Abstract
Purpose
Alopecia areata (AA) is an autoimmune disease characterized by the development of non-scarring alopecia. The prevalence is not well known, and estimates vary considerably with no recent estimates in the United States (US). The objective of this study was to define the current AA point prevalence estimate among the general population in the US overall and by severity.
Patients and Methods
We administered an online, cross-sectional survey to a representative sample of the US population. Participants self-screening as positive for AA using the Alopecia Assessment Tool (ALTO) also completed the Severity of Alopecia Tool (SALT) to measure the severity of disease as a percent of scalp hair loss. Self-reported AA participants were invited to upload photographs for adjudication of AA by 3 clinicians.
Results
The average age of participants was 43 years. Approximately half of the participants (49.2%) were male, and the majority were white (77.1%) and not of Hispanic origin (93.2%). Among the 511 self-reported AA participants, 104 (20.4%) uploaded photographs for clinician evaluation. Clinician-adjudicated point prevalence of AA was 0.21% (95% CI: 0.17%, 0.25%) overall, 0.12% (95% CI: 0.09%, 0.15%) for “mild” disease (≤50% SALT score), and 0.09% (95% CI: 0.06%, 0.11%) for “moderate to severe” disease (>50% SALT score) with 0.04% (95% CI: 0.02%, 0.06%) for the alopecia totalis/alopecia universalis (100% SALT score) “moderate to severe” subgroup. The average SALT score was 44.4% overall, 8.8% for “mild”, and 93.4% for “moderate to severe”.
Conclusion
This study suggests that the current AA prevalence in the US is similar to the upper estimates from the 1970s at approximately 0.21% (700,000 persons) with the current prevalence of “moderate to severe” disease at approximately 0.09% (300,000 persons). Given this prevalence and the substantial impact of AA on quality of life, the burden of AA within the US is considerable.
Keywords: epidemiology, Alopecia Assessment Tool, Severity of Alopecia Tool, teledermatology
Introduction
Alopecia areata (AA) is an autoimmune disease characterized by the development of patches of non-scarring hair loss.1 Studies on the prevalence of AA are limited and estimates vary considerably. The most commonly referenced study is the 1971–1974 First National Health and Nutrition Examination Survey (NHANES-I) with a period prevalence estimated between 0.1% and 0.2%.2 Two recent studies in Greece and Japan estimated the period prevalence of AA at 1.27% and 2.45%, respectively.3,4 Estimates of the prevalence of alopecia totalis (AT) and alopecia universalis (AU) among patients with AA also vary greatly, with estimates ranging from 11.5% in NHANES-I to 40% and 50% in two recent studies in the United States (US).5,6 Current treatment options are limited and are not effective in many cases. Fewer options exist for patients with “moderate to severe” AA, including the subgroups of AU or AT. In this study, we sought to estimate the current point prevalence for AA among a representative sample of the US general population with an online cross-sectional survey and clinician adjudication using a teledermatology approach.
Materials and Methods
Study Design
A cross-sectional survey was administered online to 45,016 participants with clinician evaluation of photographs from a subset of participants who self-screened as having AA. The participant survey was administered between May 9, 2017 and July 10, 2017 and clinician evaluations occurred between June 3, 2017 and July 29, 2017. This study was approved by the New England Institutional Review Board®.
Study Population
Participants were recruited from general population research panels provided by Schlesinger Associates, Iselin, New Jersey. The study’s target sample of 45,000 participants aimed to be representative of the 2015 projected US census estimates7 with respect to age, gender, race, household income, and geographic region. Census-balancing quotas were not applied for Hispanic ethnicity or among the adolescents since parent/legal guardian proxies were recruited and the age of the adolescent was unknown. Participants who reported having trichotillomania, scalp radiation therapy, or cancer with chemotherapy in the past year were excluded.
Information on adolescents (11 to 17 years) was obtained through parent/legal guardian proxy and abided by the Federal Trade Commission’s Children’s Online Privacy Protection Act. Only the survey administrator had access to personally identifiable information from their opt-in research panels. Participants qualified for compensation at each stage of the survey.
