Abstract
α-Mangostin, a xanthone derivative from the pericarp of Garcinia mangostana L., has numerous bioactivities and pharmacological properties. However, α-mangostin has low aqueous solubility and poor target selectivity in the human body. Recently, nanoparticle drug delivery systems have become an excellent technique to improve the physicochemical properties and effectiveness of drugs. Therefore, many efforts have been made to overcome the limitations of α-mangostin through nanoparticle formulations. Our review aimed to summarise and discuss the nanoparticle drug delivery systems for α-mangostin from published papers recorded in Scopus, PubMed and Google Scholar. We examined various types of nanoparticles for α-mangostin to enhance water solubility, provide controlled release and create targeted delivery systems. These forms include polymeric nanoparticles, nanomicelles, liposomes, solid lipid nanoparticles, nanofibers and nanoemulsions. Notably, nanomicelle modification increased α-mangostin solubility increased more than 10,000 fold. Additionally, polymeric nanoparticles provided targeted delivery and significantly enhanced the biodistribution of α-mangostin into specific organs. In conclusion, the nanoparticle drug delivery system could be a promising technique to increase the solubility, selectivity and efficacy of α-mangostin as a new drug candidate in clinical therapy.
Keywords: Garcinia mangostana, solubility, controlled release, targeted delivery, nanoparticle formulations, physicochemical properties
Introduction
α-Mangostin, a xanthone derivative compound isolated from Garcinia mangostana L. peel extract, has myriad pharmacological effects: antibacterial, antifungal, anti–inflammatory, antiallergic, antioxidant and anticancer activities.1–5 The anticancer activity indicates that α-mangostin might serve as a potent anticancer agent in lung, stomach, colon, cervical, pancreatic, prostate, mammary gland, chondrosarcoma, renal, skin, tongue mucoepidermoid and breast cancers.6–18 However, α-mangostin has low solubility in water (2.03 x 10−4 mg/L at 25ºC), and many efforts have been made to improve it: structure modification, co-solvation, solid dispersion, emulsion, complexation and nanoparticle drug delivery systems.19–21 Additionally, α-mangostin and other cytotoxic drugs generally have limitations that influence their effectiveness, including a first fast metabolism reaction, an efflux reaction induced by transporter intercellular, fast drug release and a non-specific target site.22–24
Drug bioavailability is an important parameter to determine how successful the drug molecules pass through in pharmacological phases such as biopharmaceutics, pharmacokinetics, and pharmacodynamics.25 To achieve the maximum bioavailability, drug solubility is one of the primary factors that can increase the drug bioavailability.26 Currently, nanoparticle drug delivery systems are the most commonly used technique for nanomedicine-mediated treatment of diseases. Their nanosize can enhance solubility by providing a large surface area, which increases the penetration rate into a cell membrane and provides a controlled release system with passive or active targeting. This effect can serve as a cancer drug delivery system.27–29 This system becomes a promising method to overcome the limitations of α-mangostin. Several types of nanoparticles have been formulated for the α-mangostin compound, including nanolipids, nanopolymerics, nanomicelles, nanoliposomes, nanofibers and metal nanoparticles.19,20,30-34 The results are substantial, with significantly improved solubility for α-mangostin. Therefore, controlled and targeted drug delivery systems can be created by modified nanoparticle technology.
There are numerous published α-mangostin studies; however, they are usually limited and only discuss its pharmacological properties and bioactivities. Dermawan et al tried to predict the increase in α-mangostin solubility using a cyclodextrin inclusion complex. The inclusion complex formation energy values for all α-mangostin/cyclodextrins were obtained using the semi-empirical PM7 method. No researchers have performed experiments to prove the results of this in silico study.35 Taken together, we believe that our review concerning nanoparticle drug delivery systems for α-mangostin, which relates to its solubility and selectivity properties, will broaden the spectrum of α-mangostin utilisation and allow for improved efficacy.
Methodology
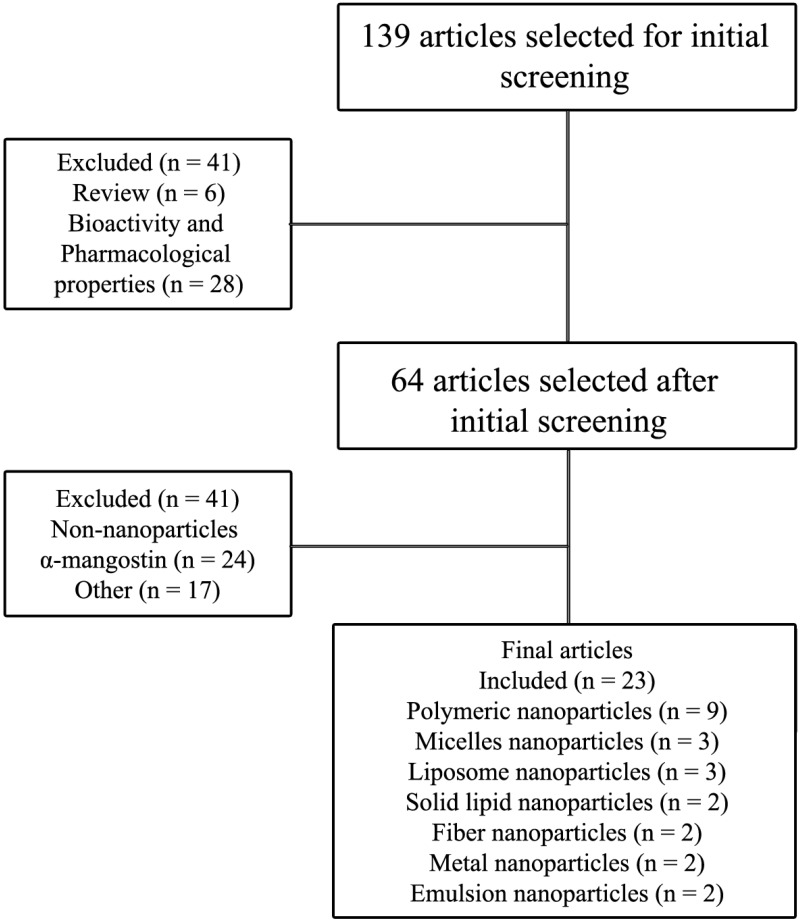
This review is based on the literature obtained from Scopus, PubMed and Google Scholar using the keyword “nanoparticle formulation of α-mangostin”, “nanoparticle drug delivery of α-mangostin”, and “α-mangostin nanoparticle.” We excluded opinions, reviews and unrelated topics such as pharmacological properties and bioactivities. The databases are limited to obtain the specific topic in pharmaceutical formulation. The flowchart of the methodology is shown in Figure 1.
Figure 1.
Flowchart of the methodology used in this review.
α-Mangostin
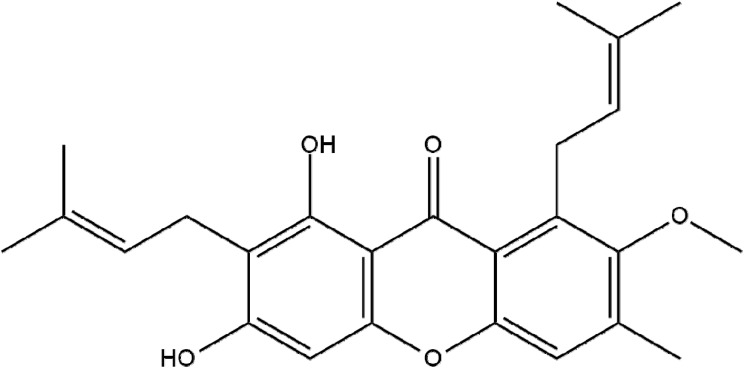
Mangosteen (G. mangostana), the queen of tropical fruits, grows in tropical rainforests of Malaysia, Thailand and Indonesia. α-Mangostin (Figure 2) is the major compound of mangosteen peel extract; it is a xanthone derivative with the chemical name 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-9H-Xanten-9-0n (Table 1). Its pharmacological activities are diverse: antibacterial, anti-allergic, anti-fungal, anti–inflammatory activity, antioxidant and anticancer.1–5
Figure 2.
Chemical structure of α-Mangostin.
