Abstract
An outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (2019 coronavirus disease, COVID-19) since December 2019, from Wuhan, China, has been posing a significant threat to global human health. The clinical features and outcomes of Chinese patients with COVID-19 have been widely reported. Increasing evidence has witnessed the frequent incident liver injury in COVID-19 patients, and it is often manifested as transient elevation of serum aminotransferases; however, the patients seldom have liver failure and obvious intrahepatic cholestasis, unless pre-existing advanced liver disease was present. The underlying mechanisms of liver injury in cases of COVID-19 might include psychological stress, systemic inflammation response, drug toxicity, and progression of pre-existing liver diseases. However, there is insufficient evidence for SARS-CoV-2 infected hepatocytes or virus-related liver injury in COVID-19 at present. The clinical, pathological and laboratory characteristics as well as underlying pathophysiology and etiology of liver injury in COVID-19 remain largely unclear. In this review, we highlight these important issues based on the recent developments in the field, for optimizing the management and treatment of liver injury in Chinese patients with COVID-19.
Keywords: SARS-CoV-2, COVID-19, Liver injury, Clinical characteristics, Mechanism
Introduction
In December 2019, a novel coronavirus (2019-nCoV), named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was discovered in Wuhan, Hubei Province, China.1–3 The SARS-CoV-2 infection and related coronavirus disease 2019 (COVID-19), has aroused great concern worldwide. The global outbreak of this disease is significantly bigger than the prior pandemic of SARS and Middle East respiratory syndrome (MERS). Within 3 months, SARS-CoV-2 spread rapidly from Wuhan city to the entire country of China, and then into more than 120 countries worldwide. Up to March 14, 2020, a total of 81,021 cases of COVID-19 with 3194 deaths in China and 63,016 cases with 2212 deaths outside of China have been confirmed. Unfortunately, the global numbers of both infected patients and fatalities will continue to grow in the future months because no specific effective antiviral therapies nor vaccine have been identified.4
With the number of cases increasing in China, abnormal liver function test results have been observed in some patients with COVID-19, making this organ the most frequently damaged outside of the respiratory system.5–7 However, the clinical characteristics and outcomes of Chinese patients with COVID-19 might be changing with time, and the clinical-pathological manifestations and mechanism of the liver injury in COVID-19 remain largely unclear.8
This review will summarize these important issues based on the recent developments in the field, for optimizing the management and treatment of COVID-19 patients with liver injury.
General clinical characteristics and outcomes of Chinese patients with COVID-19
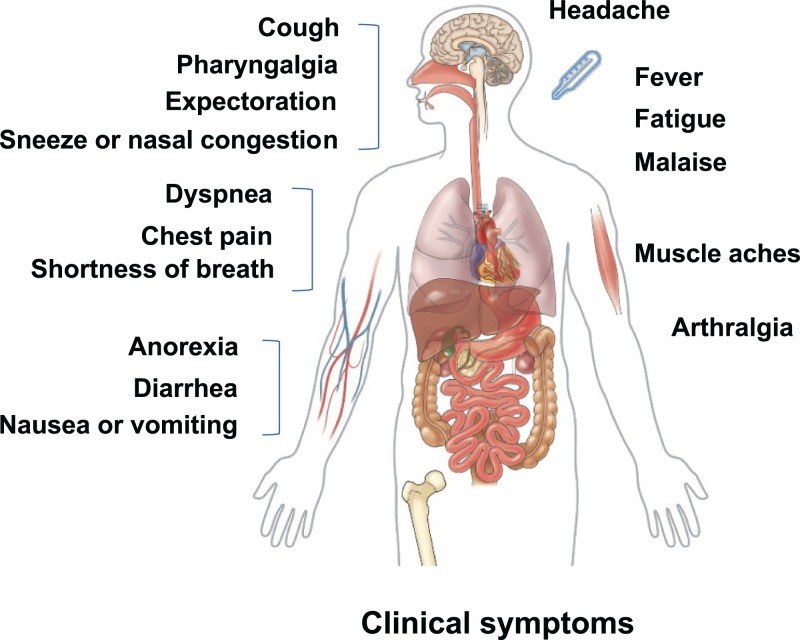
The clinical features of COVID-19 are similar to SARS and MERS, with typical manifestation of pneumonia and acute respiratory infection symptoms (Fig. 1). Most patients with SARS-CoV-2 infection usually have mild symptoms and good prognosis, with the exception of those involving patients with older age and underlying health conditions. Guan et al.9 extracted data regarding 1099 patients with laboratory-confirmed COVID-19 from 552 hospitals in mainland China through January 29, 2020. They reported that the median age of the cases was 47 years, and 41.9% of the patient population was female. The most common symptoms were fever (88.7%) and cough (67.8%). However, nausea or vomiting (5.0%) and diarrhea (3.8%) were present but uncommon. The median incubation period was 4 days, and 23.7% cases had at least one coexisting comorbid condition. Some patients rapidly developed severe pneumonia, pulmonary edema, acute respiratory distress syndrome (ARDS), acute respiratory failure, or multiple organ failure. The primary composite end-point occurred in 67 cases (6.1%), including 5.0% patients admitted to the intensive care unit (ICU), 2.3% patients who underwent invasive mechanical ventilation, and 1.4% patients who died.
