Abstract
Introduction
This investigation is a prospective cohort study examining the use of Clostridium histolyticum collagenase injection (CCH) for the treatment of Dupuytren's disease (DD) with a 7 years follow-up.
Methods
Forty-five monodigital DD patients were injected with CCH on a single joint. Assessment included measurement of residual passive extension deficit (PED), function (using QuickDASH) and patient satisfaction.
Results
86.7% of PIPJ and 65.6% of MPJ had a worsening of PED. Nevertheless, thirty-nine patients (86.7%) concluded their treatment with only one injection, without any further treatment.
Conclusion
CCH provides a long-term effective solution. Recurrence occurs, especially in PIPJ, with acceptable rates.
Keywords: Dupuytren, Recurrence rate, Hand function, Collagenase injection, Seven years
1. Introduction
Palmar fibromatosis or Dupuytren's disease (DD) is a chronic fibroproliferative disease that affects palmar and digital aponeuroses. DD develops as multi-planar and multi-depth disease, so that it involves all the structures of the aponeurosis.1 DD history usually starts with a palpable nodule at the palmar crease, that extends distally and proximally producing a pathological cord that thickens and shortens as the disease progresses. Therefore, fingers flex inward towards the palm.2 DD seriously limits daily activities and worsens quality of life. DD recurrence and disease progression are possible with any type of treatment, surgical or otherwise.3 Before the advent of collagenase of Clostridium Histolyticum (CCH), surgical treatment was the only effective treatment for this disease with a high incidence of complications and a variable recurrence rate.4, 5, 6, 7 Many non-operative treatments have been experimented over the years,8 to avoid unfavourable complications from traditional fasciectomy. CCH injection has proven to be safe and effective.9 Today CCH is increasingly used for the treatment of DD.10
In 2012, our institution performed 45 CCH injections on 45 patients. Our results have never been published before, but we considered it a valuable experience. Hence, CCH injection is the gold standard in our institution. After 7 years from the first injection series, those 45 patients are among our oldest cases. The aim of this study is to compare the results obtained 12 weeks after injection with the more recent follow up.
2. Materials and methods
2.1. Study design and aims
The present investigation consists of a prospective observational study initiated in January 2012, in the context of a multicentre trial, according to the Ministry of Health's Decree of May 8, 2003. At Orthopedics and Hand Surgery Unit of Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, 45 patients were injected with collagenase extracted from Clostridium histolyticum granted for compassionate use for treatment of DD with palpable cord. All patients expressed their written consent before the enrolment. The study was approved by our institution's ethics committee and is also in accordance with the Helsinki convention. The aim of the study is to assess long term clinical outcomes (7 years) of CCH injections in patients with DD, as well as to assess the rate of disease recurrence, the functional outcomes and the satisfaction rate of patients undergoing treatment.
2.2. Inclusion and exclusion criteria
All patients with monodigital DD with a passive extension deficit (PED) of at least 20° at the metacarpo-phalangeal joint (MPJ), any degree at the proximal interphalangeal joint (PIPJ) and a palpable cord, with at least stage II disease (according to Tubiana-Michon classification) were potentially eligible. We excluded from the study: pregnant or breast-feeding women, patients undergoing any treatment of the affected hand, patients with hypersensitivity to collagenase or any of the other components of the product, patients in therapy with anti-platelet or anticoagulant drugs, and patients with psychiatric pathologies that could reduce the compliance to the protocol.
2.3. Treatment
The procedure was performed at day-surgery (without overnight stay). All the injections were performed by two experienced hand surgeons (R.D. and G.T.). The procedure was not changed during the study. After skin disinfection, the appropriate quantity of drug was injected into the affected cords, as previously described by other authors.11,12 A sterile bulky dressing was applied after the procedure. Patients were instructed not to extend fingers themselves.
The day after the injection, after a local anaesthetic infiltration, a forced extension was performed and the pathologic cord was disrupted. A splint, made of thermoplastic material, was applied to the finger in order to immobilize it in an extended position. For any case of skin laceration, medications were applied in the following days. The splint was applied continuously for 7 days; then it was applied for 12 h per day for seven further days. Before and 7 days after the procedure, all the patients were evaluated by the surgeon and by an especially trained physiotherapist. At the 7-year follow-up visit, the examiner was the same treating surgeon.
