Abstract
Background and Aims: The World Health Organization (WHO) Western Pacific Region set a target of eliminating mother-to-child transmission (MTCT) of hepatitis B virus (HBV) by 2030. To assess the feasibility of this target in China, we carried out an epidemiological study to investigate the status quo of MTCT in the real-world setting.
Methods: One thousand and eight hepatitis B surface antigen-positive pregnant women were enrolled at 10 hospitals. Immunoprophylaxis was administered to infants. In addition, mothers with HBV DNA level >2,000,000 IU/mL were advised to initiate antiviral therapy during late pregnancy. A health application called SHIELD was used to manage the study.
Results: Nine hundred and five of the enrolled mothers, with 924 infants, completed the follow-up. Birth-dose hepatitis B vaccine and hepatitis B immunoglobulin were received by 99.7% and 99.7% of infants, respectively, within 24 h after birth. There were 446 mothers who received antiviral therapy, including 72.3% of the mothers with HBV DNA level >2,000,000 IU/mL and 21.0% of the mothers with HBV DNA level <2,000,000 IU/mL. Eight infants were infected with HBV. The overall rate of MTCT was 0.9%. Birth defects were rare (0.5% among infants with maternal antiviral exposure versus 0.7% among infants without exposure; p=1.00).
Conclusions:The MTCT rate was lower than the WHO Western Pacific Region elimination MTCT target in this real-world study, indicating that a comprehensive management composed of immunoprophylaxis to infants and antiviral prophylaxis to mothers may be a feasible strategy to achieve the 2030 WHO elimination goal.
Keywords: Mother-to-child transmission, Hepatitis B virus, Antiviral therapy, Immunoprophylaxis, Shield Project
Introduction
Mother-to-child transmission (MTCT) is the major mode of hepatitis B virus (HBV) exposure in areas with a high prevalence of HBV infection.1 MTCT among children born to hepatitis B surface antigen (HBsAg)-positive mothers will induce high rates of chronic HBV infection and is associated with high rates of severe clinical outcomes, such as liver cirrhosis, liver failure and hepatocellular carcinoma.2,3
Combined immunoprophylaxis, including hepatitis B vaccine (HepB) birth dose and hepatitis B immunoglobulin (HBIG), administered within 24 h of birth to infants born to HBsAg-positive mothers is the most effective measure for preventing MTCT of HBV.4,5 However, despite immunoprophylaxis, MTCT cannot be completely prevented, and immunoprophylaxis fails in 2-5% of infants born to mothers with high levels of HBV DNA.6–8
In 2017, the Regional Framework for Eliminating MTCT of Human Immunodeficiency Virus, Hepatitis B and Syphilis in Asia and the Pacific, 2018-2030 (Triple elimination) proposed an integrated and coordinated approach towards elimination of these three infections.9 The framework adopts the target of 0.1% HBsAg prevalence among children by 2030, as set by the Global Health Sector Strategy on Viral Hepatitis 2016-2021. It also suggests antiviral therapy for mothers with high viral load for the Regional Framework for Eliminating MTCT of hepatitis B, building upon previous successful hepatitis B vaccination programs.
In China, the HBV infection rate has been significantly decreasing since the universal immunization of newborns was initiated.10 The latest epidemiological survey (in 2014) showed that the HBsAg prevalence was as low as 0.32% among children under 5 years-old, as compared to 9.67% in 1992.11 China has a large population of HBsAg-positive pregnant women. It was reported that there were approximately one million infants born to HBsAg-positive mothers in 2015.12 It is a great challenge, however, to decrease the HBsAg prevalence further in children under 5 years-old in order to achieve the World Health Organization (WHO) 2030 goal.
In the past decade, antiviral therapy during late pregnancy in mothers with high HBV DNA has been shown to be effective in preventing MTCT of HBV7,8,13–15 and is recommended by hepatitis B guidelines for the management of pregnant women with chronic hepatitis B.1,16–18 In previous studies, no infant was infected with HBV in the group of hepatitis B e antigen (HBeAg)-positive mothers receiving antiviral therapy during late pregnancy,7,8 which indicates that elimination of HBV MTCT is possible with routine immunoprophylaxis for all infants and additional antiviral therapy for HBsAg-positive mothers with high viral load.