Fig. 1. Clinical symptoms of patients with 2019 coronavirus disease.
Recently, the Chinese Center for Disease Control and Prevention published the largest case series to date of COVID-19 in mainland China. Among 72,314 case records (updated through February11, 2020), 44,672 (62%) were classified as confirmed cases of COVID-19, 16,186 (22%) as suspected cases, 10,567 (15%) as clinically-diagnosed cases, and 889 (1%) as asymptomatic cases. In total, 87% of the cases represented ages 30 to 79 years-old. Among 44,415 confirmed cases, 14% cases were classified as severe and 5% as critical. There were 1023 deaths (2.3%) among the 44,672 confirmed cases, all having involved critical cases. The case-fatality rate (CFR) was 8.0% in those aged 70 to 79 years and 14.8% in those aged 80 years and older. The CFR was elevated among those with underlying diseases (10.5% for cardiovascular disease, 7.3% for mellitus diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension, and 5.6% for cancer).10 However, the information regarding coexisting liver disease and liver-related morbidity and mortality were not available in either article.
Furthermore, in a systematic review of 3470 patients with COVID-19, 11.5% were admitted to ICU, while the overall CFR was 3.7%. Compared to patients admitted outside of Hubei, China, those from Hubei had a significantly higher ICU admission rate (21.9% vs. 2.5%). Also, CFR attributed to COVID-19 in Hubei was significantly higher than that of non-Hubei admissions (10.4% vs. 0.6%).11
Characteristics of liver injury in Chinese patients with COVID-19
Laboratory changes of liver function test in patients with COVID-19
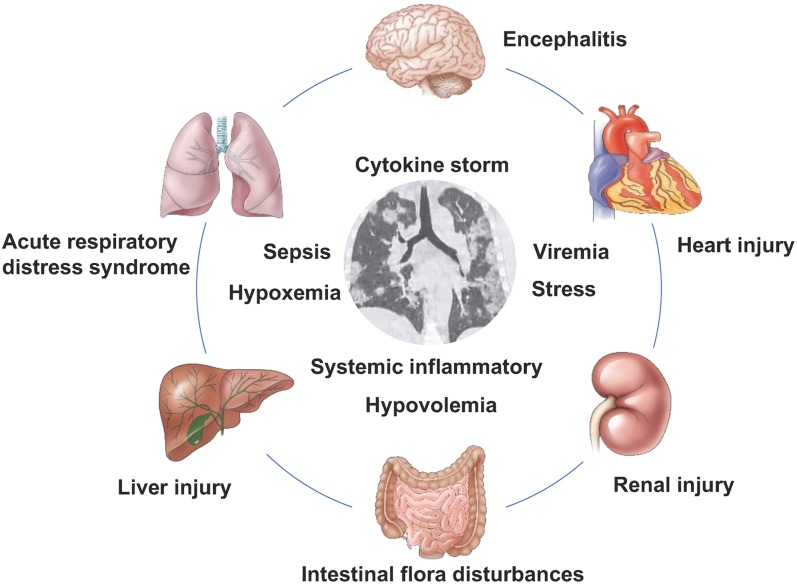
According to the diagnostic criteria, all hospitalized cases of COVID-19 were laboratory confirmed as SARS-CoV-2 infection by nucleic acid testing of respiratory tract samples. On admission, most of the patients with COVID-19 had lymphocytopenia, leukopenia, and elevated levels of C-reactive protein. Severe patients usually had blood oxygen saturation of <93%, and partial pressure of arterial oxygen to fraction of inspired oxygen ratio of <300. Severe hypoxemia and acute respiratory failure usually occurred in the critical cases. Furthermore, the severe or critical patients still had laboratory characteristic findings of septic shock and multiple organ dysfunction or failure, such as liver injury, renal injury, and heart injury (Fig. 2).