Table 1.
| Property | Description |
|---|---|
| Chemical names | 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-enyl)xanthen-9-one |
| Physical state | Solid |
| Colour/form | Faint yellow to yellow powder |
| Molecular formula | C24H26O6 |
| Molecular weight | 410.466 g/mol |
| Melting point | 180–181ºC |
| Solubility | Soluble in ethanol; in water, 2.03 x 10−4 mg/L at 25ºC |
| Log Kow | 7.71 (estimated) |
| Stability/shelf life | Stable under normal temperatures and pressures |
| Decomposition | Nitrogen oxide, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen |
| Dissociation constants | pKa 1 = 3.68 (primary carbonyl) pKa 2 = 7.69 (secondary carbonyl) pKa 3 = 9.06 (tertiary carbonyl) |
| Henry’s Law constant | 2.05 x 10−16 atm·m3/mol at 25ºC |
Previous studies demonstrated that α-mangostin can act against cancer cells via multiple pathways,38–41 including inhibiting fatty acid synthase, signalling human epidermal growth factor receptor 2 (HER2)/phosphatidylinositide 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK).6,42 Notwithstanding its excellent bioactivity, α-mangostin has limited solubility in water (2.03 x 10−4 mg/L at 25ºC). These problems become a basic consideration for developing α-mangostin with better efficacy.
Nanoparticle Drug Delivery Systems for α-Mangostin
Recent Nanoparticle Formulations for Improved Water Solubility, Modified Release and Targeted Drug Delivery
Nanomedicine, a nanotechnology application, has an important role in clinical therapy. Due to its nanosize (10−9 m), the large surface area of the nanocompounds enhances the surface contact with its solvent and improves the solubility or dissolution rate of slightly water-soluble compounds.43 Nanomedicine therapeutic interventions can be highly specific at the intermolecular scale to allow for curing diseases or repairing damaged tissues, such as nerves, muscles or bones. Liposomes, dendrimers, solid lipid nanoparticles, polymeric nanoparticles, silicon or carbon materials, metal and magnetic nanoparticles are examples of nanocarriers that have been formulated as drug delivery systems.44 A nanoparticle drug delivery system is a promising modification technique due to the combination of physics and chemical sciences. It is a proven, favourable technique to overcome the limitation of drugs with the poor solubility in water and provide the targeted drug delivery system.45
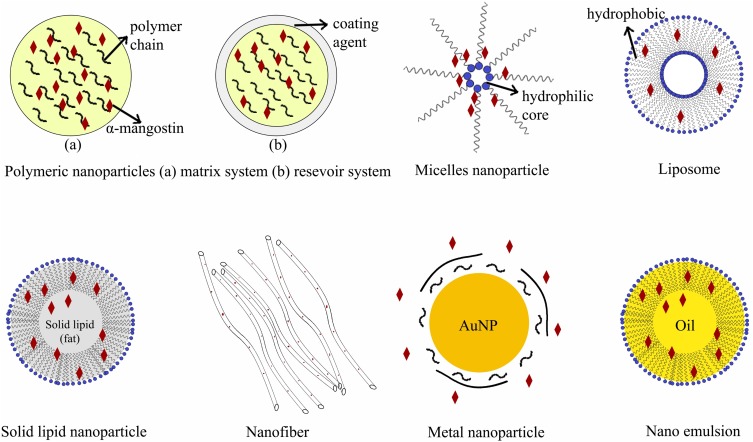
Table 2 and Figure 3 describe the various nanoparticle formulations that have made this technique a promising multifunctional drug delivery system. Nanoparticles are formulated as dendrimers, solid lipid nanoparticles, metal nanoparticles and liposomes, among others. They are commonly used to deliver drugs to specific targets, including cells, receptors and genes. The important aspects of the formulation depend on the selection of the right excipient, which play a crucial role in delivering active drug substances to the intended target. Folic acid (FA), mesenchymal stem cells (MSCs), mannose, hyaluronic acid, poly(lactic-co-glycolic acid) (PLGA) and chitosan conjugated with copolymers are the main excipients used to deliver active, high-affinity pharmaceutical ingredients. Nanoparticle formulations can also enhance absorption and the penetration rate as a function of small particle size, membrane transport, cellular uptake and bioadhesive interactions with the cell membrane.71,72 Finally, a drug’s bioavailability can be improved by nanoparticle formulations, a phenomenon consistent with the enhanced solubility, dissolution and absorption rate.73
Table 2.
Recent Nanoparticle Formulations for Improved Water Solubility, Modified Release and Targeted Drug Delivery
| No | Types of Nanoparticle | Excipients | Main Objective | Ref. |
|---|---|---|---|---|
| 1 | Nanoparticle-orodispersible films | Vinylpyrrolidone-vinyl acetate copolymer/HPMC-Glycerol | Modify the disintegration time and dissolution rate of drug particles loaded into ODFs | [46] |
| 2 | IL-polymer nanoparticle | PLGA/PVA | Formulate a hybrid IL-nanoparticle system to deliver a poorly soluble drug | [47] |
| 3 | Crystalline nanoparticle | HPC-dioctyl sulfosuccinate 141 Na | Improve solubility | [48] |
| 4 | Nanoparticle antisolvent crystallisation | Poloxamer 188 and solupus | Improve solubility and dissolution rate | [49] |
| 5 | Polymeric nanoparticle | Eudragit® RL 100 | Sustained release system | [50] |
| 6 | Gold (Au) nanoparticle | Au/Carrageenan oligosaccharide | pH-triggered anticancer drug release | [51] |
| 7 | Solid lipid nanoparticle | Decosanoic acid | Sustained release system | [52] |
| 8 | Polymeric nanoparticle | PLGA/hyaluronic acid | Controlled release and targeted drug delivery | [53] |
| 9 | Receptor-responsive nanoparticles | Amino terminal fragment (ATF) of human serum albumin (HSA) | Targeted to the urokinase receptor | [54] |
| 10 | Theranostic nanoparticle (metal nanoparticle) | Au/bovine serum albumin | Drug-dependent release and targeted drug delivery | [55] |
| 11 | Curdlan nanoparticle | Cyclodextrin | Intracellular release | [56] |
| 12 | Thermosensitive nanoparticle hydrogel (polymeric nanoparticle) | Amphiphilic copolymer poly(ε-caprolactone-co-1,4,8-trioxa [4.6]spiro-9-undecanone)-poly(ethylene glycol)-poly(ε-caprolactone-co-1,4,8-trioxa [4.6]spiro-9-undecanone) | Sustained co-delivery and early local treatment drug delivery for peri–implantitis | [57] |
| 13 | Semi-solid prodrug nanoparticles | Polymer-surfactant | Long-acting delivery | [58] |
| 14 | Integrin-based nanoparticle (liposome) | Lipoid S100, cholesterol, mPEG2000-DSPE and Mal-PEG2000-DSPE | Targeted drug delivery for hepatic stellate cells | [59] |
| 15 | Nanoparticle-conjugated microbubble | 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)- 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[succinyl(polyethylene glycol)-2000] (DSPE-PEG2k-NHS) and albumin | Targeted drug delivery for liver tumours | [60] |
| 16 | Silica nanoparticle (solid lipid nanoparticle) | (3-mercaptopropyl)-trimethoxysilane (MPTMS), β-mercaptoethylamine (MEA), Triton X-100 and tetraethyl orthosilicate (TEOS)/indocyanine green (ICG) | Targeted drug delivery for breast cancer cells | [61] |
| 17 | Self-assembling nanoparticle (poly-lysine dendrimer) | Polyglutamic acid (PGA)-polylysine/folic acid hydrate | Targeted drug delivery for breast cancer cells | [62] |
| 18 | Nano-hybrids | Bovine serum albumin (BSA), N-(3-dimethylaminopropyl), N′-ethylcarbodiimide hydrochloride (EDC·HCl), N-hydroxysuccinimide (NHS), phospholipid complex, cadmium chloride (CdCl2·2.5H2O), thioglycolic acid (TGA), and D-mannose. | Tumour-targeted drug delivery | [63] |
| 19 | Copper (Cu) nanoparticle (metal nanoparticle) | FeCl3, and CuCl2 | In vivo–targeted molecular imaging | [64] |
| 20 | Fe3O4 nanoparticle (metal nanoparticle) | Fe3O4 (iron oxide)/mesenchymal stem cells (MSC) | Targeted delivery for lung cancer | [65] |
| 21 | Hollow Au nanoparticle (metal nanoparticle) |
Human placental Au/MSCs | Targeted drug delivery | [66] |
| 22 | HSA nanoparticle | HSA/FA-N-hydroxysuccinimide (NHS) | Targeted to the folic acid receptor | [67] |
| 23 | Au nanoparticle (metal nanoparticle) | Tetrachloroauric acid (HAuCl4)-mono protected poly(ethylene glycol)-amino poly(ethylene glycol) undecyl mercaptan/chitosan low molecular weight | Targeted treatment for acute renal failure | [68] |
| 24 | Hybrid nanocarriers (liposomes) | Dipalmitoyl phosphatidylcholine (DPPC) and 1-oleoyl-2-[12-biotinyl(aminododecanoyl)]-sn-glycero-3-phosphocholine | Targeted to hepatocellular carcinoma cell lines | [69] |
| 25 | Folate-modified nanoparticle (polymeric nanoparticle) | FA, methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) and DOTAP | Targeted gene delivery system | [70] |
Figure 3.