Participant Survey and Clinician Evaluation
The participant survey included demographic questions, the ALopecia Assessment TOol (ALTO),8 and the Severity of Alopecia Tool (SALT).9 The ALTO includes five self-administered questions to which the patient is asked to respond with “yes”, “no”, or “not sure”. The questions are: 1) Have you been diagnosed with alopecia areata by a dermatologist?; 2) Have you been diagnosed with alopecia areata by a non-dermatologist health care provider (primary care physician, nurse practitioner, or physician assistant)?; 3) Have you ever had round areas of hair loss on your face or scalp?; This question is followed by two sub-questions of a) If YES, did the hair ever grow back? b) If YES, did the hair loss last longer than 6 months?; 4) Have you ever had complete loss of all the hair on your scalp?; 5) Have you ever had complete loss of all the hair on your head AND body? If the patient replies “yes” to any of the five questions, the patient is asked to choose a picture that best represents their hair loss from among four pictures of AA. The ALTO has demonstrated 89.8% (95% CI: 79.2%, 96.2%) sensitivity, 82.8% (95% CI: 76.5%, 88.0%) specificity, 63.1% positive predictive value, and 96.1% negative predictive with a sample size of 239 patients recruited from a dermatologic office setting.
The SALT is a tool used by clinicians to assess the severity of AA through an estimate of the percent of scalp hair loss (0%-100%). A visual aid shows four images of the scalp divided into quadrants (ie, left side, right side, top, and back). The percent of hair loss is assessed in each quadrant and the final SALT score is obtained by multiplying an assigned weight for each quadrant by the percent of hair loss in each quadrant, specifically, SALT score= 0.18×percent_left side + 0.18×percent_right side + 0.4×percent_top + 0.24×percent_back.
Participants who self-screened positive for AA via the ALTO completed the SALT by providing their best estimates of the percent of hair loss in each scalp quadrant using the visual aids from the SALT. In addition, they were asked to upload four photographs, one for each quadrant of the scalp, to a secure website after providing electronic consent. Participants who uploaded photographs qualified for additional compensation. Three clinicians, with experience in AA and SALT scoring, were given access to each participant’s photographs, age, gender, and self-reported age of AA first onset. They classified participants as “definitely AA”, “probably AA”, “non-AA”, “indeterminate due to quality of photographs”, or “indeterminate due to the need for further diagnostic testing". Additionally, the clinicians measured the severity of AA using the SALT.
Statistical Analysis
Self-reported point prevalence of AA was calculated as the proportion of participants who screened positive for AA based on the ALTO and reported currently having AA. For this study, “mild” AA was defined as ≤50% scalp hair loss and “moderate to severe” AA as >50% scalp hair loss, as self-reported by the participant using the SALT. Similar severity thresholds have been used in recent clinical trials.10 However, alternative thresholds may be considered in clinical practice. Lifetime prevalence was also calculated to include those who screened positive for AA and reported having AA in the past.
Participants’ photographs were evaluated separately by each of the three clinicians. For clinician-adjudicated point prevalence, “definitely AA” and “probably AA” classifications were combined and considered AA, with the clinician majority (at least 2 of 3) determining the final classification of either “AA”, “non-AA”, or “indeterminate". If there was no majority, the participant was classified as indeterminate.
To calculate the clinician-adjudicated prevalence estimate for the study population, an adjustment was made by applying the proportion classified as AA by the clinicians to the number of self-reported AA participants who did not upload photographs. Additionally, indeterminate cases were excluded from both the numerator and denominator for clinician-adjudicated prevalence. Two sensitivity analyses were conducted which classified indeterminate cases as 1) AA and as 2) non-AA. Lastly, agreement between the clinicians was assessed using Fleiss’ (unweighted) kappa coefficient.11–13 Self-reported and clinician-adjudicated severity of AA was calculated using the SALT.