Fig. 2. Complications and disease mechanisms in patients with 2019 coronavirus infection.
Abnormal liver enzymes in patients with COVID-19 was first reported by Chen et al.2 Among 99 cases with COVID-19 from Wuhan, 43 cases (43.4%) had increased serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactic dehydrogenase. Most of them had mild elevation of aminotransferase, and only one case had very high levels of aminotransferases (ALT of 7590 U/L and AST of 1445 U/L). However, no case was reported with the features of obvious intrahepatic cholestasis or liver failure. Holshue et al.12 reported the first Chinese case of COVID-19 confirmed in the USA with detailed information of liver function tests. The patient was admitted to hospital on day 4 of illness and progressed to pneumonia on day 9 of illness, and his serum levels of ALT increased from 68 to 203 U/L and AST increased from 37 to 89 U/L. In another report, among 12 severe patients with COVID-19 from Shenzhen, only 1 had abnormal liver enzymes (ALT of 107 U/L and AST of 62 U/L).13 In 62 patients with COVID-19 from Zhejiang, 16.1% patients had elevation of AST (≥40 U/L) and the mean ALT level was 22 U/L.14 Among 305 patients with COVID-19 from Wuhan, 39.1% (119/304) cases had elevation of ALT, AST, or bilirubin at admission. Of them, 24 cases had increased ALT (≥80 U/L) and 19 had increased AST (≥80 U/L), while only 6 had increased bilirubin level.15
Recently, increasing evidence has highlighted the close relationship of abnormal liver biochemistries with severity of COVID-19. In the cohort of 1099 patients with COVID-19 from mainland China, 39.4% had AST >40 U/L and 28.1% had ALT >40 U/L, and most of them occurred in severe and critical cases.9 The mean levels of serum ALT, AST, and bilirubin in severe or critical cases were significantly higher than those in control cases in another multicenter retrospective study including 32 patients.16 Serum levels of ALT and AST in severe to critical cases were significantly higher than those in mild to moderate cases among 265 patients with COVID-19 from Shanghai.17 Similarly, patients admitted to the ICU were more likely to have high levels of serum ALT, AST, and total bilirubin.18,19
In 52 critically ill patients with multiple organ damage, 35 (67%) had ARDS, 15 (29%) had acute kidney injury, 15 (29%) had liver injury, and 12 (23%) had cardiac injury.20 Among 298 patients with COVID-19 from Shenzhen, the percentage of liver injury in the severe group was substantially higher than that in the non-severe group.21 However, significant differences of serum levels of ALT, AST and total bilirubin between patients with and without ARDS were not found in a small sample cohort study from Wuhan.22 In addition, abnormal liver function test in cases of COVID-19 is often transient and often simultaneously combined with increased enzymes from muscle and heart; these laboratory changes can return to normal without liver-related morbidity and mortality.
Pathological changes of liver in Chinese patients who died from COVID-19
The reported point CFR of COVID-19 patients in mainland China has varied widely, mainly based on the geographic locality and the observation time. To date over 3100 patients have died from COVID-19 in mainland China, all of them belonging to the critical cases. However, only several cases have undergone autopsies or post-mortem tissue biopsies. Xu et al.23 first reported the pathological characteristics of post-mortem biopsy specimens from a 50-year-old man who died from critical COVID-19. The results showed moderate microvascular steatosis and mild lobular and portal activity in the liver. Liu et al.24 performed several cases of autopsies in Wuhan, China, and concluded that the histological characteristics of COVID-19 focuses on the lungs and without sufficient evidence for other organ obvious injuries. The autopsy results of the liver include hepatomegaly with dark red, hepatocyte degeneration accompanied by lobular focal necrosis and neutrophil infiltration, infiltration of lymphocytes and monocytes in the portal area, and congestion of hepatic sinuses with microthrombosis. However, neither histological features of liver failure nor bile duct injuries have been observed in these deceased cases.