Nanoparticle drug delivery systems for α-mangostin.
Polymeric Nanoparticles of α-Mangostin
Polymeric nanoparticles are generally used to solve the limitation of poorly soluble drugs, provide controlled release and targeted drug delivery. Several studies have been reported related to polymeric nanoparticle formulations for α-mangostin (Table 3). The first polymeric nanoparticle formulation of α-mangostin was reported in 2011; it included biodegradable PLGA copolymers. In that study, α-mangostin was encapsulated in PLGA using colloidal extraction solvent evaporation. The PLGA α-mangostin nanoparticle was less cytotoxic to the A549 lung cancer cell line compared to free α-mangostin. These results suggest that PLGA nanoparticles can be used as a micro-carrier system for the delivery of α-mangostin as a passive tumour-targeting agent.74 Another study from the same year reported an α-mangostin polymeric nanoparticle using PLGA with chitosan biopolymer. Interestingly, the formulation without chitosan was more toxic to A549 cells. The authors speculated that the mechanism of action is mediated by the high-affinity property of chitosan biopolymer can target the nanoparticle drug delivery system targeted in lung cancer tissues.75
Table 3.
Nanoparticle Formulations of α-Mangostin
| Formulations | Ingredients | Methods | Ref. |
|---|---|---|---|
| Polymeric Nanoparticles | PLGA | Colloidal extraction solvent evaporation | [74] |
| Chitosan, PLGA | Colloidal extraction solvent evaporation | [75] | |
| PEG, PLA | Emulsion/solvent evaporation techniques | [76] | |
| PLGA | Double emulsion solvent evaporation method | [42] | |
| EC-MC | Spray drying | [77] | |
| Chitosan, alginate and genipin | Ionotropic gelation method | [78] | |
| β-cyclodextrin | Inclusion complex technique | [79] | |
| β-cyclodextrin-chitosan | Inclusion complex | [80] | |
| β-cyclodextrin | Inclusion complex | [81] | |
| Nanomicelles | PVP | Solvent evaporation method | [82] |
| MPEG and PLA | Single-step self-assembly method | [83] | |
| MPEG and PCL | Self-assembly method | [84] | |
| Liposome nanoparticles | Transferrin | Thin film hydration | [85] |
| Soya lecithin | Phase separation coacervation method | [86] | |
| Cholesterol, Tween 60 and ethanol | Film hydration method | [87] | |
| Solid lipid nanoparticles | Lavender essential oil and cetyl palmitate | Hot and high-pressure homogenisation techniques | [88] |
| PLGA and CD44 thioaptamer | Nanoprecipitation combined with self-assembly | [89] | |
| Nanofibres | Thiolated chitosan | Electrospinning | [90] |
| PVP | Electrospinning | [91] | |
| Metal nanoparticles | Ag (silver) | Chemical reaction by using silver nitrate (AgNO3) | [92] |
| Gold, PEI, cyclodextrin and tanshinone | Chemical reaction using polyethyleneimine (PEI) | [93] | |
| Emulsion nanoparticle | Captex 200 P, Tween 80, carbopol 90 and silica | Solid self-emulsification | [94] |
| Oleic acid, isopropyl myristate, Cremophor EL, Tween 80, carboxymethylcellulose sodium | Self-microemulsion | [31] |
Verma et al also examined PLGA with α-mangostin as a drug payload. The authors aimed to improve the bioactivity of α-mangostin against pancreatic cancer. They prepared the nanoparticle using a double emulsion solvent evaporation method. Impressively, the nanoparticle system inhibited the proliferation of pancreatic cancer stem cells (CSCs) and pancreatic cancer cell lines and had no effect on normal human pancreatic ductal epithelial (HPNE) cells. Moreover, the nanoparticle inhibited colony formation, motility, migration and the invasion-induced apoptotic mechanism in vitro and in vivo.42
Another polymeric nanoparticle formulation used poly(ethylene glycol)-poly(L-lactide) (PEG-PLA) as a matrix for α-mangostin. The authors aimed to use this formulation for Alzheimer’s disease. The nanoparticle was prepared by emulsion/solvent evaporation techniques. The particle size, zeta-potential and entrapment efficiency of the nanoparticle were 94.26 ± 4.54 nm, −32 ± 0.43 mV and 50.47 ± 1.96%, respectively. In vitro, the drug was rapidly released in the first 24 h (approximately 50%), followed by a slow, continuous release until 72 h and 100% release by 96 h. These results demonstrated a significant improvement of the pharmacokinetic and biodistribution profiles of nanoparticle compared to free α-mangostin.76
Ethyl cellulose-methyl cellulose (EC-MC), a natural polymer from cellulose groups, was designed for α-mangostin polymeric nanoparticle formulation as an anti-acne therapy in a cosmeceutical form. The system was designed as a nanoreservoir system to achieve an extended release profile using a spray drying technique. In this study, the particle size, polydispersity and loading capacity were 300–500 nm, 0.111 ± 0.024 and 41.90 ± 0.79%, respectively. The nanoparticle formation exhibited lower skin irritation compared to controls. Approximately 80–100% of α-mangostin was released over more than 7 days. Impressively, the anti-acne activity of the nanoparticle system significantly decreased the acne severity index (ASI) value and inflammatory lesions (P < 0.05) compared to control.77
Another study developed a nanoparticle system based on chitosan/alginate and genipin (GP) as a crosslinker prepared using the ionotropic gelation method. The system aimed to achieve a controlled release system and increase the antitumor activity of α-mangostin. Cytotoxicity and antitumor activity studies confirmed that an increase in GP concentration significantly reduced cell viability and induced apoptosis in colorectal adenocarcinoma cells.78
Nguyen et al demonstrated that nanoparticles with β-cyclodextrin (β-CD) improved the solubility and enhanced the cytotoxic activity of α-mangostin, with a minimal inhibitory concentration (IC50) of 8.86 and 9.86 µg/mL for LU-1 (human lung adenocarcinoma) and HL-60 (human promyelocytic leukaemia), respectively.79 Another α-mangostin complex with β-CD was fabricated with grafted-chitosan. The system was prepared using high shear mixing techniques. The system exhibited a high of entrapment efficiency (>75%) and anti–inflammatory activity. The inclusion complex of α-mangostin and quaternised cyclodextrin grafted chitosan (QCD-g-CS) influenced cytokine secretion and inhibited inflammation during the first hour (60% inhibition). After 3 h, there was almost total inhibition (95%).80 Additionally, nanoparticles of a water-soluble β-CD and α-mangostin presented cytotoxic activities against A549 lung cancer cells, with an IC50 of 2.34 µg/mL.81
Nanomicelles
A micelle is an amphipathic molecule in water and suitable as a drug delivery carrier for drugs with high lipophilicity. The first nanomicelle for α-mangostin was generated by Aisha et al α-Mangostin in solid dispersion nanomicelles, combined with polyvinylpyrrolidone (PVP) as a main polymer, was produced by the solvent evaporation method. The solubility of α-mangostin markedly increased 10,000 fold, from 0.2 ± 0.2 pg/mL to 2743 ± 11 pg/mL. Self-assembly of anionic nanomicelles around α-mangostin was observed by transmission electron microscopy and dynamic light scattering; the diameter size was 99–127 nm. The nanomicelle uptake was mediated by endocytosis, a finding that indicated intracellular delivery of α-mangostin that could be associated with potential cytotoxicity (IC50 of 8.9 ± 0.2 μg/mL).82
In another study, α-mangostin nanomicelles with methoxypoly(ethylene glycol)-poly(lactide) (MPEG-PLA) were developed by a single-step self-assembly method for malignant glioma. In vitro and in vivo assays showed that the α-mangostin/MPEG-PLA nanoparticles inhibited cell growth and induced apoptosis—with cleaved caspase expression, DNA fragmentation, downregulation of anti-apoptotic molecules and up-regulation of apoptotic molecules. This study also successfully investigated the process of programmed cell death in malignant glioma cells after treatment with α-mangostin/MPEG-PLA.83
Yang et al recently generated α-mangostin with methoxypoly(ethylene glycol)-poly(ε-caprolactone) (MPEG-PCL) as an anti-melanoma agent. The system had a sustained release profile, high solubility, strong toxicity to tumour cells and low toxicity to non-tumour cells. Additionally, MPEG-PCL inhibited melanoma cell proliferation, induced apoptosis via intrinsic and extrinsic pathways, suppressed growth cells and restrained angiogenesis. These data suggest that α-mangostin/MPEG-PCL nanomicelles are promising potential chemotherapy agents for the treatment of melanoma.84
Liposome Nanoparticles