Since 2007, China established a rigorous strategy for prevention of HBV MTCT that requires immunization (timely HepB birth dose and HBIG plus two additional doses of HepB), and in 2010, universal screening of HBV in pregnant women was started. Given the increasing evidence of the effectiveness of maternal antiviral therapy in preventing MTCT, we initiated the Shield Project across China — aiming towards zero HBV transmission rate in infants born to HBsAg-positive mothers.19
Methods
Study design and population
This was a prospective, multicenter, observational cohort study to investigate the status quo of MTCT of HBV in the real-world setting (ClinicalTrials.gov No. NCT03539016). The study was conducted between July 2015 and May 2018 in 10 hospitals in China. The subjects included pregnant women at gestational weeks 4-32 and their infants. The main inclusion criteria were pregnant women with positive HBsAg test for a minimum of six months, good compliance, and they and their infants could be followed-up as planned. Pregnant women were excluded if they had a positive serologic test for human immunodeficiency virus or hepatitis C virus, any comorbidity that might reduce compliance, or if they were unable or unwilling to use the mobile health application. Immunoprophylaxis was administered to infants following the national Chinese vaccination schedule, i.e. HBIG and first dose of hepatitis B vaccine within 12 h after delivery and the other two doses of hepatitis B vaccine at 1 and 6 months of age. In addition, mothers with HBV DNA level >2,000,000 IU/mL were advised to initiate antiviral therapy during late pregnancy. Enrolled mothers and infants were prospectively followed until infant post-vaccination serologic testing (PVST) was performed at 7-12 months of age.
Data collection and laboratory testing
The information of the enrolled mothers, including their demographic data, antiviral treatment history, pregnancy and labor history, and comorbidities, were collected from prenatal medical records. HBV serologic markers, including HBsAg, antibody to HBsAg, HBeAg, antibody to HBeAg and antibody to hepatitis B core antigen, HBV DNA levels, alanine aminotransferase, aspartate aminotransaminase, total bilirubin, direct bilirubin, and albumin were measured at baseline, at gestational week 28, and at delivery for all enrolled mothers. Additional tests included liver ultrasound at baseline and PVST for infants at 7-12 months of age. The mode of delivery, neonatal characteristics (height, weight, head circumference, Apgar score, and birth defect) and breastfeeding were noted. The patient’s HBV DNA level and HBV serological markers were measured with the Roche COBAS TaqMan platform (with the lower limit of detection of 20 IU/mL) and Roche Elecsys (Basel, Switzerland). Maternal HBV DNA levels at gestational weeks 24-28 and infant HBV serological markers at 7-12 months were measured at the central laboratory set up by the research group. All other markers were measured at each participating center.
Use of the mobile health application
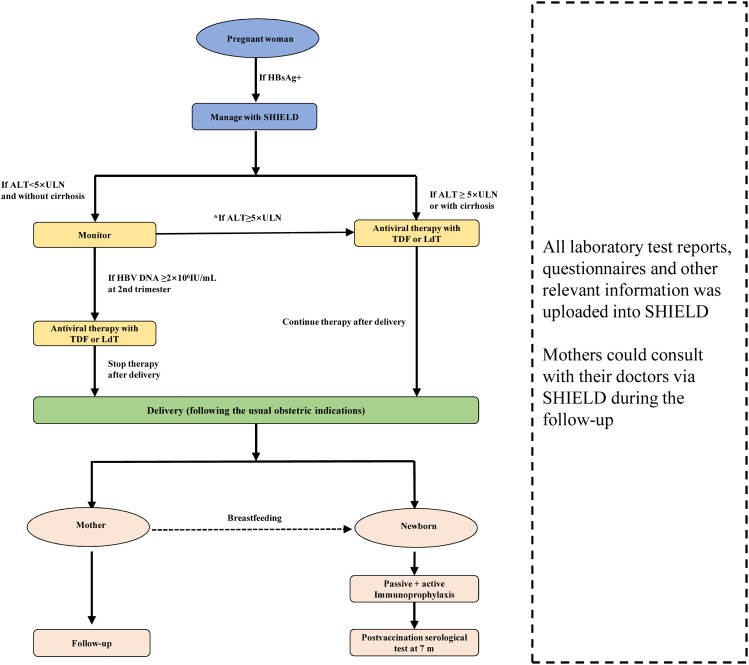
A mobile health application called “SHIELD” was developed and used in this study to collect data and provide support for communication between mothers and their doctors. All laboratory test reports, questionnaires and other relevant information was uploaded into SHIELD and made available to mothers and doctors. Mothers could consult with their doctors via SHIELD during the follow-up (Fig. 1).
Fig. 1. Use of the mobile health application SHIELD to manage this study.