Results
Survey Participants
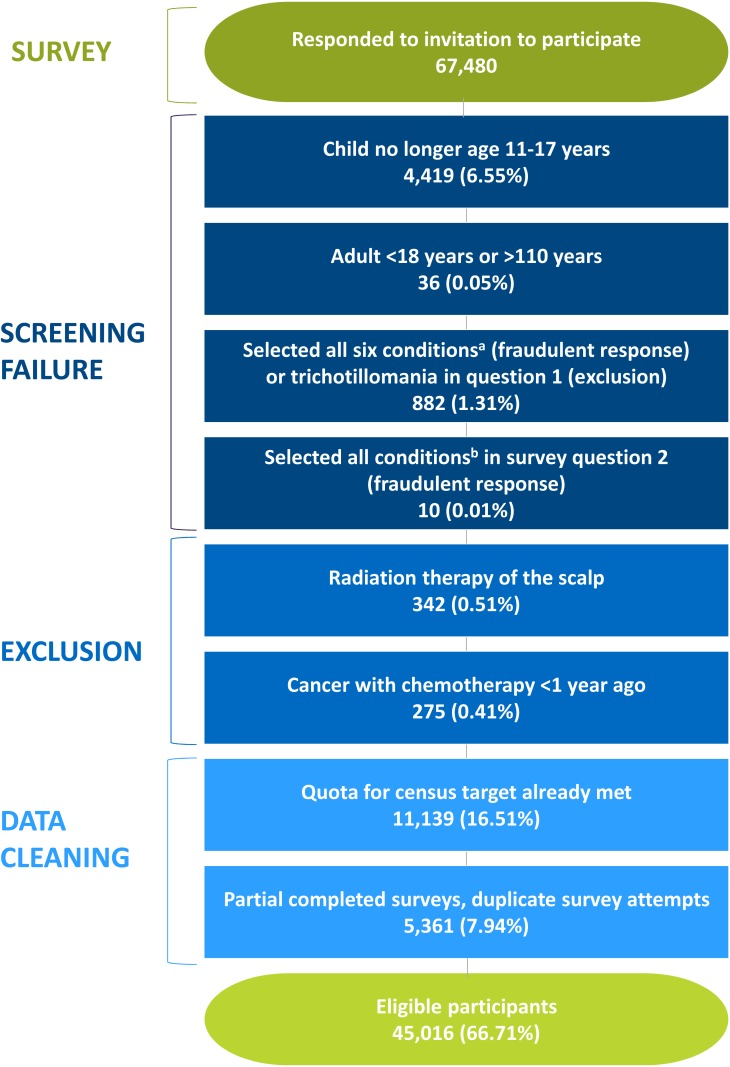
To obtain a sample size of 45,000 eligible participants, 67,480 respondents were recruited (Figure 1). Only 7.3% of respondents failed the screening criteria either because of their age, trichotillomania, or fraudulent response (ie, selected having all pre-existing skin conditions listed, indicating a highly unlikely scenario; Figure 1). Less than 1% were excluded for ever having cancer with scalp radiation therapy or chemotherapy in the past year. Nearly one-fourth were not eligible because the census quota for the demographics had been met (16.5%) or the respondent provided incomplete or duplicate surveys (7.9%). Among the respondents, 45,016 (66.7%) were included as participants (Figure 1).
Figure 1.
Participant attrition.
Notes: aConditions in question 1: atopic dermatitis, psoriasis, rosacea, vitiligo, cancer, trichotillomania. bConditions in question 2: hair loss, warts, rash, hives, brittle nails/nail fungus, argyria, dry skin patches, calluses, skin discoloration, rough skin patches, eczema, fatty skin deposits.
Abbreviations: AA, alopecia areata; HRQoL, health-related quality of life; NA, not applicable.
The average age of the participants was 43.0 years, and half (49.2%) were male (Table 1). The majority were white (77.1%) and not of Hispanic origin (93.2%). Over one-third were from the South (38.9%), followed by the Midwest (22.0%), West (20.7%), and Northeast (18.4%). The participants were representative of the US census 2015 projected population estimates (Table 1).
Table 1.
Demographics of US Population and Study Participants
| Demographic | 2015 Projected US Population (N=321M) | All Participants (N=45,016) | AA Participants (N=511) |
|---|---|---|---|
| Age | |||
| 11–12 | 3.0% | 1334 (3.0%) | 2 (0.4%) |
| 13–17 | 7.4% | 3330 (7.4%) | 9 (1.8%) |
| 18–24 | 11.3% | 5083 (11.3%) | 47 (9.2%) |
| 25–34 | 16.0% | 7186 (16.0%) | 131 (25.6%) |
| 35–44 | 14.7% | 6612 (14.7%) | 124 (24.3%) |
| 45–54 | 15.6% | 7036 (15.6%) | 96 (18.8%) |
| 55–64 | 14.8% | 6657 (14.8%) | 63 (12.3%) |
| 65+ | 17.3% | 7778 (17.3%) | 39 (7.6%) |
| Age, years | |||
| Mean (SD) | – | 43.0 (19.03) | 41.2 (14.12) |
| Median | – | 43.0 | 39.0 |
| Min-Max | – | 11–97 | 12–78 |
| Gender | |||
| Male | 49.2% | 22,145 (49.2%) | 299 (58.5%) |
| Female | 50.8% | 22,871 (50.8%) | 212 (41.5%) |
| Race | |||
| White | 77.1% | 34,715 (77.1%) | 313 (61.3%) |
| Black or African American | 13.3% | 5995 (13.3%) | 133 (26.0%) |
| Asian | 5.6% | 2519 (5.6%) | 45 (8.8%) |
| Native American or Alaskan | 1.2% | 526 (1.2%) | 8 (1.6%) |
| Pacific Islander | 0.2% | 90 (0.2%) | 2 (0.4%) |
| Other Race | 2.6% | 1171 (2.6%) | 10 (2.0%) |
| Hispanic, n (%) | 17.6% | 3043 (6.8%) | 60 (11.7%) |
| Geographic Region | |||
| Northeast | 17.5% | 8287 (18.4%) | 74 (14.5%) |
| Midwest | 21.1% | 9898 (22.0%) | 95 (18.6%) |
| West | 23.7% | 9330 (20.7%) | 119 (23.3%) |
| South | 37.7% | 17,501 (38.9%) | 223 (43.6%) |
| Household Income | |||
| $0–$14,999 | 11.6% | 4792 (10.6%) | 45 (8.8%) |
| $15,000–$24,999 | 10.5% | 4865 (10.8%) | 59 (11.5%) |
| $25,000–$34,999 | 10.0% | 4691 (10.4%) | 75 (14.7%) |
| $35,000–$49,999 | 12.7% | 5959 (13.2%) | 67 (13.1%) |
| $50,000–$74,999 | 16.7% | 7901 (17.6%) | 96 (18.8%) |
| $75,000–$99,999 | 12.1% | 5725 (12.7%) | 62 (12.1%) |
| $100,000–$149,999 | 14.1% | 6603 (14.7%) | 71 (13.9%) |