Liposome nanoparticle (nanoliposome) is a liposome with particle size around 80–300 nm. Liposome nanoparticles can improve the physicochemical properties and performance of drugs due to their capability to deliver a drug. Chen developed a liposome, with α-mangostin as a drug payload using transferrin, with the thin-film hydration method. On the intercellular distribution assay, liposomes presented a time-dependent property; approximately 210 Ω/cm2 α-mangostin crossed the blood–brain barrier (BBB), with horseradish peroxidase (HRP) permeability less than 5%.85
Chin et al developed an α-mangostin niosome to improve the skin permeation rate of α-mangostin. Proniosome was prepared with soya lecithin using a phase separation coacervation method. The system enhanced skin permeation of α-mangostin 1.8–8.0 fold compared to control. It also improved viable epidermis/dermis (VED) of the α-mangostin compound, where α-mangostin deposition in the VED layer was increased 2.5–2.9 fold compared to control. Moreover, the addition of spans and soya lecithin improved the solubility of α-mangostin in water.86
Another niosome formulation from Limpapayom et al utilised cholesterol, Tween 60 and ethanol as the main carrier system. The system was prepared by film hydration; the particle size was 213 ± 26.47 nm, polydispersity index (PDI) was 0.23 ± 0.19 and zeta potential was −12.67±0.90 mV. Subsequently, the noisome/α-mangostin was prepared in cream and serum forms with 2.5–5% α-mangostin. The particle size, PDI and zeta potential were 600–700 nm, 1.11 ± 0.01 and 0.58 ± 0.04 mV, respectively. A skin permeation study confirmed that about 10–40% of α-mangostin released over more than 24 h.87
Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLN) are spherical carrier composed of single or double lipid layer on the surface, and solid layer in the core of the system. The complex system of SLN can provide an excellent drug control released. However, only two journals publication reported regarding solid lipid nanoparticle formulation of α-mangostin. Yostawonkul et al designed a nanostructure lipid carrier for α-mangostin (AM-NLC) by hot and high-pressure homogenisation techniques for non-surgical castration of male animals. Lavender essential oil and cetyl palmitate were the carrier system in this study. AM-NLC increased the activity of caspase-3 and caspase-7 and induced germ cell degeneration within the seminiferous tubules. Shrunken tubules were greatly depleted of germ cells. Additionally, the use of AM-NLC reduced the levels of pro-inflammatory mediators (nitric oxide and tumour necrosis factor α).88
Bonafe et al developed a lipid nanoparticle formulation to increase the activity of α-mangostin in disaggregation of MCF-7 cells. PLGA and CD44 thioaptamer used as the main carrier. A nanoparticle that contained 0.5 µg/mL α-mangostin induced disaggregation of multicellular tumour spheroid (MCTS). There was a similar dissociation effect when MCTS were cultured in matrix gel under the same conditions for 48–72 hrs. Moreover, the system with the lower α-mangostin concentration triggered damage, denoted as a substantial reduction in the MCTS size and density. The reduced spheroid expansion implied that a significant number of cells died or were in cell cycle arrest.89
Nanofibers
Nanofiber is widely used for site-specific drug released to achieve the desired therapeutic effects. Nanofiber has a diameter range around 150 nm and length 50–200 µm. Nanofibers could potentially overcome the limitation of α-mangostin. A nanofibre combined with chitosan thiolated with the electrospinning method had excellent mucoadhesive properties. Additionally, the α-mangostin nanofibre improved the bactericidal rate.90 Another nanofibre formulation was prepared using polyvinylpyrrolidone (PVP) as a carrier matrix for the active compound. The PVP nanofibre (387–586 nm) was prepared using an electrospinning apparatus. The preparation exhibited antioxidant activity, and the use of high voltage in the electrospinning technique did not apparently damage the molecular structure of α-mangostin. In vitro, α-mangostin release increased from 35% to over 90% in 60 min.91
Metal Nanoparticles
Metal nanoparticle is a metal with particle size around 1–100 nm. Several studies reported that metal nanoparticles have a bioactivity as anticancer agent and a high affinity with the cancer cells. Silver α-mangostin nanoparticles were formulated in a perfect spherical shape. These nanoparticles significantly inhibited the growth of the bacteria Escherichia coli and Bacillus subtilis and the fungus Aspergillus niger. Additionally, the presence of α-mangostin substantially reduced the silver ions in the silver nanoparticle system.92
Gold α-mangostin nanoparticles were also formulated; they comprised polyethylenimine (PEI) and cyclodextrin. Tanshinone was used as competitor drug payload in this study. The α-mangostin gold nanoparticles improved the loading efficiency approximately 15–50%, with an IC50 of 17.5 μM and 6.0 μM for PC-3 and DU145 cell lines, respectively. Comparatively, the tanshinone gold nanoparticles were very active against these cells, with a 40% improvement in the IC50 value for both PC-3 and DU145 cells.93
Emulsion Nanoparticles
Emulsion nanoparticle (nanoemulsion), a colloidal particulate system, consists of oil, water, and surfactant with high kinetic stability, low viscosity, and optically transparent. In the last decade, nanoemulsion has become a promising lipophilic drugs delivery system. Solid self-emulsification is one common modification technique to enhance the solubility and dissolution rate of α-mangostin. Droplet particles obtained from this system (using liquid-self-emulsifying drug delivery system [liquid-SEDDS]) were 106.9 ± 24.3 nm. The droplet was further converted to the solid state (solid-SEDDS) using Aeroperl 300 and Sylysia 350 silica. Solid-SEDDS with Aeroperl 300 had better flowability compared to solid-SEDDS with Sylysia 350. Based on the characterisation of X-ray diffraction (XRD) and differential scanning calorimetry (DSC) analysis, the solid-SEDDS exhibited an amorphous form. The dissolution test indicated that approximately 18.82% and 7.71% of α-mangostin was released from solid-SEDDS with Aeroperl 300 and Sylysia 350, respectively, within 60 min. However, only 0.26% of the intact α-mangostin dissolved.94
The mechanism for the improved α-mangostin solubility in emulsion was due to self-microemulsion; the particle diameter size was 24.6 nm and the encapsulation efficiency was 87.26%. These factors increased the area under the curve of α-mangostin by 4.75 fold compared to the free form. The preparation also increased α-mangostin distribution in lymphatic organs. Overall, self-microemulsion as a nano delivery system can promote the digestive tract absorption of α-mangostin and provide a specific distribution. The targeted system and high oral bioavailability of α-mangostin with self-microemulsion provides excellent performance for clinical drug efficacy.31
Perspective
In drug development, nanoparticle technology represents physical modifications intended to ameliorate solubility problems. Currently, nanotechnology can be applied for drug delivery systems, such drug controlled release,95 delayed release and sustained release.96 These nanoparticle formulations are the most commonly used in drug delivery systems.97 Our objective review highlighted that the nanoparticle technology in nanomedicine applications is divided into three general classifications: increased water solubility, controlled release and targeted drug delivery. As mentioned before, nanotechnology can be used to recover the solubility problem of drugs through multiple pathways and mechanisms. Firstly, particle size reduction in nanotechnology improves the drug solubility by expanding the surface area of particles.98,99 Secondly, the use of high water-soluble excipients as the main base of nanoparticles increase the solubility of drugs mediated by hydrogen bonding interaction between excipients and water molecules.100,101 On the other hand, the use of surface-active agent (surfactant) in nanotechnology also enhances the solubility of high lipophilicity drugs through interfacial tension reduction.102,103
Considering the effects of therapy with a dose and frequency of administration that is efficient, nanoparticle technology can be utilised to provide controlled and targeted drug delivery systems, especially for cancer therapy, to increase selectivity, mitigate potentially harmful side effects and even cause death in normal cells. The physicochemical properties of α-mangostin, especially its poor water solubility profile and its low selectivity on the target cells, limits its therapeutic applicability. Therefore, nanoparticle formulations are one option to resolve these limitations.
