Dear Editor,
In December 2019, cases of pneumonia associated with a novel 2019 coronavirus emerged in Wuhan (Hubei Province, China) [1]. The causative agent was subsequently named SARS-CoV-2 and the resulting disease COVID-19. As of 17 March 2020, 80,894 cases of COVID-19 were reported in China of which 3237 (4%) were fatal. In Wuhan, 2490 (4.98%) of 50,005 cases died. In addition, 98,218 cases from 104 countries outside of China were laboratory-confirmed, of which 4189 (4.27%) were fatal. A previous study suggested that the condition of 11 (11%) patients worsened in a short period of time and they died of multiple organ failure [2], while Wang et al. [3] reported that 4.3% of COVID-19 cases were fatal. A national study of 1099 patients with COVID-19 found that 55 patients (5%) were admitted to an intensive care unit and 15 (1.36%) succumbed to the infection [4]. It is important to emphasize that most patients studied previously were hospitalized and thus the full spectrum of COVID-19 severity is still being elucidated [1–4]. We aimed to further explore the clinicolaboratory characteristics, hospital complications and treatments of 25 fatal cases of COVID-19. The clinicolaboratory characteristics of survivors (N = 149) and non-survivors were also compared.
This was a single-center retrospective analysis. All consecutive fatal cases of COVID-19 admitted to Wuhan University Zhongnan Hospital from 3 January to 24 February 2020 were included. COVID-19 was confirmed using throat swab samples by real-time RT-PCR [3, 5]. Epidemiological, clinical and laboratory data as well as information on treatments received, hospital complications and causes of death were collected. Blood samples were collected at admission. The study was approved by the Ethics Committee of Wuhan University Zhongnan Hospital and informed consent was waived by the Ethics Committee.
Twenty-five fatal COVID-19 cases were included. The median age of these patients was 70 years (interquartile range [IQR] 64–80 years) and 19 (76%) were men. The median time from onset of symptoms to hospital admission and death was 7 days (IQR 1–10 days) and 19 days (IQR 13–26 days), respectively. As shown in Table 1, fatal cases were older (70 years, IQR 64–80 years vs. 51 years, IQR 37–62 years), disproportionately male (76% vs. 40.3%) and more often suffered from comorbidities (64% vs. 24.2%; cardiovascular and cerebrovascular diseases: 32% vs. 7.4%) compared with non-fatal cases. Fatal cases were also more likely to be admitted to intensive care units (36% vs. 10.7%) and had higher medical expenses (53,745 CNY, IQR 30,286–112,268 CNY vs. 14,507 CNY, IQR 8813–27,617 CNY).
Table 1.
Baseline characteristics and laboratory results of included cases of COVID-19
| Non-survivors | Survivors | p-value | |
|---|---|---|---|
| N | 25 | 149 | – |
| Age (years) | 70 (64–80) | 51 (37–62) | < 0.001 |
| Sex—male | 19 (76.0) | 60 (40.3) | < 0.001 |
| BMI (kg/m2) | 24.6 (22.3–28.3) | 23.6 (21.6–25.6) | 0.125 |
| Temperature at admission (°C) | 38.1 (37.1–39.0) | 38.0 (37.3–38.9) | 0.383 |
| Comorbidities | |||
| Any | 16 (64.0) | 36 (24.2) | < 0.001 |
| Hypertension | 12 (48.0) | 25 (16.8) | < 0.001 |
| Diabetes | 6 (24.0) | 11 (7.4) | 0.010 |
| Cardiovascular–cerebrovascular diseases | 8 (32.0) | 8 (7.4) | < 0.001 |
| Respiratory diseases | 4 (16.0) | 8 (7.4) | 0.130 |
| Onset of symptom to (days) | |||
| Hospital admission | 7 (1–10) | 7 (3–9) | 0.381 |
| Death or discharge | 19 (13–26) | 20 (13–27) | 0.894 |
| ICU admission | 9 (36.0) | 16 (10.7) | 0.003 |
| Cost of hospitalization (CNY) | 53 745 (30 286–112 268) | 14 507 (8 813–27 617) | < 0.001 |
| Laboratory findings at admissiona | |||
| White blood cell (109/l) | 6.88 (4.96–13.48) | 4.22 (3.21–5.91) | < 0.001 |