2.4. Data collection and follow up
Before performing the procedure, the following data were collected: demographic data, Charlson Comorbidity Index (CCI), Quick Disability of the Arm, Shoulder and Hand (QuickDASH), PED and Total Passive Extension Deficit (TPED). PED and TPED were measured immediately after the extension procedure and at 1, 2, 4 and 8 weeks after. Twelve weeks, one and 7 years after surgery, QuickDASH, PED and TPED were measured. Moreover, the disease recurrence rate at 7 years was evaluated. Recurrence was defined as the worsening of PED>20° compared to the PED at 12 weeks in presence of a palpable cord,13 and with loss of hand function which needed to be treated again.
The general satisfaction was estimated with a ten-point scale (General Satisfaction Index, GSI) administered after 12 weeks and at the last follow-up visit.
2.5. End points
The primary end points of the study were efficacy and significant disease recurrence rate at 7 years of follow up. The secondary end points were maintenance of functionality at 7 years of follow up and patients’ general satisfaction for the treatment received.
2.5.1. Statistical analysis
All data shown were mean and standard deviation. Only one decimal digit was reported, as rounded up. T-Student test was used to compare parametric data. For non-parametric data, Mann–Whitney test was used. Its significance was established for a value of p < 0.05. A dedicated software (GraphPad Software - Prism 8 for Mac) was employed.
3. Results
3.1. Participants
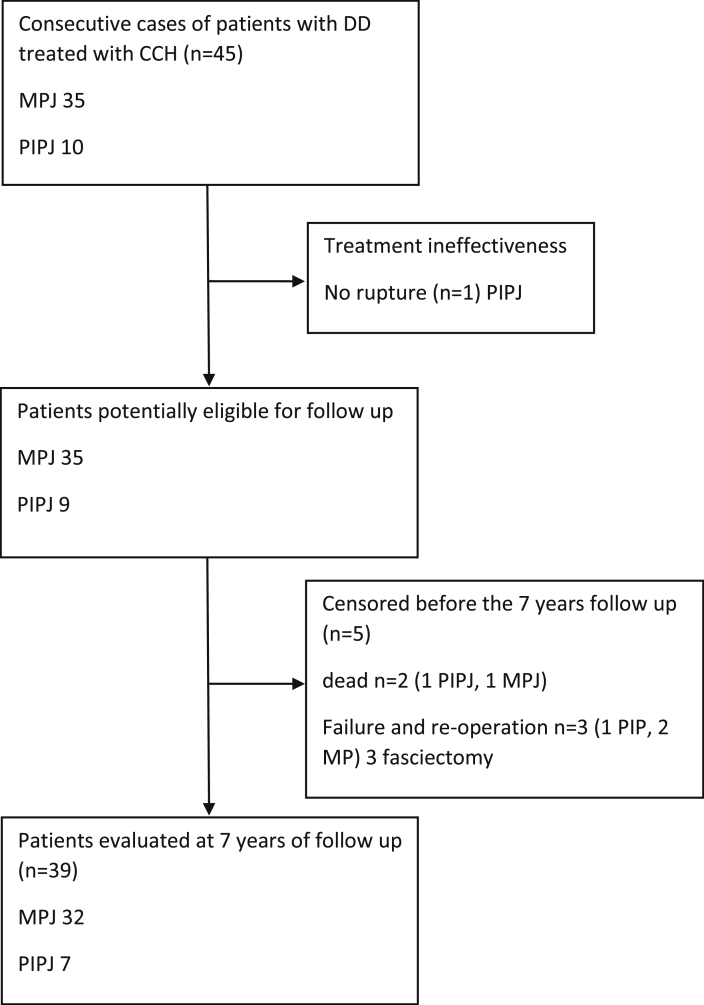
Based on the availability of the drug and the inclusion and exclusion criteria, 45 patients (38 males and 7 females) were enrolled in the study. In the period examined, 35 MPJ and 10 PIPJ were injected. Each patient received only one treatment. The mean age was 66.4 (±7.3) years. The mean BMI was 26.9 (±3.1). Seventeen (37.7%) patients were smokers. Charlson Comorbidity Index (CCI) average value was of 2.7 (±1.1). The median follow-up period was 2643 (±34.3) days (7 years). Twenty-two patients (48.8%) were “white collar” workers while the other were “blue collar” workers. Thirty-three patients (73.3%) had Tubiana stage II, 7 (15.5%) had stage III, and 5 (11.1%) had stage IV. In 44 (97.8%)patients treated, forced extension was successful and the pathologic cord was disrupted with immediate improvement of range of motion (ROM), while the remaining patients were excluded from the study. Three patients required surgical treatment before completing the established follow-up due to an unsatisfactory clinical result. Two patients died and therefore did not complete the 7-year follow-up (Fig. 1).