Numerous nanoparticle formulations have been described, including polymeric nanoparticles, solid lipid nanoparticles, nanofibers, nanomicelles and metal nanoparticles. In general, these formulations aim to increase the solubility of compounds that are poorly soluble in water through particle size modification to obtain a larger surface area. On the other hand, the type of nanoparticle and ingredients also influences the solubility of a compound. Polymeric nanoparticles are formulated with the polymer as a base for the formulations and are often made for further examinations.
Nanoparticle formulations have been developed using various nanocarriers with different techniques. Each nanocarriers are formulated by considering the aims of the studies such as to provide solubility improvement with hydrophilic polymer as a carrier,104 to provide control released system with pH-sensitive polymers or thermal-sensitive polymers,105,106 and to prevent protein degradation with liposome protection.107 In some cases, the nanocarrier is combined with targeting mediators to gain the nanoparticle targeted drug delivery system into specific target.108
Nanoparticles that mediate passive or active targeted delivery are generally prepared with ingredients that have a high affinity to the target and low affinity towards normal cells, for example, PLGA. PLGA is a copolymer formed from the combination of polymer polylactic acid (PLA) and polyglycolic acid (PGA). Research showed that PLGA has a high affinity to cancer cells, including hepatic cancer,109 prostate cancer110 and lung cancer cells,111 and many cell lines, including human umbilical vein endothelial cells,112 H1299,113 COS-7 and Cf2th.114 This high affinity allows PLGA to provide drug delivery systems or genes into the target-specific tissues or organs. Ultimately, consideration of nanoparticle shape and the materials used in the formula requires careful and thoughtful attention, especially with regards to the desired use and destination.
Conclusion
Many techniques have been considered to improve α-mangostin’s water solubility, of which nanoparticle formulations have become the most widely performed. This formulation provides many advantages. Overall, nanoparticle formulations improve α-mangostin’s water solubility and affect its biopharmaceutical, pharmacokinetic and pharmacodynamic aspects. Additionally, nanoparticle technology for α-mangostin can be a promising for controlled release and passive and active targeting. This system should help to maximise the efficacy of α-mangostin in a drug delivery system.
Acknowledgment
The authors thank the Minister of Research and Higher Education, Republic of Indonesia for the fundamental research grant (grant number 1123v/UN6.O/LT/2019), and Universitas Padjadjaran for academic leadership grant 2020.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen L, Yang L, Wang C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46:688–693. doi: 10.1016/j.fct.2007.09.096 [DOI] [PubMed] [Google Scholar]
- 2.Jung H-A, Su B-N, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 2006;54(6):2077–2082. doi: 10.1021/jf052649z [DOI] [PubMed] [Google Scholar]
- 3.Ma Y, Yu W, Shrivastava A, Srivastava RK, Shankar S. Inhibition of pancreatic cancer stem cell characteristics by α-Mangostin: molecular mechanisms involving Sonic hedgehog and Nanog. J Cell Mol Med. 2019. doi: 10.1111/jcmm.14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivaranjani M, Leskinen K, Aravindraja C, et al. Deciphering the antibacterial mode of action of alpha-mangostin on Staphylococcus epidermidis RP62A through an integrated transcriptomic and proteomic approach. Front Microbiol. 2019;10:150. doi: 10.3389/fmicb.2019.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limwikrant W, Aung T, Chooluck K, Puttipipatkhachorn S, Yamamoto K. Size reduction efficiency of Alpha-Mangostin suspension using high-pressure homogenization. Chem Pharm Bull. 2019;67(4):c18–00589. [DOI] [PubMed] [Google Scholar]
- 6.Kritsanawong S, Innajak S, Imoto M, Watanapokasin R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int J Oncol. 2016;48(5):2155–2165. doi: 10.3892/ijo.2016.3399 [DOI] [PubMed] [Google Scholar]
- 7.Krajarng A, Nakamura Y, Suksamrarn S, Watanapokasin R. α-Mangostin induces apoptosis in human chondrosarcoma cells through downregulation of ERK/JNK and Akt signaling pathway. J Agric Food Chem. 2011;59(10):5746–5754. doi: 10.1021/jf200620n [DOI] [PubMed] [Google Scholar]
- 8.Chen CM, Hsieh SC, Lin CL, Lin YS, Tsai JP, Hsieh YH. Alpha-Mangostin suppresses the metastasis of human renal carcinoma cells by targeting MEK/ERK expression and MMP-9 transcription activity. Cell Physiol Biochem. 2017;44(4):1460–1470. doi: 10.1159/000485582 [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Ying T, Chiou H, Hsieh S. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget. 2017;8(29):47425–47439. doi: 10.18632/oncotarget.17659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HN, Jang HY, Kim HJ, et al. Antitumor and apoptosis-inducing effects of α-mangostin extracted from the pericarp of the mangosteen fruit (Garcinia mangostana L.) in YD-15 tongue mucoepidermoid carcinoma cells. Int J Mol Med. 2016;37(4):939–948. doi: 10.3892/ijmm.2016.2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novilla A, Djamhuri DS, Fauziah N, Maesaroh M, Balqis B, Widowati W. Cytotoxic activity of Mangosteen (Garcinia mangostana L.) peel extract and α-mangostin toward leukemia cell lines (HL-60 and K-562). J Nat Remedies. 2016;16(2):52. doi: 10.18311/jnr/2016/842 [DOI] [Google Scholar]
- 12.Muchtaridi M, Wijaya CA. Anticancer potential of Α-Mangostin. Asian J Pharm Clin Res. 2017;10(12):440. doi: 10.22159/ajpcr.2017.v10i12.20812 [DOI] [Google Scholar]
- 13.Scolamiero G, Pazzini C, Bonafè F, Guarnieri C, Muscari C. Effects of α-mangostin on viability, growth and cohesion of multicellular spheroids derived from human breast cancer cell lines. Int J Med Sci. 2018;15(1):23–30. doi: 10.7150/ijms.22002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moongkarndi, P., Jaisupa, N., Kosem, N., Konlata, J., Samer, J., Pattanapanyasat, K. and Rodpai, E., 2015. Effect of purified α-mangostin from mangosteen pericarp on cytotoxicity, cell cycle arrest and apoptotic gene expression in human cancer cells. World J Pharm Sci, 3(8), pp.1473–84. [Google Scholar]
- 15.Kwak HH, Kim IR, Kim HJ, Park BS, Yu SB. α -Mangostin induces apoptosis and cell cycle arrest in oral squamous cell carcinoma cell. Evid Based Complement Alternat Med. 2016;2016. doi: 10.1155/2016/5352412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan TKT, Shahbazzadeh F, Pham TTH, Kihara T. Alpha-mangostin inhibits the migration and invasion of A549 lung cancer cells. PeerJ. 2018;6:e5027. doi: 10.7717/peerj.5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JJ, Sanderson BJS, Zhang W. Significant anti-invasive activities of α-mangostin from the mangosteen pericarp on two human skin cancer cell lines. Anticancer Res. 2012;32(9):3805–3816. [PubMed] [Google Scholar]