Outcomes
The primary outcome was the rate of MTCT following the administration of immunoprophylaxis and antiviral therapy. The secondary outcome was any potential adverse effect noted among infants following antiviral exposure, including birth defect and infant growth. The rate of MTCT was defined as the proportion of infants who were positive for HBsAg, while they had a detectable HBV DNA level when PVST was performed.
Statistical analysis
Continuous variables are presented as the means ± standard deviation, and the means are compared by Student’s t-test or the nonparametric Mann-Whitney U test. Categorical variables are presented as proportions and Pearson’s chi-square test or Fisher’s exact test were used to compare groups. The association between covariates and the rate of MTCT were assessed with univariate analysis. All statistical tests were two-sided. A p-value <0.05 was considered statistically significant. All data were analyzed by SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Ethics approval
The study was conducted in accordance with Good Clinical Practice, following local regulations and ethical principles described in the Declaration of Helsinki. The study protocol was approved by the ethics committee at each participating center. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Enrollment and follow-up
One thousand and twenty HBsAg-positive pregnant women were screened. Among them, 12 pregnant women were excluded due to not meeting the eligibility criteria, 23 pregnant women withdrew informed consent, 11 lost fetuses before delivery, and 69 were lost to follow-up. Therefore, a total of 905 mothers and 924 infants completed follow-up (Supplementary Fig. 1). The main reasons for loss to follow-up were moving away and parental unwillingness to let their infants undergo blood sampling.
Characteristics of the mothers and infants
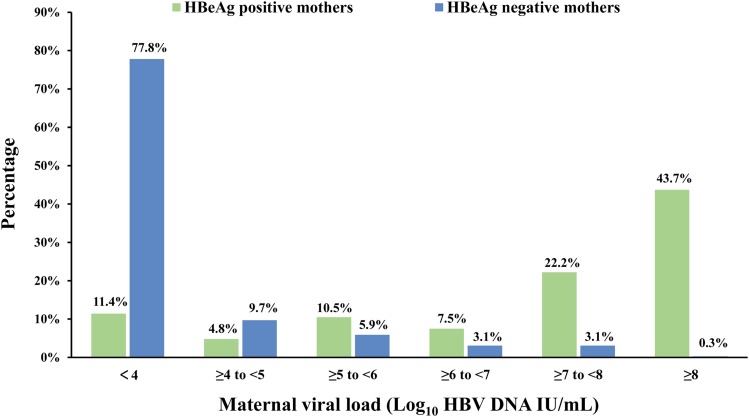
The characteristics of the mothers and infants are listed in Table 1. The distribution of viral load in HBeAg-positive mothers and HBeAg-negative mothers is depicted in Fig. 2. Up to 73.4% of HBeAg-positive mothers had an HBV DNA level of more than 6 log10 IU/mL, compared to 6.5% of HBeAg-negative mothers (p<0.001).
Table 1. Characteristics of the HBV-infected pregnant mothers and their infants enrolled in the Shield study, China, 2015-2018.
| Characteristics | Total, n=905 |
| Maternal characteristics at baseline | |
| Age in year, mean ± SD | 28.2±4.2 |
| Alanine aminotransferase | |
| No. of mothers with data* | 892 |
| Mean ± SD, U/L | 32.2±42.5 |
| Distribution, n (%) | |
| <1×ULN, 40 U/L | 732 (82.1) |
| ≥1×ULN to <2×ULN | 101 (11.3) |
| ≥2×ULN to <5×ULN | 47 (5.3) |
| ≥5×ULN | 12 (1.4) |
| HBV DNA | |
| No. of mothers with data† | 904 |
| Log10 (HBV DNA), mean±SD | 5.7±2.6 |
| Distribution as log10 HBV DNA, n (%) | |
| Undetectable | 38 (4.2) |
| Detectable to <6 | 345 (38.2) |
| ≥6 to <8 | 311 (34.4) |
| ≥8 | 210 (23.2) |
| HBeAg | |
| No. of mothers with data‡ | 893 |
| Distribution, n (%) | |
| Negative | 332 (37.2) |
| Positive | 561 (62.8) |
| Infant characteristics at birth, mean ± SD | |
| Head circumference in cm | 33.2±1.3 |
| Length in cm | 49.8±1.8 |
| Birth weight in kg | 3.2±0.5 |
| Apgar score | 9.7±0.6 |
This category excludes 13 mothers without ALT records.
This category excludes one mother without HBV DNA data.
This category excludes 12 mothers without HBeAg records.
Abbreviations: HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; SD, standard deviation; ULN, upper limit of normal.