| Elevated (> 9.5) | 10 (40.0) | 10 (6.7) | < 0.001 |
| Lymphocyte (109/l) | 0.53 (0.33–0.82) | 0.92 (0.67–1.23) | < 0.001 |
| Reduced (< 1.1 × 109/l) | 23 (92.0) | 99 (66.4) | 0.010 |
| IL-6 (pg/ml) | 108.8 (44.1–177.9) | 16.8 (4.4–76.9) | < 0.001 |
| Elevated (> 2.9 pg/ml) | 25 (100.0) | 115 (77.2) | 0.017 |
| D-dimer (ng/ml) | 3306 (1790–7512) | 660 (370–1108) | < 0.001 |
| Elevated (> 500 ng/ml) | 24 (96.0) | 89 (59.7) | < 0.001 |
| CRP (mg/l) | 118 (22–184) | 22 (6–45) | < 0.001 |
| Elevated (> 10 mg/l) | 25 (100.0) | 95 (63.8) | < 0.001 |
| Treatment | |||
| Umifenovir | 3 (12.0) | 70 (47.0) | 0.001 |
| Oseltamivir | 16 (64.0) | 61 (40.9) | 0.032 |
| Lopinavir | 5 (20.0) | 46 (30.9) | 0.269 |
| Methylprednisolone | 18 (72.0) | 71 (47.7) | 0.024 |
| Noninvasive ventilation | 4 (16.0) | 5 (3.4) | 0.031 |
| Invasive mechanical ventilation | 17 (68.0) | 2 (1.3) | < 0.001 |
| ECMO | 3 (12.0) | 3 (2.0) | 0.052 |
| CRRT | 7 (28.0) | 1 (0.7) | < 0.001 |
| Complications | |||
| Shock and/or ARDS | 23 (92.0) | 8 (5.4) | < 0.001 |
| Secondary bacterial infection | 20 (80.0) | 6 (4.0) | < 0.001 |
| Acute cardiac injuryb | 18 (72.0) | 7 (4.7) | < 0.001 |
| Acute kidney injury | 19 (76.0) | 8 (5.4) | < 0.001 |
| Acute liver injury | 15 (60.0) | 30 (20.1) | < 0.001 |
| Cause of death | |||
| MODS | 14 (56.0) | – | |
| ARDS | 2 (8.0) | – | |
| Cardiac arrest | 5 (20.0) | – | |
| Respiratory failure | 4 (16.0) | – | |
The results were presented as median (IQR) for continuous variables and number (%) for categorical variables. The different characteristics between death and survival groups were tested by Mann–Whitney U test (continuous variables) or Chi-square test (categorical variables). A two-sided a of less than 0.05 was considered statistically significant
ICU intensive care unit, BMI body mass index, CNY, China Yuan, IL-6 interleukin-6, CRP C reaction protein, ECMO extracorporeal membrane oxygenation, MODS multiple organ dysfunction syndrome, CRRT continuous renal replacement therapy, ARDS acute respiratory distress syndrome
aWe calculated the average value if one patient had multiple tests
bAcute cardiac injury was diagnosed if serum levels of cardiac biomarkers (e.g., troponin I) were above the 99th percentile upper reference limit, or new abnormalities were shown in electrocardiography and echocardiography
During the study period, 174 patients (all COVID-19-positive hospital admissions) had an outcome (death or discharge). Thus, the case fatality rate was 14.4% (95% CI 9.2–19.6%). The most common cause of death was multiple organ dysfunction syndrome (56%). Cardiac arrest (20%), respiratory failure (16%) and acute respiratory distress syndrome (16%) were other causes of death (Table 1). Acute respiratory distress syndrome (shock), secondary bacterial infection and acute cardiac/kidney/liver injury were common during hospitalization. Most patients were treated with methylprednisolone (76%), invasive mechanical ventilation (68%) and oseltamivir (64%). Fatal cases experienced hospital complications and received aggressive treatment strategies more often than non-fatal cases (Table 1). Interestingly, fatal cases were treated more often with oseltamivir and methylprednisolone, but less often with umifenovir (Table 1).
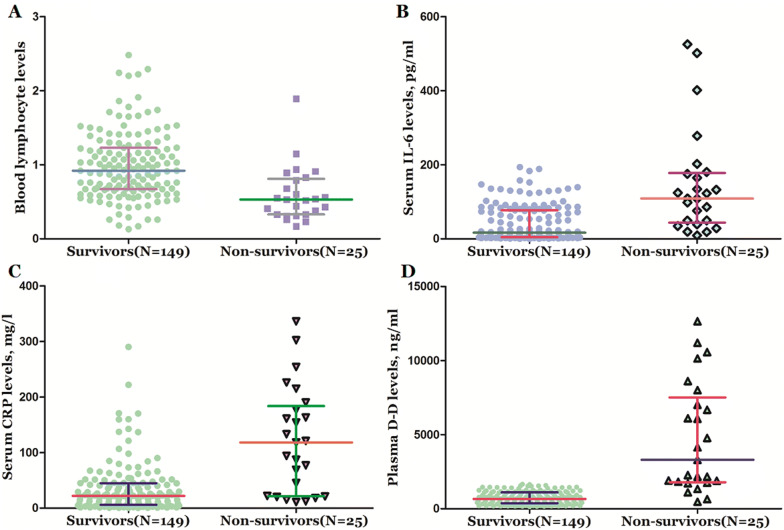
Serum levels of interleukin-6, C-reactive protein and D-dimer were higher in non-survivors than in survivors, while lymphocyte counts were lower (Table 1, Fig. 1). Nearly, all fatal cases had abnormal coagulation, and 24 (96%) fatal cases showed elevated D-dimer levels. All fatal cases showed evidence of cytokine abnormalities and establishment of an inflammatory state as demonstrated by elevated interleukin-6 and C-reactive protein levels.