- 18.Zhang C, Yu G, Shen Y. The naturally occurring xanthone α-mangostin induces ROS-mediated cytotoxicity in non-small scale lung cancer cells. Saudi J Biol Sci. 2018;25(6):1090–1095. doi: 10.1016/j.sjbs.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rungnim C, Phunpee S, Kunaseth M, et al. Co-solvation effect on the binding mode of the α-mangostin/β-cyclodextrin inclusion complex. Beilstein J Org Chem. 2015;11(1):2306–2317. doi: 10.3762/bjoc.11.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarena AS, Sankar KU. Synthesis of α− mangostin-D-glucoside in supercritical carbon dioxide media. J Food Sci Technol. 2015;52(10):6547–6555. doi: 10.1007/s13197-014-1705-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elsaid Ali AA, Taher M, Mohamed F. Microencapsulation of alpha-mangostin into PLGA microspheres and optimization using response surface methodology intended for pulmonary delivery. J Microencapsul. 2013;30(8):728–740. doi: 10.3109/02652048.2013.788081 [DOI] [PubMed] [Google Scholar]
- 22.Li L, Brunner I, Han A, Hamburger M, Kinghorn AD. Pharmacokinetics of a -mangostin in rats after intravenous and oral application. Mol Nutr Food Res. 2011;55(S1):67–74. doi: 10.1002/mnfr.201000511 [DOI] [PubMed] [Google Scholar]
- 23.Pelivan K, Frensemeier L, Karst U, et al. Understanding the metabolism of the anticancer drug triapine: electrochemical oxidation, microsomal incubation and in vivo analysis using LC-HRMS. Analyst. 2017;142(17):3165–3176. doi: 10.1039/C7AN00902J [DOI] [PubMed] [Google Scholar]
- 24.Khodadadei F, Safarian S, Ghanbari N. Methotrexate-loaded nitrogen-doped graphene quantum dots nanocarriers as an efficient anticancer drug delivery system. Mater Sci Eng C. 2017;79:280–285. doi: 10.1016/j.msec.2017.05.049 [DOI] [PubMed] [Google Scholar]
- 25.Sahoo CK, Reddy GS, Vojjala A, Reddy BV. Bioavailability enhancement for poorly soluble drugs: a review. Innoriginal Int J Sci. 2018:1–6. [Google Scholar]
- 26.Abuzar SM, Hyun S-M, Kim J-H, et al. Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical antisolvent (SAS) process. Int J Pharm. 2018;538(1–2):1–13. doi: 10.1016/j.ijpharm.2017.12.041 [DOI] [PubMed] [Google Scholar]
- 27.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544 [DOI] [PubMed] [Google Scholar]
- 28.Singh R, Lillard JJW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–223. doi: 10.1016/j.yexmp.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizvi SAA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26(1):64–70. doi: 10.1016/j.jsps.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi X, Zi C, Li H, et al. RSC advances. RSC Adv. 2018;8(January):41377–41388. doi: 10.1039/C8RA08409B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W, Jiang H, Yang K, Wang Y, Zhang Q. ScienceDirect development and in vivo evaluation of self-microemulsion as delivery system for a –mangostin. Kaohsiung J Med Sci. 2017;116–123. doi: 10.1016/j.kjms.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 32.Sodalee K, Sapsuphan P, Wongsirikul R. Preparation and evaluation of alpha-mangostin solid self-emulsifying drug delivery system. Asian J Pharm Sci. 2016;11(1):225–226. doi: 10.1016/j.ajps.2015.11.024 [DOI] [Google Scholar]
- 33.Jittamaro P, Ruktanonchai UR, Phunpee S. Effect of solvent on the complex between α -Mangostin and β -Cyclodextrin α. Int Conf Chem Civ Mater Eng. 2015:5–9. [Google Scholar]
- 34.Phunpee S, Suktham K, Surassmo S, et al. Controllable encapsulation of ␣-mangostin with quaternized -cyclodextrin grafted chitosan using high shear mixing, International Journal. Int J Pharm. 2017. doi: 10.1016/j.ijpharm.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 35.Dermawan D, Wathoni N, Muchtaridi M. Host-guest interactions of α− Mangostin with (α, β, γ)− Cyclodextrins: semi-empirical quantum mechanical methods of PM6 and PM7. J Young Pharm. 2019;11(1):31. doi: 10.5530/jyp.2019.11.7 [DOI] [Google Scholar]
- 36.Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2017;46(D1):D608–D617. doi: 10.1093/nar/gkx1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S, Chen J, Cheng T, et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2018;47(D1):D1102–D1109. doi: 10.1093/nar/gky1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P, Tian W, Ma X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol Cancer. 2014;13(1):138. doi: 10.1186/1476-4598-13-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa Y, Iinuma M, Naoe T, Nozawa Y, Akao Y. Characterized mechanism of α-mangostin-induced cell death: caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem. 2007;15(16):5620–5628. doi: 10.1016/j.bmc.2007.04.071 [DOI] [PubMed] [Google Scholar]
- 40.Shih Y-W, Chien S-T, Chen P-S, Lee J-H, Wu S-H, Yin L-T. α-Mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via αvβ3 integrin/FAK/ERK and NF-κB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem Biophys. 2010;58(1):31–44. doi: 10.1007/s12013-010-9091-2 [DOI] [PubMed] [Google Scholar]
- 41.Sato A, Fujiwara H, Oku H, Ishiguro K, Ohizumi Y. α-Mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci. 2004;95(1):33–40. doi: 10.1254/jphs.95.33 [DOI] [PubMed] [Google Scholar]
- 42.Verma RK, Yu W, Shrivastava A, Shankar S, Srivastava RK. α-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (KrasG12D, and KrasG12D/tp53R270H) mice. Sci Rep. 2016;6(May):1–13. doi: 10.1038/srep32743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thassu D, Deleers M, Pathak YV. Nanoparticulate Drug Delivery Systems. Vol. 166 CRC Press; 2007. [Google Scholar]
- 44.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–1037. doi: 10.1016/S1734-1140(12)70901-5 [DOI] [PubMed] [Google Scholar]
- 45.Langer R. Drug delivery and targeting. Nature. 1998;5–10. [PubMed] [Google Scholar]
- 46.Steiner D, Finke JH, Kwade A. Model-based description of disintegration time and dissolution rate of nanoparticle-loaded orodispersible films. Eur J Pharm Sci. 2019;132:18–26. doi: 10.1016/j.ejps.2019.02.029 [DOI] [PubMed] [Google Scholar]
- 47.Júlio A, Lima SAC, Reis S, de Almeida TS, Fonte P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J Drug Deliv Sci Technol. 2019;100915. doi: 10.1016/j.jddst.2019.01.030 [DOI] [Google Scholar]
- 48.Braig V, Konnerth C, Peukert W, Lee G. Enhanced dissolution of naproxen from pure-drug, crystalline nanoparticles: a case study formulated into spray-dried granules and compressed tablets. Int J Pharm. 2019;554:54–60. doi: 10.1016/j.ijpharm.2018.09.069 [DOI] [PubMed] [Google Scholar]