Fig. 2. Viral load distribution in hepatitis B e antigen-positive and hepatitis B e antigen-negative mothers.
Maternal antiviral therapy
There were 49.3% of the mothers enrolled in the study receiving antiviral therapy during pregnancy with either telbivudine (LdT), tenofovir disoproxil fumarate (TDF), or lamivudine (LAM). Among the three nucleot(s)ide analogues, LdT (76.8%) was the most frequently used, followed by TDF (19.9%) and LAM (3.3%). Nearly half (46.4%) of the 442 mothers received nucleot(s)ide analogues before gestational week 28, while 49.5% received between gestational weeks 28 to 32, and 4.1% after gestational week 32. A higher percentage of HBeAg-positive pregnant women received antiviral therapy compared to HBeAg-negative pregnant women (71.5% vs. 13.0%). Up to 72.3% of 499 pregnant women with an HBV DNA level of more than 2,000,000 IU/mL received antiviral therapy, compared to 21.0% of those with an HBV DNA level of less than 2,000,000 IU/mL (Supplementary Fig. 2).
Neonatal immunoprophylaxis
Data on the immunoprophylaxis was obtained from 918 infants whose records were available and complete. The coverage of HepB birth dose and HBIG was 99.8% and 99.9%, respectively. The coverage of timely HepB birth dose within 24 h after birth was 99.7%, and 98.1% within 12 h after birth. The coverage of timely HBIG within 24 h was 99.7%, and 99.1% within 12 h after birth. The immunization schedule of four out of ten hospitals involved in this study included two doses of HBIG 100 IU, administered to infants born to HBsAg mothers at birth and 1 month of age. In this cohort, 46.6% of 916 infants received two doses of HBIG 100 IU.
Rate of MTCT
In total, eight infants were found to be positive for HBsAg and had detectable HBV DNA after PVST. All eight infants were born to HBeAg-positive mothers with a high viral load. All of them received timely HepB birth dose and HBIG, except for one who was a preterm infant. The rates of MTCT were 0.9% in 905 HBsAg-positive mothers, 1.4% in 561 mothers who were both HBsAg- and HBeAg-positive mothers, and 1.6% in 499 mothers who had an HBV DNA level of more than 2,000,000 IU/mL, respectively. The characteristics of the eight infected infants and their mothers are listed in Table 2.
Table 2. Characteristics of HBV-infected infants and their mothers in the Shield study, China, 2015-2018.
| No. | Infants | Mothers | ||||||||||||
| Weight in kg | Time of HBIG | Time of HepB birth dose | Feeding practice* | Age in year | HBeAg status | HBV DNA baseline in log10 IU/mL |
HBV DNA at delivery in log10 IU/mL | Antiviral agents | Baseline ALT in U/L | Gestational week | Type of delivery | Threatened abortion/ preterm labor | Amniocentesis | |
| 1 | 3.4 | <1 h | <1 h | Breastfeeding | 38 | Positive | 8 | 7 | No | 19.0 | 38 | Cesarean section | Threatened preterm labor | No |
| 2 | 2.7 | <1 h | <12 h | Breastfeeding | 40 | Positive | 8 | NT | No | 15.0 | 40 | Vaginal | No | No |
| 3 | 2.1 | <2h | - | Breastfeeding + Formula | 33 | Positive | 8 | NT | No | 19.0 | 33 | Vaginal | No | No |
| 4 | 3.2 | <1 h | <1 h | Formula | 38 | Positive | 8 | NT | No | 10.3 | 38 | Vaginal | No | No |
| 5 | 2.4 | <1 h | <1 h | Formula | 38 | Positive | 8 | 4 | TDF at gestational week 27 | 10.2 | 38 | Cesarean section | No | No |
| 6 | 3.6 | <1 h | <1 h | Formula | 39 | Positive | 8 | 4 | LdT at gestational week 27 | 12.8 | 39 | Cesarean section | No | No |
| 7 | 3.2 | <1 h | <1 h | Formula | 39 | Positive | 8 | NT | TDF at gestational week 28 | 14.1 | 39 | Cesarean section | No | No |
| 8 | 2.9 | <1 h | <1 h | Formula | 40 | Positive | 8 | 6 | TDF at gestational week 29 | 18.0 | 40 | Vaginal | Threatened abortion | No |
Feeding practice was observed till infant post-vaccination serologic testing was done.
Abbreviations: ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBIG, hepatitis B immunoglobulin; HBV, hepatitis B virus; HepB, hepatitis B vaccine; LdT, telbivudine; NT, not tested; TDF, tenofovir disoproxil fumarate.