Fig. 1.
Blood levels of biomarkers in non-survivors and survivors of COVID-19. a Levels of lymphocyte in non-survivors and survivors; b levels of interleukin-6 in non-survivors and survivors; c levels of C reaction protein in non-survivors and survivors; d levels of D-dimer in non-survivors and survivors. All data are medians and interquartile ranges (IQR), with dot plots representing all values
In summary, COVID-19 mortality is more common in older male patients with comorbidities and is mainly caused by multiple organ dysfunction syndrome. The roles of hypercoagulability and pathological inflammatory states should not be ignored. Similarly, other literature recently published in this population also showed that the increasing odds of in-hospital death associated with older age, the presence of underlying diseases, elevated inflammatory and d-dimer greater than 1 μg/ml on admission [6, 7].
An interferon-γ-related cytokine storm may be involved in immunopathological damage in SARS patients [8]. In addition, SARS patients with early-stage disease, especially those with subsequent poor outcomes, had very high numbers of tumor necrosis factor-α- and interleukin-6-producing cells in the blood [9]. Previous studies also reported that fatal cases of COVID-19 had higher levels of clotting factors and cytokines [1–3, 7]. We speculate that the pathogenesis of fatal cases might involve uncontrolled release of immune mediators (i.e., a ‘cytokine storm’). Ruan et al. [6] also suggested that COVID-19 mortality might be due to virus-activated “cytokine storm syndrome” or fulminant myocarditis [6]. Tocilizumab (a monoclonal antibody targeting the interleukin-6 receptor) had been used to treat cytokine storm syndrome [10], and a clinical trial to assess its use in COVID-19 patients has been registered. These findings offer new insights into the characteristics of fatal cases of COVID-19, which may help identify patients at high risk of severe disease or death. The limitations of this study are listed in the supplementary material.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients included in this study. We are really grateful to all the health workers around the world. Their expertise and humanity are fundamental to stop SARS-COV-2 from spreading further. We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. We also acknowledge the contribution of the editors and reviewers who have helped us to improve the manuscript.
Author contributions
Drs W-JT and JC contributed equally as the co-author. Drs XH and QL contributed equally as senior authors. Drs W-JT and JC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design were contributed by JC, XH, LY, W-JT and QL. Acquisition, analysis, or interpretation of data were contributed by JC, XH, LY, W-JT and QL. Drafting of the manuscript was contributed by JC, W-JT and QL. Critical revision of the manuscript for important intellectual content was contributed by XH and LY. Statistical analysis was contributed by JC and W-JT. Administrative, technical, or material support was contributed by JC, XH, W-JT and QL. Supervision was contributed by JC and XH. W-JT and QL obtained funding.
Funding
This study was supported by funding from CAMS Innovation Fund for Medical Science (Dr. Liu, No. 2017-I2M-1-016; Dr. Tu, 2019-I2M-2-006); Natural Science Foundation of Tianjin (Dr. Tu, No. 19JCYBJC26600) and China Postdoctoral Science Foundation funded project (Dr. Tu, No. 2019M660921).
Data availability
Data available can be obtained from the corresponding author.
Compliance with ethical standards
Conflicts of interest
None reported.
Consent for publication
Not applicable.
Role of the funders/sponsors
The study funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen-Jun Tu and Jianlei Cao have contributed equally to this work.
Contributor Information
Xiaorong Hu, Email: cjlzn14@whu.edu.cn.
Qiang Liu, Email: liuqiang@irm-cams.ac.cn.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PubMed] [Google Scholar]
- 5.Cao JL, Hu XR, Cheng W, Yu L, Tu WJ, Liu Q. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;9:9. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-γ-related cytokine storm in SARS patients. J Med Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones BM, Ma ESK, Peiris JSM, Wong PC, Ho JCM, Lam Lai KN, Tsang KWT. Prolonged disturbances of in vitro cytokine production in patients with severe acute respiratory syndrome (SARS) treated with ribavirin and steroids. Clin Exp Immunol. 2004;135:467–473. doi: 10.1111/j.1365-2249.2003.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett D. IL-6 blockade in cytokine storm syndromes. In: Cron R, Behrens E, editors. Cytokine storm syndrome. Cham: Springer; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available can be obtained from the corresponding author.