- 49.Homayouni A, Amini M, Sohrabi M, Varshosaz J, Nokhodchi A. Curcumin nanoparticles containing poloxamer or soluplus tailored by high pressure homogenization using antisolvent crystallization. Int J Pharm. 2019;562:124–134. doi: 10.1016/j.ijpharm.2019.03.038 [DOI] [PubMed] [Google Scholar]
- 50.Öztürk AA, Yenilmez E, Yazan Y. Dexketoprofen trometamol-loaded Eudragit® RL 100 nanoparticle formulation, characterization and release kinetics. ACTA Pharm Sci. 2019;57(1). [Google Scholar]
- 51.Chen X, Han W, Zhao X, Tang W, Wang F. Epirubicin-loaded marine carrageenan oligosaccharide capped gold nanoparticle system for pH-triggered anticancer drug release. Sci Rep. 2019;9(1):6754. doi: 10.1038/s41598-019-43106-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao Y, Yang F, Meng K, et al. Exploitation of enrofloxacin-loaded docosanoic acid solid lipid nanoparticle suspension as oral and intramuscular sustained release formulations for pig. Drug Deliv. 2019;26(1):273–280. doi: 10.1080/10717544.2019.1580798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee P-C, Zan B-S, Chen L-T, Chung T-W. Multifunctional Plga-based nanoparticles as a controlled release drug delivery system for antioxidant and anticoagulant therapy. Int J Nanomedicine. 2019;14:1533. doi: 10.2147/IJN.S174962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, Yuan C, Chen J, et al. Nanoparticle binding to urokinase receptor on cancer cell surface triggers nanoparticle disintegration and cargo release. Theranostics. 2019;9(3):884. doi: 10.7150/thno.29445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saurabh CK, Gupta S, Variyar PS, Sharma A. Effect of addition of nanoclay, beeswax, tween-80 and glycerol on physicochemical properties of guar gum films. Ind Crops Prod. 2016;89:109–118. doi: 10.1016/j.indcrop.2016.05.003 [DOI] [Google Scholar]
- 56.Basha RY, Sampath Kumar TS, Doble M. Dual delivery of tuberculosis drugs via cyclodextrin conjugated curdlan nanoparticles to infected macrophages. Carbohydr Polym. 2019;218:53–62. doi: 10.1016/j.carbpol.2019.04.056 [DOI] [PubMed] [Google Scholar]
- 57.Chen W, Zhi M, Feng Z, et al. Sustained co-delivery of ibuprofen and basic fibroblast growth factor by thermosensitive nanoparticle hydrogel as early local treatment of peri-implantitis. Int J Nanomedicine. 2019;14:1347. doi: 10.2147/IJN.S190781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobson JJ, Al-khouja A, Curley P, et al. Semi-solid prodrug nanoparticles for long-acting delivery of water-soluble antiretroviral drugs within combination HIV therapies. Nat Commun. 2019;10(1):1413. doi: 10.1038/s41467-019-09354-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Pu S, Liu Q, et al. An integrin-based nanoparticle that targets activated hepatic stellate cells and alleviates liver fibrosis. J Control Release. 2019;303:77–90. doi: 10.1016/j.jconrel.2019.04.022 [DOI] [PubMed] [Google Scholar]
- 60.Lee JH, Moon H, Han H, et al. Antitumor effects of intra-arterial delivery of albumin-doxorubicin nanoparticle conjugated microbubbles combined with ultrasound-targeted microbubble activation on VX2 rabbit liver tumors. Cancers (Basel). 2019;11(4):581. doi: 10.3390/cancers11040581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, Li Y, Wei M, Liu C, Yu T, Yang J. Cetuximab-modified silica nanoparticle loaded with ICG for tumor-targeted combinational therapy of breast cancer. Drug Deliv. 2019;26(1):129–136. doi: 10.1080/10717544.2018.1564403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei M, Sha S, Wang X, et al. Co-delivery of paclitaxel and gemcitabine via a self-assembling nanoparticle for targeted treatment of breast cancer. RSC Adv. 2019;9(10):5512–5520. doi: 10.1039/C9RA00276F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zayed DG, Ebrahim SM, Helmy MW, et al. Combining hydrophilic chemotherapy and hydrophobic phytotherapy via tumor-targeted albumin–QDs nano-hybrids: covalent coupling and phospholipid complexation approaches. J Nanobiotechnology. 2019;17(1):7. doi: 10.1186/s12951-019-0445-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernández-Barahona I, Gutiérrez L, Veintemillas-Verdaguer S, et al. Cu-doped extremely small iron oxide nanoparticles with large longitudinal relaxivity: one-pot synthesis and in vivo targeted molecular imaging. ACS Omega. 2019;4(2):2719–2727. doi: 10.1021/acsomega.8b03004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Chen H, Zeng X, et al. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm Sin B. 2019;9(1):167–176. doi: 10.1016/j.apsb.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sancho-Albero M, Navascués N, Mendoza G, et al. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J Nanobiotechnology. 2019;17(1):16. doi: 10.1186/s12951-018-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun Y, Zhao Y, Teng S, et al. Folic acid receptor-targeted human serum albumin nanoparticle formulation of cabazitaxel for tumor therapy. Int J Nanomedicine. 2019;14:135. doi: 10.2147/IJN.S181296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zayed GM, El-feky GS. Growth factor loaded functionalized gold nanoparticles as potential targeted treatment for acute renal failure. Int J Appl Pharm. 2019;11(1):66–70. [Google Scholar]
- 69.AlQahtani SA, Harisa GI, Badran MM, et al. Nano-erythrocyte membrane-chaperoned 5-fluorouracil liposomes as biomimetic delivery platforms to target hepatocellular carcinoma cell lines. Artif Cells Nanomed Biotechnol. 2019;47(1):989–996. doi: 10.1080/21691401.2019.1577887 [DOI] [PubMed] [Google Scholar]
- 70.Liu X, Wang B, Li Y, et al. Powerful anticolon tumor effect of targeted gene immunotherapy using folate-modified nanoparticle delivery of CCL19 to activate the immune system. ACS Cent Sci. 2019;5:277–289. doi: 10.1021/acscentsci.8b00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng L, Qin C, Wang W, Chi W, Li W. Absorption and distribution of chitosan in mice after oral administration. Carbohydr Polym. 2008;71(3):435–440. doi: 10.1016/j.carbpol.2007.06.016 [DOI] [Google Scholar]
- 72.Yan C, Gu J, Lv Y, Shi W, Huang Z, Liao Y. 5β-cholanic acid/glycol chitosan self-assembled nanoparticles (5β-CHA/GC-NPs) for enhancing the absorption of FDs and insulin by rat intestinal membranes. AAPS PharmSciTech. 2019;20(1):30. doi: 10.1208/s12249-018-1242-6 [DOI] [PubMed] [Google Scholar]
- 73.Pi J, Wang S, Li W, et al. A nano-cocrystal strategy to improve the dissolution rate and oral bioavailability of baicalein. Asian J Pharm Sci. 2019;14(2):154–164. doi: 10.1016/j.ajps.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elsaid Ali AA. Development of Chitosan-α-mangostin loaded nanoparticles as an anticancer agent; 2011.
- 75.Elsaid Ali AA. Development of alpha Mangostin-PLGA nanoparticles as an anticancer agent; 2011.