Pathogenesis and etiology of liver injury in patients with COVID-19
Systemic inflammatory responses and pulmonary injury associated with coronaviruses are triggered by the innate and adaptive immune system. Expression of SARS-CoV S protein in animals is associated with strong inflammatory reaction and enhanced hepatitis. During SARS-CoV infection, host factors trigger an immune response, which can inhibit virus replication, promote virus clearance, and trigger a prolonged adaptive immune response against the viruses. In SARS-CoV-infected patients, CD4+ T cells activate B cells then promote the production of virus-specific antibodies. A large amount of CD8+ T cells can be found in the pulmonary interstitium, which play a vital role in clearing the coronaviruses in infected cells and inducing immune injury.25 However, it is important to note that an immune response out of control can induce immunopathogenesis, which may result in lung tissue damage and functional impairment in COVID-19 patients. However, reports about the underlying immune mechanism of liver injury in COVID-19 are still limited.
Coronavirus-related liver injury in COVID-19
Abnormal liver biochemistries have also been reported in patients with SARS and MERS,26,27 implying that potential liver injury is closely associated with corona virus infection. But it’s unclear whether the liver injury can be caused directly by the coronavirus itself. It is well known that SARS-CoV-2 is closely related to SARS-CoV, and they share the same receptor, angiotensin converting enzyme 2 (ACE2), and lung is the main target organ of the corona virus infection. Previous RNA-seq data in the human protein atlas database demonstrates expression of ACE2 in the liver.28 However, only a low frequency of ACE2 expression is observed in cholangiocytes, but not in hepatocytes, Kupffer cells, and endothelial cells.29 Recently, the single-cell RNA-seq data from two independent cohorts suggest cholangiocyte-specific expression of ACE2 in human liver samples. Similarly, single cell sequencing and immunohistochemistry study showed that ACE2 was only expressed in bile duct epithelial cells of normal liver tissues, and very minimally in hepatocytes.
In a mouse model of acute liver injury with partial hepatectomy, ACE2 expression in the liver was down-regulated on the first day, but it was elevated up to twice of the normal level on the third day and returned to normal level on seventh day when the liver recovered and hepatocyte proliferation stopped. The experimental results implied the up-regulation of ACE2 expression in the liver was caused by compensatory proliferation of hepatocytes derived from bile duct epithelial cells during acute liver injury.30,31 Considering the COVID-19 patients with liver injury mainly manifested as elevation of serum aminotransferases but alkaline phosphatase, SARS-CoV-2 might or might not affect the cholangiocytes through ACE2 damage to the hepatocytes. Liver injury in COVID-19 may thus have alternative pathophysiology and etiology.
Stress and systemic inflammation-related liver injury in COVID-19
Under physiological conditions, the liver is the important organ that meets and filters a large amount of alien material, and then maintains immune tolerance through gut-liver axis. However, immune tolerance is interrupted under psychological stress conditions in patients with severe COVID-19. Hyperactivated immune responses and cytokine storm-related systemic inflammation in SARS-CoV-2 infection can affect and damage many organs, including the gut and liver. Peripheral blood levels of Th17 and CD8 T cells, interleukin-2, interleukin-6, interleukin-7, interleukin-10, tumor necrosis factor-α, granulocyte-colony stimulating factor, interferon-inducible protein-10, monocyte chemotactic protein 1, macrophage inflammatory protein 1 alpha in severe patients were significantly higher than those in control patients.18,21,22,32,33 In the same way, abnormal liver biochemistries have also occurred often in severe COVID-19 cases. Stress-induced liver injury might be associated with hypoxia-reoxygenation, over-activation of Kupffer cells and oxidative stress, intestinal endotoxemia, and activation of the sympathetic nervous and adrenocortical system in COVID-19 patients.