Fig. 1.
Patient flow-chart. DD: Dupuytren's Disease. CCH: Collagenase Clostridium histolitycum. MPJ: metacarpophalangeal joint; PIPJ: proximal interphalangeal joint.
3.2. Outcomes and follow-up
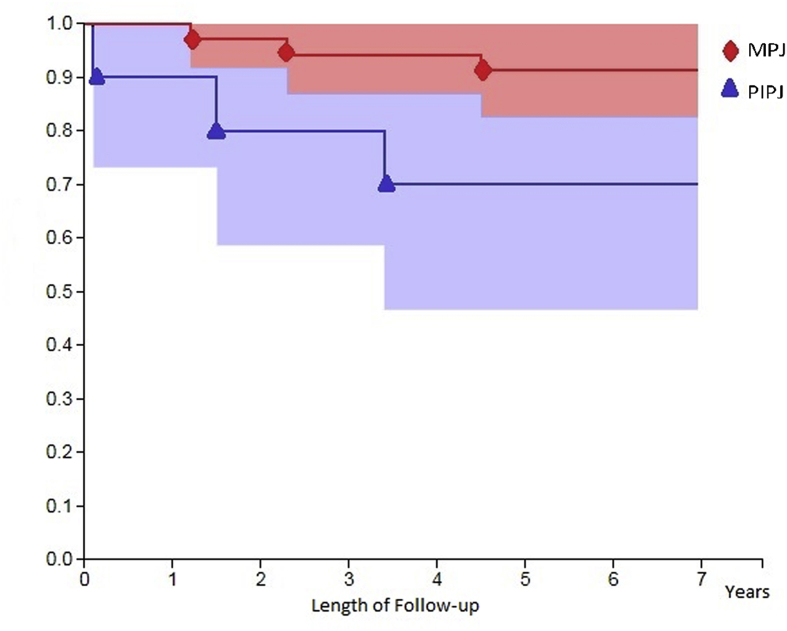
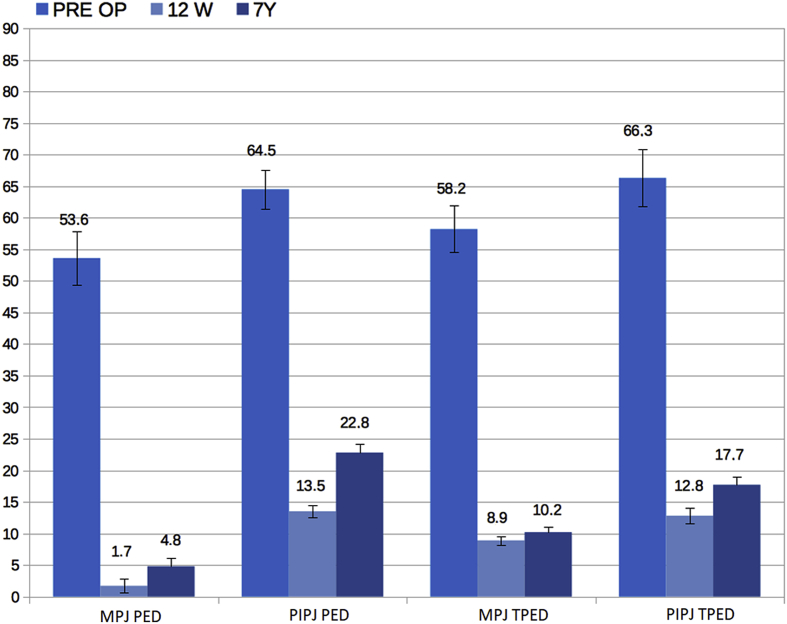
Among evaluated patients we observed an improvement in the PED on the MPJ from 53.6° (±4.2) to 1.7° (±1.1) (p = 0.003) and on the PIPJ from 64.5° (±3.1) at 13.5° (±0.9) (p = 0.002) at 12 weeks after treatment, with an average improvement in 96.8% of patients for MPJ and in 79.1% for PIPJ (Fig. 2). Thirty-nine patients (86.7%) completed the 7-year follow-up without further treatment. Among these, 32 were MPJ (corresponding to 91.4% of the initial sample) and 7 were PIPJ (corresponding to 70.0% of the initial sample). Six PIPJ (86.7%) and 21 MPJ (65.6%) had a worsening of the PED at 7 years (PIPJ: 22.8° ±4.1; MPJ: 4.8 ± 1.7°), of these 2 PIPJ out of 7 (28.6%) had a PED>20° and no MPJ had a PED>20°. Considering the single finger treated in the patients who completed the follow-up, the TPED varied from a mean preoperative value of 64.2° (±4.3) to 11.4° (±1.1) to 12 weeks (p = 0.02) and 14.7° (±1.3) after 7 years (p = 0.07). After treatment the function of the affected hand was improved. QuickDASH improved from 23.6 (±5.2) to 7.6 (±3.2) points after 12 weeks and changed to 8.4 (±4.1) after 7 years (Fig. 3). No statistically significant variation was observed in the QuickDASH between treated patients on the PIPJ and on the MPJ.