| $150,000–$199,999 | 6.2% | 2557 (5.7%) | 21 (4.1%) |
| $200,000 and over | 6.1% | 1923 (4.3%) | 15 (2.9%) |
Abbreviations: AA, alopecia areata; US, United States; SD, standard deviation.
Among the 45,016 participants, 511 self-reported having AA using the ALTO. The average age was 41.2 years and over half (58.5%) were male (Table 1). The majority were white (61.3%), not of Hispanic origin (88.3%), and from the South (43.6%), followed by the West (23.3%), Midwest (18.6%), and Northeast (14.5%) (Table 1).
Self-Reported Prevalence and Severity
The self-reported point prevalence of AA was 1.14% (95% CI: 1.04%, 1.24%) overall, 1.03% (95% CI: 0.94%, 1.12%) for “mild” disease (≤50% SALT score), 0.11% (95% CI: 0.08%, 0.14%) for “moderate to severe” disease (>50% SALT score), and 0.04% (95% CI: 0.02%, 0.06%) for AT/AU (100% SALT score) (Table 2). Among self-reported “moderate to severe” AA participants, 37.5% were AT/AU, the most severe among those with “moderate to severe” disease (Table 2).
Table 2.
Estimates of the Point Prevalence and Severity of AA in the US
| AA Group | n | Prevalence Estimate | SALT Score |
|---|---|---|---|
| (95% CI) | Mean (SD) | ||
| Clinician-adjudicated (N=104)a | |||
| Point prevalence | |||
| AA overall | 19 | 0.21% (0.17, 0.25) | 44.4% (43.93) |
| “mild” AA | 11 | 0.12% (0.09, 0.15) | 8.8% (8.12) |
| “moderate to severe” AA | 8 | 0.09% (0.06, 0.11) | 93.4% (11.08) |
| AT/AUb | 4 | 0.04% (0.02, 0.06) | – |
| Sensitivity analysis of AA overall | |||
| Lower bound (indeterminate cases classified as non-AA) | 19 | 0.21% (0.17, 0.25) | – |
| Upper bound (indeterminate cases classified as AA) | 44 | 0.48% (0.42, 0.54) | – |
| Self-reported (N=45,016) | |||
| Point prevalence | |||
| AA overall | 511 | 1.14% (1.04, 1.24) | 25.4% (23.10) |
| “mild” AA | 463 | 1.03% (0.94, 1.12) | 19.3% (12.59) |
| “moderate to severe” AA | 48 | 0.11% (0.08, 0.14) | 84.7% (16.58) |
| AT/AU (SALT=100%)b | 18 | 0.04% (0.02, 0.06) | – |
| AT/AU (SALT≥90%)c | 23 | 0.05% (0.03, 0.07) | – |
| Lifetime prevalenced | |||
| AA overall | 1132 | 2.51% (2.37, 2.65) | – |
| AT/AU | 345 | 0.77% (0.69, 0.85) | – |
Notes: a104 participants uploaded photographs for clinician evaluation resulting in the following classifications by clinician majority: 19 AA cases, 60 non-AA cases, and 25 indeterminate cases (18 due to poor photo quality, 4 due to need for further testing, 3 due to poor photo quality or need for further testing). Using the clinician-adjudicated estimate for overall AA prevalence as an illustrative example, the proportion of clinician-adjudicated AA cases among those participants who uploaded photographs (19 out of 104) is multiplied by the estimated number of self-reported AA cases [(19 ÷ 104) × 511]. This calculation adjusts the numerator in the clinician-adjudicated prevalence estimate for those participants who did not upload photographs. To obtain the final overall estimate of 0.21%, the adjusted numerator is divided by an adjusted denominator which removes the 25 indeterminate cases {45,016 – [(25 ÷ 104) × 511]}. bAT/AU is a subset of moderate to severe AA and defined as self-reported SALT=100%. cAT/AU is a subset of moderate to severe AA and defined as self-reported SALT≥90%. dBased on ALTO alone and self-report of currently having AA or having AA in the past. AT/AU defined based on the ALTO; SALT was not used for the definition of AT/AU.
