the KCa3.1 channel in mechanical stretch‐induced proliferation of rat bone marrow‐derived mesenchymal stem cells. J Cell Mol Med. 2020;24:3739–3744. 10.1111/jcmm.15014
DATA AVAILABILITY STATEMENT
Data sets generated or analysed during the current study are included in the article.
REFERENCES
- 1. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143‐147. [DOI] [PubMed] [Google Scholar]
- 2. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341‐347. [DOI] [PubMed] [Google Scholar]
- 3. Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707‐715. [DOI] [PubMed] [Google Scholar]
- 4. Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE. 2008;3:e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359‐368. [DOI] [PubMed] [Google Scholar]
- 6. Song G, Ju Y, Soyama H, Ohashi T, Sato M. Regulation of cyclic longitudinal mechanical stretch on proliferation of human bone marrow mesenchymal stem cells. Mol Cell Biomech. 2007;4:201‐210. [PubMed] [Google Scholar]
- 7. Yuan L, Luo Q, Yang L, Song GB. Role of FAK‐ERK1/2 signaling pathway in proliferation of rat bone‐marrow mesenchymal stem cells stimulated by cyclic stretching. J Med Biol Eng. 2013;33:229‐238. [Google Scholar]
- 8. Sforna L, Megaro A, Pessia M, Franciolini F, Catacuzzeno L. Structure, Gating and Basic Functions of the Ca2+‐activated K Channel of Intermediate Conductance. Curr Neuropharmacol. 2018;16:608‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tao R, Lau CP, Tse HF, Li GR. Regulation of cell proliferation by intermediate‐conductance Ca2+‐activated potassium and volume‐sensitive chloride channels in mouse mesenchymal stem cells. Am J Physiol Cell Physiol. 2008;295:C1409‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deng XL, Lau CP, Lai K, Cheung KF, Lau GK, Li GR. Cell cycle‐dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif. 2007;40:656‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vigneault P, Naud P, Qi X, et al. Calcium‐dependent potassium channels control proliferation of cardiac progenitor cells and bone marrow‐derived mesenchymal stem cells. J Physiol. 2018;596:2359‐2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brakemeier S, Kersten A, Eichler I, et al. Shear stress‐induced up‐regulation of the intermediate‐conductance Ca(2+)‐activated K(+) channel in human endothelium. Cardiovasc Res. 2003;60:488‐496. [DOI] [PubMed] [Google Scholar]
- 13. Hayabuchi Y, Nakaya Y, Mawatari K, Inoue M, Sakata M, Kagami S. Cell membrane stretch activates intermediate‐conductance Ca2+‐activated K+ channels in arterial smooth muscle cells. Heart Vessels. 2011;26:91‐100. [DOI] [PubMed] [Google Scholar]
- 14. Huang Y, Jia X, Bai K, Gong X, Fan Y. Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells. Arch Med Res. 2010;41:497‐505. [DOI] [PubMed] [Google Scholar]
- 15. Si H, Grgic I, Heyken WT, et al. Mitogenic modulation of Ca2+ ‐activated K+ channels in proliferating A7r5 vascular smooth muscle cells. Br J Pharmacol. 2006;148:909‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jia X, Yang J, Song W, et al. Involvement of large conductance Ca(2+)‐activated K (+) channel in laminar shear stress‐induced inhibition of vascular smooth muscle cell proliferation. Pflugers Arch. 2013;465:221‐232. [DOI] [PubMed] [Google Scholar]
- 17. Kearney EM, Farrell E, Prendergast PJ, Campbell VA. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann Biomed Eng. 2010;38:1767‐1779. [DOI] [PubMed] [Google Scholar]
- 18. Sumanasinghe RD, Bernacki SH, Loboa EG. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP‐2) mRNA expression. Tissue Eng. 2006;12:3459‐3465. [DOI] [PubMed] [Google Scholar]
- 19. Pchelintseva E, Djamgoz MBA. Mesenchymal stem cell differentiation: Control by calcium‐activated potassium channels. J Cell Physiol. 2018;233:3755‐3768. [DOI] [PubMed] [Google Scholar]
- 20. Hu K, Sun H, Gui B, Sui C. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed Pharmacother. 2017;91:841‐848. [DOI] [PubMed] [Google Scholar]
- 21. Riddle RC, Taylor AF, Rogers JR, Donahue HJ. ATP release mediates fluid flow‐induced proliferation of human bone marrow stromal cells. J Bone Miner Res. 2007;22:589‐600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sets generated or analysed during the current study are included in the article.