Sepsis is not uncommon in severe and critical COVID-19 cases, especially in patients with intestinal microflora imbalance and existing liver cirrhosis.34 Sepsis is a dysregulated immune response to an infection that leads to psychological stress and multiple organ dysfunction.35 The pathophysiology of sepsis-related liver injury includes hypoxic liver injury due to ischemia and shock, cholestasis due to altered bile metabolism, hepatocellular injury due to drug toxicity or overwhelming inflammation.36 Hence, sepsis in COVID-19 patients might be one of the etiologies of liver injury and substantially impairs the prognosis of COVID-19. Furthermore, severe hypoxia and hypovolemia are the major cause of ischemic/hypoxic liver injury in COVID-19 cases with acute lung failure and/or shock. Ischemic/hypoxic liver injury is associated with metabolic acidosis, calcium overloading, and changes of mitochondrial membrane permeability, and has thus far usually manifested as very high aminotransferase concentrations in serum.37
Drug-induced liver injury or existing liver disease in COVID-19
Moderate microvascular steatosis with mild hepatic inflammation in a COVID-19 patient indicates the possibility of drug-induced liver injury.23 In clinical practice, a large number of patients had history of using antipyretic drugs for treatment of fever.38 Most antipyretic drugs contain paracetamol, which is generally recognized as a common reason for liver injury.39 In addition, many patients with COVID-19 have history of simultaneous use of multiple antiviral drugs, i.e. oseltamivir, abidol, and lopinavir/ritonavir.40,41 These antiviral drugs can induce liver injury as well. A clinical trial of lopinavir/ritonavir or abidol for treatment of COVID-19 from Shanghai showed that two cases of each group occurred liver injury among two-hundred and sixty-two patients.42 Thus, if abnormality of liver enzymes occurs after using a hepatotoxic drug, drug-induced liver injury should first be confirmed or ruled out. In addition, given the high burden of chronic liver disease in China, nonalcoholic fatty liver disease, chronic hepatitis B, and liver cirrhosis might be the major alternative causes of liver injury in Chinese patients with COVID-19.43 SARS-CoV-2 infection and related immune changes might be regarded as a “second hit” to simple fatty liver, and can induce incident liver injury and steatohepatitis. Stress and sepsis are particularly problematic in cirrhotic patients, as either can trigger acute-on-chronic liver failure. However, the information of pre-existing liver diseases has not been outlined in most studies of COVID-19 and the interaction between existing liver disease and SARS-CoV-2 infection has not yet been studied.36
Summary and future perspective
In conclusion, the SARS-CoV-2 infection outbreak has become a global threat to human health over the past 3 months. The COVID-19 disease itself can result in severe and even fatal respiratory diseases, and in mainland China 2.3% of CFR is attributed to ARDS and multiple organ failure. Increasing evidence has demonstrated frequent incident liver injury in COVID-19, especially in patients with multiple organ injury. The liver injury itself has often manifested as transient elevation of serum aminotransferases. However, acute liver failure and obvious intrahepatic cholestasis have been reported in the available studies, but seldomly prevalence of abnormal aminotransferases is significantly higher in severe cases than in mild cases, according to many studies of COVID-19. The underlying mechanisms of liver injury in cases of COVID-19 might include psychological stress, systemic inflammation response, drug toxicity, and progression of pre-existing liver diseases, i.e. from simple fatty liver to steatohepatitis. Currently, there is insufficient evidence for SARS-CoV-2 infected hepatocytes or virus-related liver injury in COVID-19 patients, and it is difficult to summarize the role of the immune system in causing liver damage by the virus at present. Therefore, the pathophysiology and implication of liver injury have not yet been fully determined in COVID-19, as reports about liver injury as well as underlying mechanisms are limited.
Being that liver is the most frequently affected outside of the respiratory system in COVID-19, more intensive surveillance or individually tailored therapeutic approaches is needed for severe cases, especially among those with pre-existing advanced liver disease. Mechanistic understanding of the relationship of SARS-CoV-2 infection with liver injury is important for the clinical practice of managing COVID-19 patients with hepatic injury. Further study should focus on the mechanism of incident liver injury in COVID-19 and the effect of underlying liver disease on treatment and outcome of COVID-19 in the future.