Abbreviations: AA, alopecia areata; ALTO, Alopecia Assessment Tool; AT, alopecia totalis; AU, alopecia universalis; AT/AU, AT and AU combined; CI, confidence interval; “mild” AA, self-reported SALT score ≤50% hair loss; “moderate to severe” AA, self-reported SALT score >50% hair loss; SALT, Severity of Alopecia Tool; US, United States.
Self-reported lifetime prevalence was 2.51% (95% CI: 2.37%, 2.65%) (Table 2). The average SALT scores were 25.4% overall, 19.3% for “mild” disease, and 84.7% for “moderate to severe” disease (Table 2).
Clinician-Adjudicated Prevalence and Severity
Among the 511 self-reported AA participants, 104 (20.4%) uploaded photographs for clinician evaluation. Demographic characteristics and the presence of other skin conditions (eczema, atopic dermatitis, rosacea, psoriasis, and vitiligo) among these 104 participants were similar to those who did not upload photographs (data not shown). Clinician majority classified 19 of 104 as AA, 60 as non-AA, and 25 as indeterminate. Clinician-adjudicated prevalence of AA was 0.21% (95% CI: 0.17%, 0.25%) overall, 0.12% (95% CI: 0.09%, 0.15%) for “mild” disease, 0.09% (95% CI: 0.06%, 0.11%) for “moderate to severe” disease, and 0.04% (95% CI: 0.02%, 0.06%) for the “moderate to severe” subgroup of AT/AU (Table 2). Among “moderate to severe” AA, clinician-adjudicated AT/AU was 50%. Among the 19 AA cases, the average SALT score reported by the clinicians was 44.4% overall, 8.8% for “mild”, and 93.4% for “moderate to severe” (Table 2).
In the sensitivity analysis, the clinician-adjudicated prevalence remained at 0.21% (95% CI: 0.17%, 0.25%) when setting the indeterminate cases to non-AA, given the small effect on the denominator. However, when setting indeterminate cases to AA, the clinician-adjudicated prevalence increased to 0.48% (95% CI: 0.42%, 0.54%; Table 2).
There was moderate agreement among the three clinicians with respect to AA assessment, with Fleiss’ kappa coefficient (unweighted) equal to 0.49 (95% CI: 0.41, 0.57). Additionally, all adjudicated AA severity assessments (ie, “mild” disease, “moderate to severe” disease, AT/AU) agreed with the participant self-reported assessments of severity made by the participants.
Discussion
This cross-sectional, online survey study utilized research panels and census balancing in an aim to best represent the US population and support generalizability. This methodology provides the largest known sample of the US population for examining the prevalence of AA (NHANES-I was 20,749)2 overall and for the subgroups of “mild” and “moderate to severe”. It also is likely a less biased estimate of the point prevalence of AA in the US compared to past studies that are regionally based or conducted in dermatologic offices or hospital settings. As summarized in a systematic review, two prior population studies in the US may not be representative of the true population at risk of AA since both were based in Minnesota,14 and past hospital-based studies may fail to provide an unbiased sample of the population at risk with respect to exposure status.1
These data provide a recent estimate of the point prevalence of AA based on a sample reflective of the current US population. Clinician-adjudicated point prevalence of AA overall was 0.21% and as high as 0.48% with the sensitivity analysis; and “mild” AA was 0.12%, “moderate to severe” was 0.09%, and AT/AU was 0.04%. Prevalence estimates of “moderate to severe” AA were similar between self-reported (0.11%) and clinician-adjudicated (0.09%), and prevalence estimates of the most severe subgroup of AT/AU were the same between self-reported (0.04%) and clinician-adjudicated (0.04%). Self-reported lifetime prevalence was 2.51%. Compared to the most commonly cited study conducted in the US almost 5 decades ago,2 the clinician-adjudicated prevalence was similar to the upper bound of the NHANES-I estimate (0.21% vs 0.20%) but lower than estimates from Greece (1.27%) and Japan (2.45%).3,4 Additionally, self-reported lifetime prevalence of 2.51% in this study was higher than an estimate from a community-based study conducted in Minnesota reporting 1.7%14 but closer to a more recent study reporting 2.1%.15
These results must be interpreted in the context of the study design. Differences in these findings compared to results from prior studies may reflect variations in the included population, a changing incidence over time, or misclassification bias. Although the self-reported (0.04%) and clinician-adjudicated (0.04%) prevalence estimates for AU/AT were consistent, a large difference was observed between clinician-adjudicated (0.21%) and self-reported (1.14%) prevalence for AA overall. Several factors may account for this.