Fig. 2.
Survival without repeated treatment. MPJ: metacarpophalangeal joints; PIPJ: proximal interphalangeal joints.
Fig. 3.
PED and TPED values by type of articulation expressed in degrees and referred to pre-operative measurements, after 12 weeks and after 7 years of follow-up. PED: Passive Extension Deficit; TPED: Total Passive Extension Deficit.MPJ: metacarpophalangeal joints; PIPJ: proximal interphalangeal joints.
All patients demonstrated a high grade of satisfaction for the treatment received, in fact the GSI was 8.4 (±0.9) 12 weeks after the treatment and changed to 8.3 (±1.2) at the 7-year follow-up.
3.3. Complications and adverse or unanticipated events
All patients had at least one complication. Bruising or ecchymoses were the most frequent (39 cases in one week), and all resolved within two weeks. Swelling was observed in 28 cases at one week; the majority resolved within four weeks, and all resolved within three months. Other frequent complications were oedema, local lymphadenitis and skin lesion. The latter, caused by forced extension, was the complication that endured the longest, but it did not impair hand function excessively. No cases of systemic allergic reaction have been recorded. The average number of complications per patient was 2.7 at the time of forced extension, 1.7 at one week, 0.6 at two weeks, 0.3 at four weeks, and 0.2 at eight weeks. Hence 95% of patients returned to work within two weeks after injection. No further complications were found at the 7-year follow-up.
4. Discussion
4.1. Background
Many studies have evaluated efficacy and safety of CCH.9 Only a few studies have been published with a very long follow up (5 years or more).14, 15, 16 Watt et al. published an 8-year follow-up study with a very small sample (8 patients).16 Six out of eight patients had recurrence, but it was less severe than the starting contracture. Peimer et al. published the retrospective results of CORDLESS study. They reported a recurrence rate of 47% that was comparable with surgical treatment. They surgically re-treated patients with a 30° contracture increase and with a palpable cord, thus 32% of MPJ and 46% of PIPJ needed to be treated again.15 Werlinrud et al. prospectively evaluated 107 injected joints. After 5 years, 71 joints (79% MPJ and 49% PIJ) did not undergo a second treatment and patients who did not undergo a second treatment were generally satisfied, in spite of relapsed disease.14 A very long follow up is useful to understand whether CCH could be a definitive treatment for DD, and if it can replace traditional surgery.17, 18, 19 In the USA, where CCH has been extensively used for years, the number of patients receiving fasciotomies and fasciectomies progressively decreased from 2007 to 2014, especially in patients with more comorbidities and in elderly patients.10 The percentage of recurrence after open surgery is not well defined, but it is not negligible, considering a follow-up of more than 5 years, up to more than 10 years.4,5,20 Neither CCH injection nor fasciectomy prevent relapsing of DD: recurrence and progression of disease are not clearly predictable.4,5,21 Relapse could be related to histological patterns.22,23
4.2. Present investigation
In our study, relapse was evident in almost all PIPJ we injected, but it was significant only in two cases (more than 20° contracture increase).Patients who received a further treatment (open surgery limited fasciectomy) had a good result from treatment, even if they were finally excluded. This means that a CCH injection can control the disease for a defined period of time, without compromising the result of any secondary procedure. There is still inadequate evidence regarding treating recurrence using CCH injection.17 Among patients injected in PIPJ, only two worsened more than 20°on PED, but they did not ask to be treated again within 7 years, because they did not feel the need for it. Therefore, we assumed that contractures increasing in a single joint do not necessarily reflect clinical impairment. Moreover, it should also not be forgotten that at last follow-up TPED values were biased from recurrence and reactivation (progression) of disease in other joints not previously injected. Our results suggest that CCH can control DD for a long period of time, leading to a good recovery of hand function.