Abbreviations
- 2019-nCoV
2019 novel coronavirus
- ACE2
angiotensin converting enzyme 2
- ALT
alanine aminotransferase
- ARDS
acute respiratory distress syndrome
- AST
aspartate aminotransferase
- CFR
case-fatality rate
- COVID-19
2019 coronavirus disease
- ICU
intensive care unit
- MERS
Middle East respiratory syndrome
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
References
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares Global Emergency: A review of the 2019 Novel Coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu LL, Wang WJ, Zhu QJ, Yang L. [Novel coronavirus pneumonia related liver injury: etiological analysis and treatment strategy] Zhonghua Gan Zang Bing Za Zhi. 2020;28:E001. doi: 10.3760/cma.j.issn.1007-3418.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology. 2020:200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren M, Jie L, Jun S, Subrata G, Liang-Ru Z, Hong Y, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhangfu F, Fang Y, Kang W, Kefang L, Xizhuo S, Nanshan Z, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19): an updated systematic review. medRxiv 2020 (v2) doi: 10.1101/2020.03.07.20032573. [DOI] [Google Scholar]
- 12.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang D, Ma J, Guan J, Wang M, Song Y, Tian D, et al. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: a single-center, descriptive study. Chin J Dig 2020. doi: 10.3760/cma.j.issn.0254-1432.2020.0005. [DOI] [Google Scholar]
- 16.Liu C, Jiang ZC, Shao CX, Zhang HG, Yue HM, Chen ZH, et al. [Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study] Zhonghua Gan Zang Bing Za Zhi. 2020;28:148–152. doi: 10.3760/cma.j.issn.1007-3418.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Lu H, Ai J, Shen Y, Li Y, Li T, Zhou X, et al. A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. medRxiv 2020 (v1) doi: 10.1101/2020.02.19.20025031. [DOI] [Google Scholar]
- 18.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. medRxiv 2020 (v1) doi: 10.1101/2020.02.17.20024018. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Sun W, Li J, Chen L, Wang Y, Zhang L, et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv 2020 (v3) doi: 10.1101/2020.02.17.20024166. [DOI] [Google Scholar]
- 23.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020. pii: S2213-2600(20)30076-X doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Wang R, Qu G, Wang Y, Liu P, Zhu Y, et al. General anatomy report of novel coronavirus pneumonia patients. Journal of Forensic Medicine. 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 29.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, et al. [Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia] Zhonghua Gan Zang Bing Za Zhi. 2020;28:E002. doi: 10.3760/cma.j.issn.1007-3418.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv 2020 (v1) doi: 10.1101/2020.02.06.20020974. [DOI] [Google Scholar]
- 32.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv 2020 (v1) doi: 10.1101/2020.02.10.20021832. [DOI] [Google Scholar]
- 33.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19) medRxiv 2020 (v1) doi: 10.1101/2020.02.18.20024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joung JY, Cho JH, Kim YH, Choi SH, Son CG. A literature review for the mechanisms of stress-induced liver injury. Brain Behav. 2019;9:e01235. doi: 10.1002/brb3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14:417–427. doi: 10.1038/s41581-018-0005-7. [DOI] [PubMed] [Google Scholar]
- 36.Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Li RJ, Lv GY, Liu HQ. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2015;19:2036–2047. [PubMed] [Google Scholar]
- 38.Deng SQ, Peng HJ. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9:575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun C, Fan JG. [Strengthen clinical research on liver injury in patients with novel coronavirus pneumonia] J Prac Hepatol. 2020;23:153–155. doi: 10.3969/j.issn.1672-5069.2020.02.001. [DOI] [Google Scholar]
- 40.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020. pii: S2468-1253(20)30057-1 doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19) J Gen Intern Med 2020. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Ling Y, Xi X, Liu P, Li F, Li T, et al. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chin J Infect Dis. 2020;38:E008. doi: 10.3760/cma.j.cn311365-20200210-00050. [DOI] [Google Scholar]
- 43.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]