First, the original validation of the ALTO was undertaken in a single dermatological office setting among a group of patients (<300) whose rate of AA was disproportionate to the general US population. The ALTO’s positive predictive value in a population with a lower AA prevalence would be less than in the testing cohort (83.7%). These factors strongly supported the inclusion of teledermatology and clinician adjudication in this study design. The ALTO tool was originally validated by a clinician performing a physical examination, whereas participant-supplied photographs were used in this study. While a novel approach, the use of teledermatology for this purpose has not been validated and studies examining the use of teledermatology for the diagnosis of AA are limited and include only a few patients to date.16,17
Second, the accuracy of the SALT for use by patients to determine disease severity has not been assessed previously, to the authors’ knowledge, and a margin of error is to be expected in self-classification of the participants as having “mild” disease (≤50% SALT score) or “moderate to severe” disease (>50% SALT score). However, only participants with “mild” hair loss around 50% are likely to be subject to possible misclassification. Additionally, there was complete agreement between the clinicians’ adjudicated assessments of disease severity and the participants’ self-reported assessments of disease severity in this study, which gives some support to patient evaluation of their percent of hair loss using the SALT images for guidance.
Third, in this study, image quality led to the inability to evaluate 21 participants who uploaded photographs; and in cases where photographs were deemed to be of sufficient quality, cases of “mild” AA may have presented as smaller lesions, which are more difficult to identify without an in-person clinical evaluation. This is suggested by the lower average SALT score among adjudicated “mild” cases (SALT score=8.8%) versus self-reported “mild” cases (SALT score=19.3%). It also may be difficult to distinguish “mild” AA cases from androgenic alopecia using photographs when both disorders present concurrently. In such cases, the ALTO may identify suspected AA cases which are determined to be androgenic alopecia after clinical review. This issue may have been compounded by the higher number of male self-reported AA cases in this study. Together, these factors may explain why the self-reported estimate for “mild” AA was much higher than the clinician-adjudicated estimate, whereas, self-reported and clinician-adjudicated estimates for “moderate to severe” AA and the most severe subgroup of AT/AU were similar.
This study is subject to other limitations, including the possible omission of approximately 15% of US households who do not have access to the Internet.18 As lower socioeconomic status and stress have been observed to be associated with the onset and persistence of AA in some populations,19–22 the exclusion of persons without Internet access may have resulted in underestimating the prevalence of AA. Further, participants with possible diffuse AA also were excluded per the ALTO, given the difficulty in differentiating it from age-related hair loss using photographic evidence. In addition, although the sample of participants in this study closely mirrors the US population with respect to age, gender, race, household income, and geographic region, it was not a perfect replication and did not include other population characteristics such as clinical or environmental characteristics that may influence the prevalence of AA.
In this study, clinician evaluation may be subject to variation depending on level of training, years of clinical practice, experience with teledermatology, and experience with AA.23 This was minimized by requiring a majority decision among the three independent clinician reviewers regarding the participant’s AA classification. All three clinicians had significant experience researching and treating AA, and had experience with the SALT to measure disease severity. Finally, the clinician-adjudicated estimate is conditional upon participants who self-reported AA.