In the 499 mothers with an HBV DNA level of more than 2,000,000 IU/mL, 72.3% received antiviral therapy during late pregnancy. Four HBV-infected infants had maternal antiviral exposure and four were without maternal antiviral exposure. Thus, the rates of MTCT were 1.1% and 2.9% in 361 mothers with antiviral therapy and 138 without antiviral exposure, respectively (p=0.15).
Risk factors for MTCT
Based on the data for the entire cohort, we analyzed the risk factors of MTCT. The results of univariate analyses are listed in Table 3. In the univariate analyses, maternal viral load and HBeAg status were found to be associated with MTCT. We did not find an association between MTCT and mode of delivery, feeding practice, history of threatened abortion and threatened preterm birth, and history of invasive procedures during pregnancy.
Table 3. Univariate analyses on risk factors of mother-to-child transmission.
| Variables | Mother-to-child transmission | p-value | |
| Yes | No | ||
| Total | 8 (0.9%) | 897 (99.1%) | |
| HBeAg | 0.03 | ||
| Positive | 8 (1.4%) | 553 (98.6%) | |
| Negative | 0 (0.0%) | 332 (100.0%) | |
| HBV DNA level at gestational week 28 in log10 IU/mL | 0.002 | ||
| <6 | 1 (0.2%) | 449 (99.8%) | |
| ≥6 to <8 | 0 (0.0%) | 183 (100.0%) | |
| ≥8 | 7 (2.9%) | 237 (97.1%) | |
| Initiation time of antiviral therapy | 1.00 | ||
| <28 weeks | 2 (1.0%) | 203 (99.0%) | |
| ≥28 weeks to <32 weeks | 2 (0.9%) | 215 (99.1%) | |
| ≥32 weeks | 0 (0.0%) | 20 (100.0%) | |
| Mode of delivery | 0.54 | ||
| Vaginal | 4 (0.7%) | 552 (99.3%) | |
| Cesarean section | 4 (1.2%) | 355 (98.9%) | |
| Feeding practice | 0.48 | ||
| Breastfeeding | 3 (0.6%) | 499 (99.4%) | |
| Formula feeding | 5 (1.3%) | 394 (98.8%) | |
| History of threatened abortion/threatened preterm labor | 0.28 | ||
| Yes | 2 (1.7%) | 116 (98.3%) | |
| No | 6 (0.8%) | 781 (99.2%) | |
| History of invasive procedure | 1.00 | ||
| Yes | 0 (0.0%) | 7 (100.0%) | |
| No | 8 (0.9%) | 890 (99.1%) | |
Abbreviations: HBeAg, hepatitis B e antigen; HBV, hepatitis B virus.
Infant safety outcomes
Among the liveborn infants, there were no significant differences in the rate of birth defect between infants with maternal antiviral exposure during pregnancy and those without maternal antiviral exposure (0.5% vs. 0.7%, p=1.00). The gestational age, mode of delivery, weight, height, head circumference and Apgar score at birth were similar in the two groups (Table 4).
Table 4. Characteristics and birth defects of the infants.
| Variable | Maternal antiviral therapy | p-value | |
| Yes, n=446 | No, n=459 | ||
| Head circumference in cm | 33.1±1.3 | 33.2±1.3 | 0.14 |
| Length in cm | 49.9±1.8 | 49.8±1.9 | 0.05 |
| Birth weight in kg | 3.2±0.5 | 3.2±0.5 | 0.39 |
| Apgar score | 9.7±0.5 | 9.7±0.7 | 0.10 |
| Gestational age in week | 39.4±1.5 | 39.1±1.7 | 0.12 |
| No. of cesarean section | 181 (40.4%) | 179 (38.2%) | 0.49 |
| No. of birth defect | 2 (2/440, 0.5%) | 3 (3/447, 0.7%) | 1.00 |
Discussion
A real world study enrolling 1008 HBsAg-positive mothers and their infants was prospectively conducted to investigate the status quo of MTCT in the real world. The results show that the rate of MTCT among HBV-exposed infants was 0.9%, in the context of high coverage of birth dose immunization and maternal antiviral intervention during pregnancy.