- 76.Yao L, Gu X, Song Q, et al. Nanoformulated alpha-mangostin ameliorates Alzheimer’s disease neuropathology by elevating LDLR expression and accelerating amyloid-beta clearance. J Control Release. 2016;226:1–14. doi: 10.1016/j.jconrel.2016.01.055 [DOI] [PubMed] [Google Scholar]
- 77.Pan-In P, Wongsomboon A, Kokpol C, Chaichanawongsaroj N, Wanichwecharungruang S. Depositing α-mangostin nanoparticles to sebaceous gland area for acne treatment. J Pharmacol Sci. 2015;129(4):226–232. doi: 10.1016/j.jphs.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 78.Samprasit W, Akkaramongkolporn P, Jaewjira S, Opanasopit P. Design of alpha mangostin-loaded chitosan/alginate controlled-release nanoparticles using genipin as crosslinker. J Drug Deliv Sci Technol. 2018;46:312–321. doi: 10.1016/j.jddst.2018.05.029 [DOI] [Google Scholar]
- 79.Nguyen PTM, Tran LD, Dang NK, Nguyen DT. Synthesis of polymeric nanoparticles of α - mangostin and its cytotoxicity to human cancer cell lines. Biomed Res Ther. 2017;4:15419. doi: 10.15419/bmrat.v4iS.307 [DOI] [Google Scholar]
- 80.Phunpee S, Suktham K, Surassmo S, et al. Controllable encapsulation of α-mangostin with quaternized β-cyclodextrin grafted chitosan using high shear mixing. Int J Pharm. 2018;538(1–2):21–29. doi: 10.1016/j.ijpharm.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 81.Phương TNM, Phuong NT, Dai Lam T, Mai TT, Hop NT. Cytotoxicity of α- mangostin encapsulated polymeric nanoparticles against lung cancer cells. Tap Chi Sinh Hoc. 2018;40(1):108–114. doi: 10.15625/0866-7160/v40n1.10504 [DOI] [Google Scholar]
- 82.Aisha AFA, Ismail Z, Abu-salah KM, Malik A, Abdul S. Solid dispersions of α -Mangostin improve its aqueous solubility through self-assembly of nanomicelles. J Pharm sci. 2012;101(2):815–825. doi: 10.1002/jps [DOI] [PubMed] [Google Scholar]
- 83.Zheng S, Liu J, Faried A, Richard SA, Gao X. Novel chemically synthesized, alpha-Mangostin-loaded nano-particles, enhanced cell death through multiple pathways against malignant glioma. J Biomed Nanotechnol. 2018;14(11):1866–1882. doi: 10.1166/jbn.2018.2627 [DOI] [PubMed] [Google Scholar]
- 84.Yang S, Gao X, He Y, Hu Y, Xu B, Cheng Z. Applying an innovative biodegradable self-assembly nanomicelles to deliver α -mangostin for improving anti-melanoma activity. Cell Death Dis. 2019. doi: 10.1038/s41419-019-1323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z, Huang M, Wang X, et al. Transferrin-modified liposome promotes α-Mangostin to penetrate the blood-brain barrier. Nanomedicine. 2015. doi: 10.1016/j.nano.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 86.Chin GS, Todo H, Kadhum R, Hamid A, Sugibayashi K. In vitro permeation and skin retention of α -mangostin proniosome. Chem Pharm Bull. 2016;64(12):1666–1673. doi: 10.1248/cpb.c16-00425 [DOI] [PubMed] [Google Scholar]
- 87.Limphapayom W, Loylerd K, Leabwan N, Sukhasem S. Encapsulation of alpha-mangostin in cosmetic production by using nanotechnology. Int Symp Durian Other Humid Trop Fruits. 2017;189–192. doi: 10.17660/ActaHortic.2017.1186.29 [DOI] [Google Scholar]
- 88.Yostawonkul J, Surassmo S, Namdee K, Khongkow M. Nanocarrier-mediated delivery of α -mangostin for non-surgical castration of male animals. Sci Rep. 2017;1–10. doi: 10.1038/s41598-017-16563-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bonafè F, Pazzini C, Marchionni S, Guarnieri C, Muscari C. Complete disaggregation of MCF-7-derived breast tumour spheroids with very low concentrations of α -Mangostin loaded in CD44 thioaptamer-tagged nanoparticles. Int J Med Sci. 2019;16(1):33. doi: 10.7150/ijms.28135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Samprasit W, Rojanarata T, Akkaramongkolporn P, Ngawhirunpat T, Kaomongkolgit R, Opanasopit P. Fabrication and in vitro/in vivo performance of mucoadhesive electrospun nanofiber mats containing α -Mangostin. AAPS PharmSciTech. 2015;16(5):1140–1152. doi: 10.1208/s12249-015-0300-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miftahul M. Mangosteen pericarp extract embedded in electrospun PVP nanofiber mats: physicochemical properties and release mechanism of α –mangostin. Int J Nanomedicine. 2018;13:4927–4941. doi: 10.2147/IJN.S167670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karthiga P, Soranam R, Annadurai G. Alpha-mangostin, the major compound from Garcinia mangostana Linn. Responsible for synthesis of Ag nanoparticles: its characterization and evaluation studies. Res J Nanosci Nanotechnol. 2012;2(2):46–57. doi: 10.3923/rjnn.2012.46.57 [DOI] [Google Scholar]
- 93.Iia T, Cyclodextrin PEI, Qiu S, et al. Delivery of tanshinone IIA and α-mangostin from Gold/PEI/cyclodextrin nanoparticle platform designed for prostate cancer chemotherapy. Bioorg Med Chem Lett. 2016. doi: 10.1016/j.bmcl.2016.03.097 [DOI] [PubMed] [Google Scholar]
- 94.Sodalee K, Sapsuphan P, Wongsirikul R, Puttipipatkhachorn S. Preparation and evaluation of alpha-mangostin solid self-emulsifying drug delivery system. Asian J Pharm Sci. 2016;11(1):225–226. doi: 10.1016/j.ajps.2015.11.024 [DOI] [Google Scholar]
- 95.Liu T, Qiao Z, Wang J, et al. Molecular imprinted S-nitrosothiols nanoparticles for nitric oxide control release as cancer target chemotherapy. Colloids Surf B Biointerfaces. 2019;173:356–365. doi: 10.1016/j.colsurfb.2018.09.078 [DOI] [PubMed] [Google Scholar]
- 96.Kashyap S, Singh A, Mishra A, Singh V. Enhanced sustained release of furosemide in long circulating chitosan-conjugated PLGA nanoparticles. Res Pharm Sci. 2019;14(2):93–106. doi: 10.4103/1735-5362.253356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma G, Parchur AK, Jagtap JM, Hansen CP, Joshi A. Hybrid nanostructures in targeted drug delivery In: Hybrid Nanostructures for Cancer Theranostics. Elsevier; 2019: 139–158. [Google Scholar]
- 98.Sun J, Wang F, Sui Y, et al. Effect of particle size on solubility, dissolution rate, and oral bioavailability: evaluation using coenzyme Q10 as naked nanocrystals. Int J Nanomedicine. 2012;7:5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khadka P, Ro J, Kim H, et al. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2014;9(6):304–316. doi: 10.1016/j.ajps.2014.05.005 [DOI] [Google Scholar]
- 100.Agarwal R, Singh V, Jurney P, Shi L, Sreenivasan SV, Roy K. Scalable imprinting of shape-specific polymeric nanocarriers using a release layer of switchable water solubility. ACS Nano. 2012;6(3):2524–2531. doi: 10.1021/nn2049152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):701–705. doi: 10.1016/j.addr.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Attwood D. Surfactant Systems: Their Chemistry, Pharmacy and Biology. Springer Science & Business Media; 2012. [Google Scholar]
- 103.Nagarajan R. Self-Assembly: From Surfactants to Nanoparticles. Wiley; 2019. [Google Scholar]
- 104.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 105.Sahoo B, Devi KSP, Banerjee R, Maiti TK, Pramanik P, Dhara D. Thermal and pH responsive polymer-tethered multifunctional magnetic nanoparticles for targeted delivery of anticancer drug. ACS Appl Mater Interfaces. 2013;5(9):3884–3893. doi: 10.1021/am400572b [DOI] [PubMed] [Google Scholar]
- 106.Yoshida T, Lai TC, Kwon GS, Sako K. pH-and ion-sensitive polymers for drug delivery. Expert Opin Drug Deliv. 2013;10(11):1497–1513. doi: 10.1517/17425247.2013.821978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rusdin A, Wathoni N, Motoyama K, Joni IM, Lesmana R. Nanoparticles targeted drug delivery system via epidermal growth factor receptor. J Pharm. 2019;1(3):77–91. [Google Scholar]
- 109.Kou G, Gao J, Wang H, et al. Preparation and characterization of paclitaxel-loaded PLGA nanoparticles coated with cationic SM5-1 single-chain antibody. BMB Rep. 2007;40(5):731–739. doi: 10.5483/BMBRep.2007.40.5.731 [DOI] [PubMed] [Google Scholar]
- 110.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt (IV) prodrug-PLGA–PEG nanoparticles. Proc Natl Acad Sci. 2008;105(45):17356–17361. doi: 10.1073/pnas.0809154105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr C-M. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine. 2007;3(3):173–183. doi: 10.1016/j.nano.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 112.Danhier F, Vroman B, Lecouturier N, et al. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release. 2009;140(2):166–173. doi: 10.1016/j.jconrel.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 113.Nguyen J, Steele TWJ, Merkel O, Reul R, Kissel T. Fast degrading polyesters as siRNA nano-carriers for pulmonary gene therapy. J Control Release. 2008;132(3):243–251. doi: 10.1016/j.jconrel.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koby G, Ofra B, Dganit D, Marcelle M. Poly (d, l-lactide-co-glycolide acid) nanoparticles for DNA delivery: waiving preparation complexity and increasing efficiency. Biopolym Orig Res Biomol. 2007;85(5–6):379–391. [DOI] [PubMed] [Google Scholar]