Conclusion
This study suggests that AA prevalence in the US has remained reasonably constant over the past 50 years. It is similar to the upper limit estimates in the 1970s at approximately 0.21% and with “moderate to severe” disease estimated at 0.09% and possible lifetime prevalence as high as 2.51%. With current census population numbers,24 the prevalence estimates indicate that there are approximately 700,000 (or between 561,000 and 825,000) persons in the US with AA and approximately 300,000 (or between 198,000 and 363,000) persons with moderate to severe AA, of which up to half of those within the “moderate to severe” category may have AT or AU. Additionally, as many as 8.2 million have had AA in their lifetime. Given the estimated prevalence and the substantial impact of AA on quality of life,25 the burden of this disease within the US is considerable. Recent advances in the understanding of the pathophysiology of AA must be incorporated into ongoing efforts for overall disease management, for which targeted, approved treatments will be critical.
Acknowledgment
This study was presented as a poster at the International Investigative Dermatology, May 16-19, 2018, in Orlando, Florida. The abstract was published with other conference abstracts in the Journal of Investigative Dermatology. 138. B3. 10.1016/j.jid.2018.06.015.
Funding Statement
Pfizer Inc. sponsored and funded this study. The funding source had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions
Daniel and Kauffman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Udall, Anastassopoulos, Daniel, Wahl, Mostaghimi. Acquisition, analysis, and interpretation of data: Daniel, Anastassopoulos, Cappelleri, Lapthorn, Kauffman, Mostaghimi. Drafting of the manuscript: Anastassopoulos, Daniel, Lapthorn. Critical revision of the manuscript for important intellectual content: Benigno, Cappelleri, Mostaghimi, Udall, Chen. Statistical analysis: Daniel, Anastassopoulos, Cappelleri, Wahl, Kauffman. Obtained funding: Udall, Benigno. Administrative, technical, or material support: Peeva, Lapthorn. Study supervision: Anastassopoulos, Benigno, Udall. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Michael Benigno, MA, Margarita Udall, MPH, Joseph C. Cappelleri, PhD, Pratibha Chander, MPH, and Elena Peeva, MD, are employees of Pfizer Inc. Michael Benigno reports non-financial support from Covance Market Access Services Inc., during the conduct of the study; Pfizer has an asset in development for the treatment of alopecia areata. Kathryn P. Anastassopoulos, MS, Shoshana R. Daniel, PhD, Jennifer Lapthorn, MS, and Laura Kauffman, MS, are employees of Covance Market Access Services Inc., which was contracted to conduct the study and lead the development of the manuscript. Peter M. Wahl, MLA, MS, ScD was an employee of Covance Market Access Services Inc. during the conduct of the study.
Arash Mostaghimi, MPA, MPH, MD is an employee of Brigham and Women’s Hospital, Harvard University and served as a paid consultant to Pfizer. Dr. Mostaghimi also received royalties for licensing of ALTO and participated in the clinical trials for Aclaris, Lilly, Concert, and Incyte. Linda Chen is an independent researcher who was employed at Pfizer during the conduct of the study and is a shareholder of Pfizer stock. The authors report no other conflicts of interest in this work.
References
- 1.Fricke ACV, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397–403. doi: 10.2147/CCID.S53985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safavi K. Prevalence of alopecia areata in the first national health and nutrition examination survey. Arch Dermatol. 1992;128(5):702. doi: 10.1001/archderm.1992.01680150136027 [DOI] [PubMed] [Google Scholar]
- 3.Kyriakis KP, Paltatzidou K, Kosma E, et al. Alopecia areata prevalence by gender and age. J Eur Acad Dermatol Venereol. 2009;23(5):572–573. doi: 10.1111/j.1468-3083.2008.02956.x [DOI] [PubMed] [Google Scholar]
- 4.Furue M, Yamazaki S, Jimbow K, et al. Prevalence of dermatological disorders in Japan: a nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J Dermatol. 2011;38(4):310–320. doi: 10.1111/j.1346-8138.2011.01209.x [DOI] [PubMed] [Google Scholar]
- 5.Shellow W, Edwards JE, Koo J. Profile of alopecia areata: a questionnaire analysis of patient and family. Int J Dermatol. 1992;31(3):186–189. doi: 10.1111/ijd.1992.31.issue-3 [DOI] [PubMed] [Google Scholar]
- 6.Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20(9):1055–1060. doi: 10.1111/j.1468-3083.2006.01676.x [DOI] [PubMed] [Google Scholar]
- 7.US Census Bureau website. Available from: https://www.census.gov. Accessed November9, 2016.