For all infants, free three-dose HepB was provided by the Chinese government, guided by the National Immunization Schedule. In this cohort, the timely HepB birth dose and HBIG were 99.7% and 99.7%, respectively. In addition, antiviral therapy was administered to 49.3% of mothers in this cohort. In this study, LdT is the most frequently used NA, followed by TDF and LAM. LdT, LAM and TDF were recommended for maternal antiviral therapy in the previous Chinese chronic hepatitis B guideline.1 In June 2014, TDF was approved in China. Before that, LdT and LAM were used in preventing MTCT.8,13,14 However, TDF and LdT are preferably recommended for preventing MTCT of HBV, guided by the latest Chinese recommendation,20 while TDF is preferably recommended in the American Association for the Study of Liver Diseases and European Association for the Study of the Liver guidelines.16,17
Eight infants were found to be infected by HBV who were all born to HBeAg-positive mothers with an HBV DNA level of more than 2,000,000 IU/mL. Four infants were born to mothers who received antiviral therapy and HBV DNA level was less than 5 log10 IU/mL in two of four mothers and was more than 6 log10 IU/mL in one of four mothers at delivery. This result indicated that in the real world, even if HBeAg-positive mothers with a high viral load received antiviral therapy and their infants received immunoprophylaxis, perinatal infection cannot be completely prevented. One explanation for this is that intrauterine infection occurred in the infants. In fact, one of the four infants was found to be positive for HBV DNA at birth, which indicated an intrauterine HBV infection. Another possible explanation is that these infants’ infections were caused by vaccine-escape mutants with altered binding capacity for the antibody to HBsAg.21,22 Another four infected infants were born to mothers who had an HBV DNA level of 100,000,000 IU/mL but without antiviral therapy during pregnancy. One infant did not receive birth dose vaccine due to prematurity. Delayed immunoprophylaxis for premature or low-birthweight (<2000 g) infants is most likely the cause of these infections. It is recommended that premature infants should also receive timely immunoprophylaxis.23,24
In this study, the rates of MTCT were 0.9% and 1.4% in HBsAg-positive mothers and in HBsAg- and HBeAg-positive mothers, respectively, which was much lower than the rates reported in previous studies.10,25 Besides that, the MTCT rates were 1.1% and 2.9% in HBsAg-positive mothers with high viral load with antiviral exposure and those without antiviral exposure, respectively. Although an apparent reduction in the rate of MTCT was found, there was no significant difference in the rates of MTCT between the two groups. The reason may be the low rate of MTCT and that the sample size is not large enough in this study. Similar to our study, a recent study in Thailand demonstrated that the immunoprophylaxis failure rate was 2.0% in the group without maternal antiviral therapy.7
The threshold HBV DNA level for maternal antiviral therapy varies among hepatitis B guidelines. An HBV DNA level of more than 2,000,000 IU/mL is recommended in the China hepatitis B guidelines as the threshold for antiviral therapy to prevent MTCT,1 whereas HBV DNA level of more than 200,000 IU/mL has been recommended in the American Association for the Study of Liver Diseases and European Association for the Study of the Liver guidelines.16,17 In addition, in Australia and New Zealand, the threshold is set at 10,000,000 IU/mL.26 In this real-world study, all eight HBV-infected infants were born to mothers with HBV DNA level of more than 100,000,000 IU/mL. This result implied that the threshold for antiviral therapy may be higher than the current level in the context of high coverage of timely HepB birth dose and HBIG. On the contrary, in the area where coverage of timely HepB birth dose and HBIG is low, antiviral therapy during pregnancy might be a compensatory approach for preventing MTCT. Currently, it is important to objectively evaluate the significance of maternal antiviral therapy in preventing MTCT of HBV. Based on immunoprophylaxis, maternal antiviral therapy could be used to complement current prophylaxis in mothers with high viral load to prevent MTCT. On the one hand, we should avoid overuse of maternal antiviral therapy in those who are unlikely to transmit to their infant perinatally. On the other hand, we should do our best to prevent MTCT and its consequent outcomes in HBsAg-positive mothers with a high viral load and in their infants.
Univariate analysis showed that HBeAg positivity and high viral load were strongly associated with MTCT. To identify mothers who have a high risk of MTCT, HBeAg and HBV DNA are the most important factors. Ideally, all HBsAg-positive mothers should have a measurement of HBV DNA levels so that antiviral prophylaxis can be administered to those with a high viral load. However, HBV DNA quantification is expensive and may not be available in remote or under-developed areas due to its cost and technical requirements. In our study, 73.4% of HBeAg-positive mothers had an HBV DNA level of more than 2,000,000 IU/mL, compared to 6.5% of HBeAg-negative mothers. Thus, if HBeAg was utilized to screen high-risk mothers for antiviral therapy, 6.5% of HBeAg-negative mothers with an HBV DNA level of more than 2,000,000 IU/mL will be missed, whereas 26.6% of HBeAg-positive mothers with an HBV DNA level of less than 2,000,000 IU/mL will be over-treated. Considering that few infants born to HBsAg-positive and HBeAg-negative mothers would be perinatally infected by HBV after timely immunoprophylaxis at birth,11,25 HBeAg screening might be an acceptable alternative to HBV DNA quantification, especially in areas where HBV DNA testing is unavailable.