Considering all our 43 patients (excluding two patients who died before the end of the study), only four patients needed to be treated again within 7 years. Hence 86.7% of our patients concluded their treatment with only one injection 7 years earlier. We cannot exclude that some of our patients may need to be treated again in the future, therefore a longer follow up is necessary. Nevertheless, the final outcome at 7 years is acceptable to us, in accordance with the findings of function and satisfaction.
This study is a prospective study with a very long follow-up, the longest one in Italy. Our Hand Surgery Unit was one of the first to perform CCH injections on a considerable number of patients in Italy. In agreement with other studies,14,24 our study investigates the effect of CCH on the whole function of the upper limb, and it demonstrates that CCH improves function, even if recurrence occurs. Our results confirm, with a very long follow-up, that PIPJ improve less than MPJ.14,15,25 Our study had some limitations. The cohort is relatively small, and no control group has been considered. Hand function was measured using subjective scores.
5. Conclusion
Currently CCH injection is considered as a first choice for treatment of DD in our centre. Fasciectomy is considered as a second choice, useful in case of recurrence and disease progression. CCH injection is technically less difficult than fasciectomy, but it should be used by an expert surgeon to minimize complications risk. Recurrence is possible within 7 years, but CCH injection allowed a long period of time with an acceptable hand function.
Source of funding
The authors report no source of funding.
Seven-year clinical outcomes after collagenase injection in patients with Dupuytren's disease: a prospective study.
Compliance with ethical standards and informed consent: All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee (Ethics Committee of the Policlinico Universitario “Agostino Gemelli”, Rome; Protocol P/488-857-872-1041-1113/CE/2012) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Declartion of competing interest
The authors report no conflicts of interest.
Acknowledgments
The authors thank Carmen Innes, an independent medical writer, who provided language editing and journal styling prior to submission on behalf of Springer Healthcare Communications. This editorial assistance was supported by Swedish Orphan Biovitrum s.r.l. (Sobi).
References
- 1.McFarlane R.M. Patterns of the diseased fascia in the fingers in Dupuytren's contracture. Displacement of the neurovascular bundle. Plast Reconstr Surg. 1974;54(1):31–44. doi: 10.1097/00006534-197407000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Andrew J.G. Contracture of the proximal interphalangeal joint in Dupuytren's disease. J Hand Surg Br. 1991;16(4):446–448. doi: 10.1016/0266-7681(91)90024-i. [DOI] [PubMed] [Google Scholar]
- 3.Eaton C. Evidence-based medicine: Dupuytren contracture. Plast Reconstr Surg. 2014;133(5):1241–1251. doi: 10.1097/PRS.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 4.Becker G.W., Davis T.R.C. The outcome of surgical treatments for primary Dupuytren's disease--a systematic review. J Hand Surg Eur. 2010;35(8):623–626. doi: 10.1177/1753193410376286. [DOI] [PubMed] [Google Scholar]
- 5.Werker P.M.N., Pess G.M., Van Rijssen A.L., Denkler K. Correction of contracture and recurrence rates of Dupuytren contracture following invasive treatment: the importance of clear definitions. J Hand Surg Am. 2012;37(10):2095–2105. doi: 10.1016/j.jhsa.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong J.R., Hurren J.S., Logan A.M. Dermofasciectomy in the management of Dupuytren's disease. J Bone Joint Surg Br. 2000;82(1):90–94. doi: 10.1302/0301-620x.82b1.9808. [DOI] [PubMed] [Google Scholar]
- 7.Hall P.N., Fitzgerald A., Sterne G.D., Logan A.M. Skin replacement in Dupuytren's disease. J Hand Surg Br. 1997;22(2):193–197. doi: 10.1016/s0266-7681(97)80061-7. [DOI] [PubMed] [Google Scholar]
- 8.Hurst L.C., Badalamente M.A. Nonoperative treatment of Dupuytren's disease. Hand Clin. 1999;15(1):97–107. (vii) [PubMed] [Google Scholar]