- 8.Li DG, Huang KP, Xia FD, et al. Development and pilot-testing of the alopecia areata assessment tool (ALTO). PLoS One. 2018;13(6):e0196517. doi: 10.1371/journal.pone.0196517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen EA. Investigative guidelines for alopecia areata. Dermatol Ther. 2011;24(3):311–319. doi: 10.1111/dth.2011.24.issue-3 [DOI] [PubMed] [Google Scholar]
- 10.Bioniz Therapeutics. Phase 2 Trial of BNZ-1 in patients with moderate to severe alopecia areata. Available from: https://clinicaltrials.gov/ct2/show/NCT03532958. Accessed March18, 2019.
- 11.Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76(5):378–382. doi: 10.1037/h0031619 [DOI] [Google Scholar]
- 12.Chen B, Zaebst D, Seel L. A macro to calculate kappa statistics for categorizations by multiple raters. SUGI Paper; 2005:30–155. [Google Scholar]
- 13.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biom. 1977;33(1):159–174. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 14.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ. 3rd. incidence of alopecia areata in olmsted county, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70(7):628–633. doi: 10.4065/70.7.628 [DOI] [PubMed] [Google Scholar]
- 15.Mirzoyev SA, Schrum AG, Davis MD, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by rochester epidemiology project, 1990–2009. J Invest Dermatol. 2014;134(4):1141–1142. doi: 10.1038/jid.2013.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips CM, Burke WA, Shechter A, Stone D, Balch D, Gustke S. Reliability of dermatology teleconsultations with the use of teleconferencing technology. J Am Acad Dermatol. 1997;37(3):398–402. doi: 10.1016/S0190-9622(97)70139-7 [DOI] [PubMed] [Google Scholar]
- 17.Tran K, Ayad M, Weinberg J, et al. Mobile teledermatology in the developing world: implications of a feasibility study on 30 Egyptian patients with common skin diseases. J Am Acad Dermatol. 2011;64(2):302–309. doi: 10.1016/j.jaad.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 18.10% of Americans don’t use the internet. who are they? Pew research center; April 22, 2019. Available from: https://www.pewresearch.org/fact-tank/2019/04/22/some-americans-dont-use-the-internet-who-are-they/. Accessed December6, 2019.
- 19.Sehgal VN, Srivastava G, Aggarwal A, Sethi G, Adhikari T. Alopecia areata in the Indian subcontinent. Skinmed. 2007;6(2):63–69. doi: 10.1111/skm.2007.6.issue-2 [DOI] [PubMed] [Google Scholar]
- 20.Farajzadeh S, Rahnama Z, Esfandiarpour I, et al. Clinical and demographic profile of childhood alopecia areata in Iran. J Pak Assoc Dermatol. 2013;23(1):20–27. [Google Scholar]
- 21.Karimkhani C, Boyers LN, Prescott L, et al. Global burden of skin disease as reflected in cochrane database of systematic reviews. JAMA Dermatol. 2014;150(9):945–951. doi: 10.1001/jamadermatol.2014.709 [DOI] [PubMed] [Google Scholar]
- 22.Shi Q, Duvic M, Osei JS, et al. Health-related quality of life (HRQoL) in alopecia areata patients – a secondary analysis of the national alopecia areata registry data. J Investig Dermatol Symp Proc. 2013;16(1):S49–S50. doi: 10.1038/jidsymp.2013.18 [DOI] [PubMed] [Google Scholar]
- 23.Kahan BC, Feagan B, Jairath V. A comparison of approaches for adjudicating outcomes in clinical trials. Trials. 2017;18(1):266. doi: 10.1186/s13063-017-1995-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US and World Population Clock. US Census Bureau website. US population of 330,100,145 as of December 6, 2019 at 17:15 UTC Available from: https://www.census.gov/popclock/. Accessed December6, 2019.
- 25.Rencz F, Gulacsi L, Pentek M, Wikonkal N, Baji P, Brodszky V. Alopecia areata and health-related quality of life: a systematic review and meta-analysis. Br J Dermatol. 2016;175(3):561–571. doi: 10.1111/bjd.14497 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- US Census Bureau website. Available from: https://www.census.gov. Accessed November9, 2016.
- Bioniz Therapeutics. Phase 2 Trial of BNZ-1 in patients with moderate to severe alopecia areata. Available from: https://clinicaltrials.gov/ct2/show/NCT03532958. Accessed March18, 2019.
- 10% of Americans don’t use the internet. who are they? Pew research center; April 22, 2019. Available from: https://www.pewresearch.org/fact-tank/2019/04/22/some-americans-dont-use-the-internet-who-are-they/. Accessed December6, 2019.
- US and World Population Clock. US Census Bureau website. US population of 330,100,145 as of December 6, 2019 at 17:15 UTC Available from: https://www.census.gov/popclock/. Accessed December6, 2019.