The SHIELD mobile application was innovatively developed in this study. First, this new tool was used as an electronic data capture system to collect data including laboratory test reports, immunoprophylaxis and antiviral therapy information, etc. In addition, SHIELD broke down barriers among hepatologists, gynecologists and HBV-infected mothers, providing a platform for communicating without restrictions of time and space. SHIELD is exploring a new model of management for preventing MTCT.
The WHO has set the goal to eliminate viral hepatitis as a major public health threat by 2030, which includes reducing the prevalence of HBsAg among 5 year-old children to 0.1%.27 The WHO Regional Office for the Western Pacific set 2.0% as the target for eliminating MTCT of HBV. In this study, the rate of MTCT could achieve this target in the real world, in the context of high coverage of birth dose immunization and over 70% maternal antiviral intervention in mothers with high viral load. The overall coverage of hepatitis B vaccination has increased steadily, having reached 95% in urban areas in China by 2005.28 Besides that, the recommendation for maternal antiviral intervention has been widely accepted. Thanks to the government’s policy, the prices of antivirals fell sharply in China, improving the affordability and availability of antivirals. We believe that the 2030 elimination target would be achievable nationwide in the future.
China has achieved major success in the prevention and control of hepatitis B.29 To further reduce the rate of MTCT of HBV, actions have already been taken by both government sectors and non-government organizations in China. In December 2017, the National Health Commission (Ministry of Health) officially launched the aptly-named Triple Elimination of MTCT for Hepatitis B, Human Immunodeficiency Virus, and Syphilis Initiative. In July 2015, we launched the Shield Project with the aim of reducing or even eliminating MTCT of HBV. A collaborative network for preventing MTCT has been established and 29,420 HBsAg-positive mothers and 1630 doctors were enrolled in 124 hospitals nationwide by September 2019. The Shield Project will provide high-level evidence for implementing public health policies to prevent MTCT of HBV and will facilitate the process of eliminating MTCT of HBV in China and globally.
The limitation of this study is that the percentage of HBeAg-positive mothers in this study could not represent the natural distribution in pregnant women with chronic HBV infection due to more HBeAg-positive mothers being willing to participate in our study compared to HBeAg-negative mothers.
In conclusion, The MTCT rate was lower than the WHO Western Pacific Region elimination MTCT target in this real-world study, indicating that a comprehensive management composed of immunoprophylaxis to infants and antiviral prophylaxis to mothers may be a feasible strategy to achieve the 2030 WHO elimination target.
Supplementary information
(A) Percentage of maternal antiviral therapy in HBeAg-positive and HBeAg-negative mothers. (B) Percentage of maternal antiviral therapy in mothers with HBV DNA level more than 2,000,000 IU/mL and less than 2,000,000 IU/mL. (C) Percentage of maternal antiviral therapy in all enrolled mothers. (D) Percentage of three nucleot(s)ide analogues used in mothers with antiviral therapy.
Acknowledgments
We thank Dr. Lan Zhang for her professional advice on this study, when she worked in the World Health Organization’s China Office. We thank all the participants who contributed to the study, with the commitment to elimination of HBV mother-to-child transmission.
Abbreviations
- HBeAg
hepatitis B e antigen
- HBIG
hepatitis B immunoglobulin
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HepB
hepatitis B vaccine
- LAM
lamivudine
- LdT
telbivudine
- MTCT
mother-to-child transmission
- PVST
post-vaccination serologic testing
- TDF
tenofovir disoproxil fumarate
- WHO
World Health Organization
References
- 1.Hou J, Wang G, Wang F, Cheng J, Ren H, Zhuang H, et al. Guideline of prevention and treatment for chronic hepatitis B (2015 Update) J Clin Transl Hepatol. 2017;5:297–318. doi: 10.14218/JCTH.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamberth JR, Reddy SC, Pan JJ, Dasher KJ. Chronic hepatitis B infection in pregnancy. World J Hepatol. 2015;7:1233–1237. doi: 10.4254/wjh.v7.i9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 4.Wiesen E, Diorditsa S, Li X. Progress towards hepatitis B prevention through vaccination in the Western Pacific, 1990-2014. Vaccine. 2016;34:2855–2862. doi: 10.1016/j.vaccine.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 5.Chen DS. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50:805–816. doi: 10.1016/j.jhep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. Cochrane Database Syst Rev 2006:CD004790. doi: 10.1002/14651858.CD004790.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Jourdain G, Ngo-Giang-Huong N, Harrison L, Decker L, Khamduang W, Tierney C, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911–923. doi: 10.1056/NEJMoa1708131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Pan CQ, Pang Q, Tian R, Yan M, Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014;60:468–476. doi: 10.1002/hep.27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Eliminating mother to child transmission of HIV, hepatitis and syphilis. Available from: https://www.who.int/westernpacific/activities/eliminating-mother-to-child-transmission-of-hiv-hepatitis-syphilis .