- 9.Smeraglia F., Del Buono A., Maffulli N. Collagenase clostridium histolyticum in Dupuytren's contracture: a systematic review. Br Med Bull. 2016;118(1):157–166. doi: 10.1093/bmb/ldw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipman M.D., Carstensen S.E., Deal D.N. Trends in the treatment of dupuytren disease in the United States between 2007 and 2014. Hand. 2017;12(1):13–20. doi: 10.1177/1558944716647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurst L.C., Badalamente M.A., Hentz V.R. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med. 2009;361(10):968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 12.Gilpin D., Coleman S., Hall S., Houston A., Karrasch J., Jones N. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren's disease. J Hand Surg Am. 2010;35(12) doi: 10.1016/j.jhsa.2010.08.007. 2027-2038.e1. [DOI] [PubMed] [Google Scholar]
- 13.Felici N., Marcoccio I., Giunta R. Dupuytren contracture recurrence project: reaching consensus on a definition of recurrence. Handchir Mikrochir Plast Chir. 2014;46(6):350–354. doi: 10.1055/s-0034-1394420. [DOI] [PubMed] [Google Scholar]
- 14.Werlinrud J.C., Hansen K.L., Larsen S., Lauritsen J. Five-year results after collagenase treatment of Dupuytren disease. J Hand Surg Eur. 2018;43(8):841–847. doi: 10.1177/1753193418790157. [DOI] [PubMed] [Google Scholar]
- 15.Peimer C.A., Blazar P., Coleman S., Kaplan F.T.D., Smith T., Lindau T. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [collagenase option for reduction of dupuytren long-term evaluation of safety study]): 5-ysear data. J Hand Surg Am. 2015;40(8):1597–1605. doi: 10.1016/j.jhsa.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Watt A.J., Curtin C.M., Hentz V.R. Collagenase injection as nonsurgical treatment of Dupuytren's disease: 8-year follow-up. J Hand Surg Am. 2010;35(4):534–539. doi: 10.1016/j.jhsa.2010.01.003. 539.e1. [DOI] [PubMed] [Google Scholar]
- 17.Nordenskjold J., Lauritzson A., Walden M., Kopylov P., Atroshi I. Surgical fasciectomy versus collagenase injection in treating recurrent Dupuytren disease: study protocol of a randomised controlled trial. BMJ Open. 2019;9(2) doi: 10.1136/bmjopen-2018-024424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raisanen M.P., Karjalainen T., Goransson H. DupuytrEn Treatment EffeCtiveness Trial (DETECT): a protocol for prospective, randomised, controlled, outcome assessor-blinded, three-armed parallel 1:1:1, multicentre trial comparing the effectiveness and cost of collagenase clostridium histolyticum, percutaneous needle fasciotomy and limited fasciectomy as short-term and long-term treatment strategies in Dupuytren's contracture. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-019054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proietti L., Scaramuzzo L., Schiro G.R., Sessa S., Tamburrelli F.C., Cerulli G. Degenerative facet joint changes in lumbar percutaneous pedicle screw fixation without fusion. Orthop Traumatol Surg Res. 2015;101(3):375–379. doi: 10.1016/j.otsr.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Proietti L., Schiro G.R., Sessa S., Scaramuzzo L. The impact of sagittal balance on low back pain in patients treated with zygoapophysial facet joint injection. Eur spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2014;23(Suppl 6):628–633. doi: 10.1007/s00586-014-3559-5. [DOI] [PubMed] [Google Scholar]
- 21.Simon-Perez C., Alia-Ortega J., Garcia-Medrano B. Factors influencing recurrence and progression of Dupuytren's disease treated by Collagenase Clostridium histolitycum. Int Orthop. 2018;42(4):859–866. doi: 10.1007/s00264-017-3690-0. [DOI] [PubMed] [Google Scholar]
- 22.Rombouts J.J., Noel H., Legrain Y., Munting E. Prediction of recurrence in the treatment of Dupuytren's disease: evaluation of a histologic classification. J Hand Surg Am. 1989;14(4):644–652. doi: 10.1016/0363-5023(89)90183-4. [DOI] [PubMed] [Google Scholar]
- 23.Balaguer T., David S., Ihrai T., Cardot N., Daideri G., Lebreton E. Histological staging and Dupuytren's disease recurrence or extension after surgical treatment: a retrospective study of 124 patients. J Hand Surg Eur. 2009;34(4):493–496. doi: 10.1177/1753193409103729. [DOI] [PubMed] [Google Scholar]
- 24.Bradley J., Warwick D. Patient satisfaction with collagenase. J Hand Surg Am. 2016;41(6):689–697. doi: 10.1016/j.jhsa.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Hansen K.L., Werlinrud J.C., Larsen S., Ipsen T., Lauritsen J. Difference in success treating proximal interphalangeal and metacarpophalangeal joints with collagenase: results of 208 treatments. Plast Reconstr surgery Glob open. 2017;5(4) doi: 10.1097/GOX.0000000000001275. [DOI] [PMC free article] [PubMed] [Google Scholar]