- 10.Wang F, Zhang G, Zheng H, Miao N, Shen L, Wang F, et al. Post-vaccination serologic testing of infants born to hepatitis B surface antigen positive mothers in 4 provinces of China. Vaccine. 2017;35:4229–4235. doi: 10.1016/j.vaccine.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Liang XF, Wang FZ, Yan L, Li RC, Li YP, et al. Hepatitis B vaccine alone may be enough for preventing hepatitis B virus transmission in neonates of HBsAg (+)/HBeAg (-) mothers. Vaccine. 2017;35:40–45. doi: 10.1016/j.vaccine.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 12.Cui F, Woodring J, Chan P, Xu F. Considerations of antiviral treatment to interrupt mother-to-child transmission of hepatitis B virus in China. Int J Epidemiol. 2018;47:1529–1537. doi: 10.1093/ije/dyy077. [DOI] [PubMed] [Google Scholar]
- 13.Pan CQ, Han GR, Jiang HX, Zhao W, Cao MK, Wang CM, et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10:520–526. doi: 10.1016/j.cgh.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55:1215–1221. doi: 10.1016/j.jhep.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 16.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan R, Yin X, Liu Z, Liu Z, Lau G, Hou J. A hepatitis B-free generation in China: from dream to reality. Lancet Infect Dis. 2016;16:1103–1105. doi: 10.1016/S1473-3099(16)30327-9. [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Cui F, Ding Y, Dou X, Duan Z, Han G, et al. Management algorithm for interrupting mother-to-child transmission of hepatitis B virus. Clin Gastroenterol Hepatol. 2019;17:1929–1936.e1. doi: 10.1016/j.cgh.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, et al. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325–329. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- 22.Ma Q, Wang Y. Comprehensive analysis of the prevalence of hepatitis B virus escape mutations in the major hydrophilic region of surface antigen. J Med Virol. 2012;84:198–206. doi: 10.1002/jmv.23183. [DOI] [PubMed] [Google Scholar]
- 23.Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018;67:1–31. doi: 10.15585/mmwr.rr6701a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization Hepatitis B vaccines: WHO position paper, July 2017- Recommendations. Vaccine. 2019;37:223–225. doi: 10.1016/j.vaccine.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 25.Chen HL, Lin LH, Hu FC, Lee JT, Lin WT, Yang YJ, et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology. 2012;142:773–781.e2. doi: 10.1053/j.gastro.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489–492. doi: 10.5694/j.1326-5377.2009.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization Draft global health sector strategy on viral hepatitis, 2016-2021 – The first of its kind. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_32-en.pdf?ua=1 .
- 28.Lu FM, Zhuang H. Prevention of hepatitis B in China: achievements and challenges. Chin Med J (Engl) 2009;122:2925–2927. doi: 10.3760/cma.j.issn.0366-6999.2009.24.001. [DOI] [PubMed] [Google Scholar]
- 29.Cui F, Luo H, Wang F, Zheng H, Gong X, Chen Y, et al. Evaluation of policies and practices to prevent mother to child transmission of hepatitis B virus in China: results from China GAVI project final evaluation. Vaccine. 2013;31(Suppl 9):J36–J42. doi: 10.1016/j.vaccine.2012.11.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Percentage of maternal antiviral therapy in HBeAg-positive and HBeAg-negative mothers. (B) Percentage of maternal antiviral therapy in mothers with HBV DNA level more than 2,000,000 IU/mL and less than 2,000,000 IU/mL. (C) Percentage of maternal antiviral therapy in all enrolled mothers. (D) Percentage of three nucleot(s)ide analogues used in mothers with antiviral therapy.